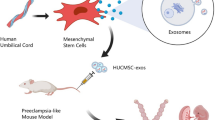
Abstract
Preeclampsia (PE) is a common morbid complication during pregnancy, affecting 2%-8% of pregnancies globally and posing serous risks to the health of both mother and fetus. Currently, the only effective treatment for PE is timely termination of pregnancy, which comes with increased perinatal risks. However, there is no effective way to delay pathological progress and improve maternal and fetal outcomes. In light of this, it is of great significance to seek effective therapeutic strategies for PE. Exosomes which are nanoparticles carrying bioactive substances such as proteins, lipids, and nucleic acids, have emerged as a novel vehicle for intercellular communication. Mesenchymal stem cell-derived exosomes (MSC-Exos) participate in various important physiological processes, including immune regulation, cell proliferation and migration, and angiogenesis, and have shown promising potential in tissue repair and disease treatment. Recently, MSC-Exos therapy has gained popularity in the treatment of ischaemic diseases, immune dysfunction, inflammatory diseases, and other fields due to their minimal immunogenicity, characteristics similar to donor cells, ease of storage, and low risk of tumor formation. This review elaborates on the potential therapeutic mechanism of MSC-Exos in treating preeclampsia, considering the main pathogenic factors of the condition, including placental vascular dysplasia, immunological disorders, and oxidative stress, based on the biological function of MSC-Exos. Additionally, we discuss in depth the advantages and challenges of MSC-Exos as a novel acellular therapeutic agent in preeclampsia treatment.
Similar content being viewed by others
Background
Preeclampsia (PE), characterized by new-onset hypertension after 20 weeks of gestation, is a common pregnancy complication concomitant with multisystem functional obstacles such as elevated liver enzymes, thrombocytopenia, proteinuria, renal insufficiency, persistent severe headache, and seizures [1]. It is becoming increasingly common in the developed countries and remains a major cause of maternal and fetal morbidity and mortality in the developing countries [2]. The pathogenesis of PE is complex, involving dysfunctions of trophoblasts, deficient spiral arterial remodeling, maldevelopment of the placental vasculature leading to maternal–fetal perfusion deficiency, oxidative stress, imbalance of maternal–fetal immune regulation [3]. The only effective treatment for PE is pregnancy termination, but it comes with risks such as fetal growth restriction and preterm delivery. Moreover, pregnant women have an increased risk of cardiovascular and kidney diseases after delivery [3]. Therefore, identifying a treatment that can effectively delay the pathological progression of PE is important for improving maternal and neonatal outcomes. Mesenchymal stem cell-derived exosomes (MSC-Exos) exert a vital regulatory influence on endothelial cell [4] and trophoblast [5] function, immune response [6], and antioxidant stress [7]. They hold great promise as an alternative therapeutic option to MSCs, offering a novel therapeutic avenue for the treatment of PE.
MSCs possess multidirectional differentiation potential, and exhibit advantages such as rapid self-renewal, stable doubling time, and high proliferation capacity [8]. MSCs attenuate the pathological progression of PE and enhance outcomes for both mother and infant [9, 10]. Initially, MSCs were believed to rely on homing and differentiation for their therapeutic effects; however, subsequent studies have underscored the role of paracrine action [11]. As one of the main paracrine pathways of MSCs, exosomes transport a diverse range of bioactive substances secreted by MSCs and serve various transport functions. MSC-Exos offer advantages over MSCs, including enhanced biological stability, reduced immunogenicity, lower lung interception, and the ability to traverse the placental barrier [12, 13]. Moreover, they mitigate potential risks associated with chromosomal variations, tumourigenicity, thrombosis, and immune rejection that may arise during MSCs therapy [14]. MSC-Exos have progressively emerged as a focal point of research in numerous fields, encompassing inflammation [15], autoimmunity [16], ischaemia [17], cerebrovascular, and neurodegenerative disorders [18]. Preclinical data indicate that treating disorders with MSC-Exos may offer greater safer and more versatility compared to MSC-base therapies [19].
Exosomes of mesenchymal stem cells
Exosomes, initially discovered in sheep reticulocytes in 1983, originate from the nuclear endosome lysosome system [20]. Their production involves intricate molecular mechanisms encompassing endocytosis, content sorting, and trafficking. The key molecules involved in this process include ceramide [21], tetra-spanning membrane proteins [22, 23], and the endosomal sorting complex required for transport [24]. Exosomes are extracellular vesicles with a bilayered lipid membrane structure similar to that of the plasma membrane. With a diameter of 40–100 nm and a density of 1.13–1.19 g/mL, these vesicles contain proteins, mRNA, miRNA, DNA, lipids, cytokines, transcription factor receptors, and other genetic material. Exosomes proficiently transfer bioactive substances, which are prone to inactivation or degradation through various pathways, to target cells, modulating their biochemical characteristics and participating in regulatory processes such as tissue repair, tumour diagnosis and treatment, and immunomodulation [20, 25]. The interaction between exosomes and target cells encompasses three main pathways: direct activation of target cell membrane receptors, modification of the extracellular environment surrounding target cells, fusion with the cell membrane, and release of bioactive molecules into the target cells [26]. Under transmission electron microscopy, exosomes exhibit a distinctive cup-shaped appearance, while they appear as isolated spheres under low-temperature electron microscopy [27]. Compared to synthetic vectors like liposomes and nanoparticles, exosomes possess unique advantages in disease diagnosis and treatment due to their inherent nature and heterogeneity [20]. They manifest diverse functions, including promoting cell proliferation and migration, regulating immune and anti-inflammatory responses, and are widely used in disease repair, including facilitating endometrial repair in uterine adhesion disease [28], ameliorating symptoms of ovarian insufficiency and polycystic ovary syndrome [29], treating PE [30, 31], repairing spinal cord injury [32], and expediting cutaneous wound healing in diabetic mice [33, 34].
Exosomes exist in multiple body fluids such as blood, breast milk, semen, and saliva [35]. Almost all types of normal cells, including human umbilical vein endothelial cells (HUVECs), MSCs, T cells, B cells, macrophages, dendritic cells, and natural killer cells, produce exosomes. Among these cells, MSCs are multipotent stem cells with self-renewing and multidirectional differentiation capabilities. They exhibit a strong paracrine activity and secret many exosomes [20]. MSCs can be exacted from various sources, such as placenta, umbilical cord blood, amniotic fluid, adipose tissue, bone marrow, and even brain tissue[36]. Compared to other types of stem cells (embryonic stem cells and induced pluripotent stem cells), MSCs have several advantages: 1) relatively easy extraction from various tissues such as bone marrow, peripheral blood, and adipose tissue, 2) low cost of isolation and culture, 3) immunosuppressive ability, 4) versatile treatments for patients with allotransplantation and autologous transplantation [37], and 5) low immunogenicity owing to the lack of MHC-II and low expression of MHC-I, similar to their parental cells [16]. Since 2010, over 100 registered clinical trials have been conducted to evaluate the effectiveness of MSCs in treating various diseases [38], such as osteoarthritis [21], COVID-19 [39], cerebral palsy [40], and heart failure [41].
Therapeutic potential of MSC-Exos in PE
MSC-Exos have the potential to therapeutically to delay the progression of PE and enhance outcomes by enhancing trophoblast function [31], promoting placental angiogenesis [42], regulating immune responses [6], reducing inflammatory [43] and oxidative stress [7] (Table 1).
MSC-Exos promote trophoblast proliferation, migration, and invasion
The placenta is crucial for the exchange of gases, nutrients, and metabolites between mother and fetus. Proper development of placental vessels are the premise and basis for the smooth progression of pregnancy and adequate blood perfusion to the fetus. PE is characterized by impaired placentation, abnormal function of extravillous trophoblast cell, and compromised uterine spiral artery remodeling during placental development [44,45,46].
MSC-Exos have been shown to enhance the biological functions of trophoblasts. For instance, patients with PE have higher expression of let-7b and lower expression of FOXO1 in the placental tissues compared to control. H19 acting as a competitive RNA for let-7b directly targets FOXO1. MSCs-Exos can transport H19 to trophoblasts, resulting in reduced let-7b expression, increased FOXO1 expression, activation the AKT signaling pathway, and ultimately enhancing the invasion and migration of trophoblasts and inhibiting their apoptosis. This study offers new insights into PE treatment [47]. In another study, exosomal miR-139-5p from human umbilical cord mesenchymal stem cells (hUCMSC-Exos) has been demonstrated to accelerate trophoblast invasion and migration, inhibited trophoblast apoptosis by downregulating protein tyrosine phosphatase expression, and activated the ERK/MMP-2 pathway, thereby improving PE symptoms in rats [30]. Additionally, a study discovered that the expression of miR-18b was downregulated while Notch2, TIM3, and mTORC1 levels were increased in the placenta of patients with PE. hUCMSC-Exos promoted trophoblast migration by secreting microRNA-18b to inhibit Notch2 expression in trophoblasts. They further applied hUCMSC-Exos to a rat model of PE and found that they improved the symptoms of PE in pregnant rats [48] (Fig. 1). Furthermore, hUCMSC-Exos were found to promote trophoblast migration and invasion by transferring miR-101 to trophoblasts and inhibiting BRD4 expression. In addition to promoting trophoblast migration and invasion, MSC-Exos promoted autophagy and trophoblast proliferation under hypoxic conditions [49]. Jiang et al. discovered that hUCMSC-Exos inhibited FSTL3 expression by transmitting miR140-5p, and further suppressed trophoblast inflammation under hypoxic conditions and promoted proliferation, migration, and invasion of hypoxic trophoblasts [50]. Exosomes derived from amniotic MScs enhance autophagy in trophoblasts by inhibiting the EZH2/mTOR signalling pathway, thus promoting their proliferation [5].
In summary, MSC-Exos promote the proliferation, migration, and invasion of trophoblasts and improve spiral artery remodeling, offering a new avenue for research on PE treatment.
Mesenchymal stem cell exosomes promote angiogenesis by regulating endothelial cell function
Endothelial cells maintain vascular integrity, regulate thrombosis, and transport and perform barrier functions. They are an essential component of placental blood vessels and crucial for the proper functioning of the placenta. In patients with preeclampsia, placental dysfunction is often accompanied by impaired HUVEC function [20]. Therefore, targeted modulation of HUVEC function may be an effective therapeutic option for treating PE.
It has been found that hUCMSC-Exos can restore soluble fms-like tyrosine kinase-1(sFlt-1)-induced endothelial dysfunction in preeclampsia, thereby improving adverse pregnancy outcomes in PE mice [51]. Zhu et al. demonstrated that adipose tissue mesenchymal stem cell exosomes (ADMSC-Exos) accelerate the proliferation of HUVEC in a concentration-dependent manner [52]. In addition to the aforementioned effects, ADMSC-Exos transfer microRNA-125a to HUVEC, directly inhibiting its downstream target delta-like ligand 4 and promoting endothelial tip cell formation and angiogenesis [53]. Bone marrow-derived exosomes (BMMSC-Exos) upregulate vascular endothelial growth factor (VEGF) and human angiopoietin-1 (Ang-1) expression in HUVECs via microRNA-126. They also activate the PI3K/AKT signaling pathway by targeting PIK3R2, thereby promoting angiogenesis [54]. Gao et al. conducted observations revealing that after MSC-Exos derived from the placental tissues of patients with gestational diabetes with knockdown of microRNA-130b-3p upregulated ICAM-1 expression, leading to enhanced proliferation, migration, and angiogenesis ability of HUVECs [55]. Qu et al. revealed that hUCMSC-Exos overexpressing miR-126-3p downregulated the expression of SPRED-1 and PIK3R2, activating the ERK1/2 and AKT signaling pathways, and promoting the proliferation, migration, and angiogenesis of HUVECs [56]. Additionally, proteins carried by MSC-Exos play crucial roles in endothelial cells. As an illustration, hUCMSC-Exos activated β-catenin signaling pathway in endothelial cells through the Wnt4 protein, thereby promoting proliferation, migration, and angiogenesis of endothelial cells. Furthermore, in a rat skin-burn model, hUCMSC-Exos accelerated wound healing in vivo by promoting angiogenesis [57].
Overall, MSC-Exos enhance the proliferation, migration, and angiogenesis of endothelial cells through diverse molecular mechanisms and have the potential to promote placental angiogenesis and improve placental blood perfusion (Fig. 2).
Immunomodulatory and anti-inflammatory effects of exosomes of mesenchymal stem cells
Immune regulation is crucial for ensuring a safe pregnancy. In solid organ transplantation, the recipient’s immune system recognizes alloantigens expressed by grafts, leading to immune attacks and rejection of transplanted organs, which can only be prevented by therapeutic immunosuppression. Similarly, during pregnancy, the maternal immune system may recognize antigens expressed by the fetus from father, potentially leading to rejection. The maternal immune tolerance mechanism suppresses such immune rejection. Therefore, maintaining normal maternal immune tolerance is essential for a successful pregnancy. Disruption of normal immune tolerance leads to pathological conditions such as PE, which has been associated with immune imbalance at the maternal–foetal interface [58]. In addition, placental ischaemia and hypoxia can cause an increase in reactive oxygen species (ROS) and ATP deficiency, ultimately promoting excessive production of inflammatory mediators, and contributing to PE [59].
MSC-Exos have emerged as a potential treatment for PE by regulating the uterine immune microenvironment. Taglauer et al. utilized a mouse model with preeclampsia-like features and administered hUCMSC-Exos during early pregnancy, revealing that hUCMSC-Exos promote the local recruitment of NK cells and macrophages in the uterus, as well as the expression of immune factors such as interleukin-10 (IL-10), interferon-gamma, and tumour necrosis factor-α (TNF-α). By regulating the immune microenvironment at the maternal–fetal interface, pregnancy outcomes were improved [60]. Additionally, MSC-Exos can exert anti-inflammatory effects by modulating the phenotype of inflammatory immune cells, such as inducing dendritic cells (DCs) and M1 macrophages to transform into tolerant dendritic cells (tolDCs) [61] and M2 macrophages [62], respectively. Studies have shown that ADMSC-Exos could induce mouse bone marrow-derived DCs to become tolDCs, thereby suppressing the immune response [61]. Furthermore, miRNAs enriched in MSC-exos are associated with macrophage immunoregulation and their immunomodulatory function can promote endometrial regeneration and fertility recovery [62]. In vitro and in vivo study have demonstrated that MSC-Exos inhibited the expression of pro-inflammatory cytokines such as IL-1β, IL-6, IL-12 and TNF-α, while increasing the expression of anti-inflammatory cytokines such as IL-10 and TGF-β [63, 64].
Hence, MSC-Exos play a crucial role in immune modulation and anti-inflammatory effects, presenting vast prospects for their application in the treatment of preeclampsia (Fig. 3).
The possible modulatory effect of MSC-Exos on the dysregulated immune system components and oxidative stress in the experiments. The expression of oxidative stress factors can be reduced by hUC-MSC-Exos. BM-MSC-Exos and hUC-MSC-Exos can reduce over-expressed proinfammatory cytokines and increase the expression of anti-inflammatory cytokines. hAD-MSC-Exos can induce dendritic cells (DC) to transform into tolerant dendritic cells (tolDC) hUC-MSC-Exos and BM-MSC-Exos can induce M1 macrophages to transform into M2 macrophages
Antioxidant properties of mesenchymal stem cell exosomes
Oxidative stress plays a crucial role in the pathophysiology of PE [3]. Insufficient placental vascular perfusion resulting in ischaemia and hypoxia can lead to an increased release of ROS and reactive nitrogen species and decreased secretion of antioxidant factors. This imbalance between pro-oxidative and antioxidant capacities induces oxidative stress-related damage to proteins, lipids, and DNA, ultimately contributing to pregnancy complications such as PE [65]. Therefore, effective treatments that inhibit the release of oxidative stress factors and control oxidative stress response should be identified.
Several studies have indicated that MSC-Exos can effectively reduce oxidative stress levels, and alleviate tissue ischemia–reperfusion injury, and inhibit ROS-induced cell apoptosis [66, 67]. Under high glucose-induced oxidative stress conditions, hUCMSC-exos demonstrated concentration-dependent reduction in the expression of the oxidative stress factors NOX1 and NOX4 in HUVECs, indicating their ability to ameliorate oxidative stress damage [68]. Extracellular vesicles secreted by human decidual MSCs significantly reduced malondialdehyde level(a by-product of lipid peroxidation that causes systemic endothelial dysfunction in pregnant women with PE) and suppressed IL-6 expression in HUVECs treated with PE serum. This inhibition of oxidative stress and inflammation, consequently enhanced the proliferation and angiogenesis of HUVECs [69]. These findings suggest that MSC-Exos may have therapeutic potential for addressing oxidative stress in PE.
Experimental research on enhancing pregnancy outcomes using mesenchymal stem cells-derived exosomes
PE is a serious pregnancy complication, and preterm delivery or miscarriage may occur during disease progression. MSC-Exos effectively reduced premature delivery and abortion rates. MSC derived from human amniotic fluid (AF-MSC) have been shown to inhibit inflammatory responses in trophoblasts induced by lipopolysaccharides (LPS) through the paracrine pathway. AF-MSC-Exos, which are rich in miR-146a-5p, miR-548e-5p, may serve as molecular mediators in reducing the LPS-induced trophoblast inflammation [59]. In rat models of abortion, the injection of BMMSC-Exos into the uterine horns resulted in significantly lower expression levels of IL-12, TNF-α, and IFN- γ at the maternal-foetal interface while the expression levels of IL-4 and IL-10 were adverse. This suggests that MSC-Exos can regulate the uterine immune response by inhibiting the Th1-type immune response and promoting the Th2-type immune response, thereby reducing the abortion rate [70]. These findings offer new hope for improving PE pregnancy outcomes.
Enhancing the therapeutic effect of MSC-Exos
The composition of biomolecules in exosomes varies depending on the microenvironment and source cell conditions. Exosome therapy shows promise as a method for treating PE due to its advantages, including lower immunogenicity, enhanced biological stability, controllable graft doses, and efficient targeted delivery to cells. However, despite a higher homing rate compared to MSC therapy, most intravenously infused exosomes become trapped in the liver by the mononuclear phagocytic system, preventing them from reaching the target site of injury [71]. To overcome this limitation and maximize therapeutic effects, various optimization methods have been explored, such as genetic modification of MSCs to release functional exosomes, direct modification of exosomes with homing peptides to enhance targeted homing ability, pretreatment of MSCs with bioactive molecules, or modification of MSC culture conditions to enhance the therapeutic potential of MSC-Exos.
Multiple studies have demonstrated the therapeutic effects of hypoxic preconditioned MSCs in various animal disease models [72,73,74]. Hypoxic preconditioning of MSCs can elevate the levels of paracrine factors in MSC-Exos, thereby enhancing their reparative actions [75,76,77,78,79]. This includes increased upregulation of pro-angiogenic proteins like VEGF, epidermal growth factor, fibr0blast growth factor, and their receptors [77], promoting proliferation, migration, and tubular ability of HUVECs.
Genetic engineering is also a valuable approach to enhance the therapeutic effects of MSC-Exos [80]. Lentivirus-infected MSCs carrying an overexpression vector of HIF-1α produce exosomes that upregulate the expression of pro-angiogenic factors (VEGF, Ang-1, and PDGF) in hypoxia-treated HUVECs, effectively reversing the impaired migration, proliferation, and pro-angiogenic ability caused by hypoxia treatment [81]. These findings highlight the efficacy of genetic modification in enhancing the functional properties of MSC-Exos, particularly in promoting the function of endothelial cells and trophoblasts.
Despite the promising potential of exosomes in therapeutic applications, their targeting ability in animal experiments is limited, leading to challenges such as short half-life and reduced therapeutic efficacy. Biochemical engineering offers a simpler, faster, and more effective approach by directly modifying exosomes without the need for cell manipulation, thereby enhancing specific exosome secretion [18]. In a study, a central nervous system-specific rabies virus glycoprotein (RVG) peptide was conjugated to MSC-Exos surfaces using G-protein coupling, which outperformed unmodified exosomes in enhancing cognitive function in APP/PS1 mouse model [82]. Another example is the use of a tumor-homing peptide iRGD, which selectively binds to the placental surface in humans and mice without interfering with normal development. Hence, iRDG-exosomes may contain vital proteins or genes that specifically target the placenta and play crucial roles in PE treatment [83].
Considering the potential impact of multiple chemical reactions involved in biochemical engineering on exosome function, it is crucial to explore less invasive methods for modifying exosomes and enhancing their targeting abilities. One promising strategy is to load exogenous substances into the interior of exosomes instead of performing surface modifications [18]. In a study, iron oxide nanoparticles (IONP) were incubated with MSCs to generate exosome-mimetic nanovesicles (NV-IONP). These NV-IONPs can be magnetically guided to target injured spinal cord tissue with the aid of an external magnetic field (MF). Furthermore, the IONPs slowly release iron ions to activate the JNK and c-Jun signaling cascade in MSCs, enabling NV-IONPs to carry more therapeutic growth factors and improve the proliferation and migration capabilities of HUVECs [84].
MSCs loaded with specific drugs can optimize the composition of their secreted exosomes, leading to improved therapeutic outcomes. Various compounds such as atorvastatin (ATV) [85], pioglitazone (PGZ) [86], baicalin [87], oxytocin [88], curcumin [89], and hemin [90] have been explored to enhance MSCs’ survival rates and functions. For instance, exosomes derived from ATV-pretreated MSCs enhanced HUVECs’ migration and angiogenesis via the upregration of lncRNAH19 expression [91].
Discussion
PE can result in adverse maternal and fetal pregnancy outcomes, which are common pregnancy complications [3]. However, the treatment options for PE are limited, often leading to pregnancy termination due to ineffective control of disease progression [92]. MSC-Exos carry numerous proteins, nucleic acids, and lipids that can be transported across cell membranes to modify signal transduction and gene expression in the target cells [33], thereby regulating the biological function of the target cells and promoting angiogenesis by improving the function of trophoblasts and endothelial cells. Furthermore, MSC-Exos play crucial roles in immune response regulation[6], anti-inflammatory processes [43], and antioxidant stress [7]. In conclusion, MSC-Exos have the potential to effectively treat PE by regulating multiple aspects of its pathogenesis.
Owing to potential chromosomal variations, tumourigenicity, thrombosis, immune rejection, and other challenges, progress in using MSCs for PE treatment has limitations. Exosomes, the crucial paracrine product of MSCs, serve as carriers to transport bioactive components to the surrounding cells and the circulatory system, even capable of traversing the blood–brain barrier [82]. Nanoparticles can also cross the placental barrier, exerting therapeutic effects simultaneously on both the mother and fetus [13]. So far, numerous clinical trials have been conducted on MSC-Exos, with cancer patients confirming their positive effects and absence of observed side effects, indicating the safety and tolerability of exosome therapy [93]. In terms of tumourigenicity and immune rejection, MSC-Exos transplantation outperforms MSCs. Sun et al. conducted a study evaluating the safety of hUCMSC-Exos transplantation and suggested that they were pyrogen-free, causing no side effects on haemolysis, liver and kidney function, or haematological indices. Notably, they do not induce vascular or muscle stimulation or systemic allergic reactions. The results demonstrated the excellent tolerance of hUCMSC-Exos in animal models [94]. Moreover, exosomes, as inert vesicles, reduce the risk of long-term side effects, such as arrhythmias, calcification, and thrombosis [95, 96]. Unlike MSCs, exosomes lack replication capability, thereby avoiding uncontrolled division and greatly reducing the risk of tumour formation during proliferation [97]. Additionally, the surface of MSC-Exos can be modified to engineer them into ligand-bound exosomes, enabling evasion of immune responses by binding to specific cells targeting damaged tissue [98]. Regarding preservation conditions, exosomes exhibit greater stability, ease of storage, and transport in vitro, with the ability to be stored at -20℃ for 6 months without significant changes in biochemical activity [12].
MSC-Exos encounter several challenges as therapeutic agents. One major challenge is the accurate quantification of microRNAs entering and exiting exosomes, given their minute quantities [99]. Currently, three main platforms are used for microRNA quantification: quantitative reverse transcriptase-polymerase chain reaction (RT-PCR), microarrays, and next-generation sequencing techniques. While next-generation sequencing allows unbiased analysis of miRNAs, it does not provide RNA quantification. Microarray analysis and quantitative RT-PCR assays requires predesigned primers, which may overlook previously unidentified microRNAs. Furthermore, although high levels of sphingomyelin have been detected in exosomes, comprehensive liposome analysis and characterization of exosomal lipids is a recent development [100]. It is crucial to eliminate the interference from unknown secretory factors in exosomes as harmful cytokines in MSCs can also be secreted by paracrine cells [101]. Clinical applications demand time-saving, low-cost, and convenient methods. However, current strategies for isolating exosomes present opposite features, including laborious and inefficient processes, limited capacity, and short-term viability, which restrict the clinical application of exosomes. Additionally, unresolved technical issues such as side effects, optimal therapeutic dosage, and administration routes pose significant challenges for exosome therapy [100]. Further experimental studies on MSC-Exos are necessary before extensive clinical trials can be conducted.
Conclusions
The pathogenesis of preeclampsia remains unclear, and effective treatment methods are currently lacking. MSC-Exos play vital roles in its potential pathogenesis. Further research on specific molecules within MSC-Exos not only enhances our understanding of preeclampsia’s pathogenesis, but also enables the identification of more specific and sensitive biomarkers for its onset. Additionally, it holds promise for the treatment of preeclampsia. However, both basic and clinical research on the mechanism of action of MSC-Exos in preeclampsia are still in its early stages, necessitating further in vivo and in vitro exploration in the future.
Availability of data and materials
Not applicable.
Abbreviations
- PE:
-
Preeclampsia
- MSC-Exos:
-
Mesenchymal stem cell-derived exosomes
- MSCs:
-
Mesenchymal stem cells
- miRNA:
-
MicroRNA
- HUVECs:
-
Human umbilical vein endothelial cells
- MHC-II:
-
Major histocompatibility complex II
- MHC-I:
-
Major histocompatibility complex I
- COVID-19:
-
Corona virus disease 2019
- let-7b:
-
Lethal-7b
- FOXO1:
-
Forkhead box protein O1
- AKT:
-
Protein kinase B
- hUCMSC-Exos:
-
Exosomes derived from human umbilical cord mesenchymal stem cells
- ERK:
-
Extracelluar signal-regulated kinase
- MMP2:
-
Matrixmetalloproteinase2
- TIM3:
-
T cell immunoglobulin domain and mucin domain-3
- mTORC1:
-
Mammalian target of rapamycin 1
- BRD4:
-
Bromodomain-containing protein 4
- FSTL3:
-
Recombinant follistatin like protein 3
- EZH2:
-
Enhancer of zeste homolog 2
- mTOR:
-
Machanistic target of rapamycin
- ADMSC-Exos:
-
Adipose tissue mesenchymal stem cell exosomes
- BMMSC-Exos:
-
Bone marrow-derived exosomes
- VEGF:
-
Vascular endothelial growth factor
- Ang-1:
-
Human angiopoietin-1
- PIK3R2:
-
Phosphoinositide-3-kinase regulatory subunit 2
- PI3K:
-
Phosphatidylinostiol3-kinase
- ICAM-1:
-
Intercelluar cell adhesion molecule-1
- SPRED-1:
-
Sprouty-related,EVH1 domain 1
- Wnt4:
-
Wingless-type MMTV integration site family,member 4
- ROS:
-
Reactive oxygen species
- ATP:
-
Adenosine-triphosphate
- IL:
-
Interleukin
- DC:
-
Dendritic cells
- tolDC:
-
Tolerant dendritic cells
- Treg:
-
Regulatory T cells
- TNF-α:
-
Tumour necrosis factor-α
- TGF-β:
-
Transforming growth factor β
- NOX:
-
NAPDH oxidase
- AF-MSC:
-
MSC derived from human amniotic fluid
- LPS:
-
Lipopolysaccharides
- IFN-γ:
-
Interferonγ
- HIF-1α:
-
Hypoxia inducible factor-1α
- PDGF:
-
Platelet-derived growth factor
- RVG:
-
Rabies virus glycoprotein
- DOPE/NHS:
-
Dioleoyl-phosphatidyl ethanolamine N-hydroxy succinimide
- IONP:
-
Incubated iron oxide nanoparticles
- MF:
-
Magnetic field
- ATV:
-
Atorvastatin
- PGZ:
-
Pioglitazone
- RT-PCR:
-
Quantitative reverse transcriptase-polymerase chain reaction
References
Duley L. The global impact of pre-eclampsia and eclampsia[J]. Semin Perinatol. 2009;33(3):130–7.
Phipps E, Prasanna D, Brima W, Jim B. Preeclampsia: updates in pathogenesis, definitions, and guidelines[J]. Clin J Am Soc Nephrol. 2016;11(6):1102–13.
Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia[J]. Lancet. 2021;398(10297):341–54.
Huang LH, Rau CS, Wu SC, Wu YC, Wu CJ, Tsai CW, et al. Identification and characterization of hADSC-derived exosome proteins from different isolation methods[J]. J Cell Mol Med. 2021;25(15):7436–50.
Chu Y, Chen W, Peng W, Liu Y, Xu L, Zuo J, et al. Amnion-derived mesenchymal stem cell exosomes-mediated autophagy promotes the survival of trophoblasts under hypoxia through mTOR pathway by the downregulation of EZH2[J]. Front Cell Develop Biol. 2020;8:545852.
Wei Z, Hang S, WireduOcansey DK, Zhang Z, Wang B, Zhang X, et al. Human umbilical cord mesenchymal stem cells derived exosome shuttling mir-129–5p attenuates inflammatory bowel disease by inhibiting ferroptosis[J]. J Nanobiotechnol. 2023;21(1):188.
Xia C, Dai Z, Jin Y, Chen P. Emerging antioxidant paradigm of mesenchymal stem cell-derived exosome therapy[J]. Front Endocrinol. 2021;12:727272.
Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function[J]. Stem Cell Res Ther. 2016;7(1):125.
Wang L-L, Yu Y, Guan H-B, Qiao C. Effect of human umbilical cord mesenchymal stem cell transplantation in a rat model of preeclampsia[J]. Reprod Sci. 2016;23(8):1058–70.
Zhang D, Fu L, Wang L, Lin L, Yu L, Zhang L, et al. Therapeutic benefit of mesenchymal stem cells in pregnant rats with angiotensin receptor agonistic autoantibody-induced hypertension: Implications for immunomodulation and cytoprotection[J]. Hypertens Pregnancy. 2017;36(3):247–58.
Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Mesenchymal Stem Cells. Methods in Molecular Biology 2016. 123–46
Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells[J]. Int J Mol Sci. 2014;15(3):4142–57.
Zou X, Yuan M, Zhang T, Zheng N, Wu Z. EVs containing host restriction factor IFITM3 inhibited ZIKV infection of fetuses in pregnant mice through trans-placenta delivery[J]. Mol Ther. 2021;29(1):176–90.
Poulos J. The limited application of stem cells in medicine: a review[J]. Stem Cell Res Ther. 2018;9(1):1.
Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases[J]. Cells. 2019;8(12):1605.
Baharlooi H, Azimi M, Salehi Z, Izad M. Mesenchymal stem cell-derived exosomes: a promising therapeutic ace card to address autoimmune diseases[J]. Int J Stem Cells. 2020;13(1):13–23.
Wang X, Tang Y, Liu Z, Yin Y, Li Q, Liu G, et al. The application potential and advance of mesenchymal stem cell-derived exosomes in myocardial infarction[J]. Stem Cells Int. 2021;2021:5579904.
Xu M, Feng T, Liu B, Qiu F, Xu Y, Zhao Y, et al. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies[J]. Theranostics. 2021;11(18):8926–44.
Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use[J]. Bone Marrow Transplant. 2019;54(Suppl 2):789–92.
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications[J]. Int J Nanomedicine. 2020;15:6917–34.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M,. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science. 2008;319(5867):1244–7.
Rana S, Zöller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis[J]. Biochem Soc Trans. 2011;39(2):559–62.
Larios J, Mercier V, Roux A, Gruenberg J. ALIX- and ESCRT-III–dependent sorting of tetraspanins to exosomes[J]. J Cell Biol. 2020;219(3):e201904113.
Juan T, Fürthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles[J]. Semin Cell Dev Biol. 2018;74:66–77.
Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment[J]. Life Sci. 2019;233:116733.
Mitchell MD, Peiris HN, Kobayashi M, Koh YQ, Duncombe G, Illanes SE, et al. Placental exosomes in normal and complicated pregnancy[J]. Am J Obstet Gynecol. 2015;213(4):S173–81.
Clotilde Théry SA, Graça R, Aled C. Isolation and characterization of exosomes from cell culture supernatants and biological fluids[J]. Curr Protoc Cell Biol. 2006;30(1):3–22.
Ebrahim N, Mostafa O, El Dosoky RE, Ahmed IA, Saad AS, Mostafa A, et al. Human mesenchymal stem cell-derived extracellular vesicles/estrogen combined therapy safely ameliorates experimentally induced intrauterine adhesions in a female rat model[J]. Stem Cell Res Ther. 2018;9(1):1–15.
Sun L, Li D, Song K, Wei J, Yao S, Li Z, et al. Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro[J]. Sci Rep. 2017;7(1):2552.
Liu H, Wang F, Zhang Y, Xing Y, Wang Q. Exosomal microRNA-139-5p from mesenchymal stem cells accelerates trophoblast cell invasion and migration by motivation of the ERK/MMP-2 pathway via downregulation of protein tyrosine phosphatase[J]. J Obstetr Gynaecol Res. 2020;46(12):2561–72.
Wang D, Na Q, Song GY, Wang L. Human umbilical cord mesenchymal stem cell-derived exosome-mediated transfer of microRNA-133b boosts trophoblast cell proliferation, migration and invasion in preeclampsia by restricting SGK1[J]. Cell Cycle. 2020;19(15):1869–83.
Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization[J]. J Neuroinflam. 2020;17(1):1–22.
Ha DH, Kim H-k, Lee J, Kwon HH, Park G-H, Yang SH, et al. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration[J]. Cells. 2020;9(5):1157.
Huang B, Lu J, Ding C, Zou Q, Wang W, Li H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD[J]. Stem Cell Res Ther. 2018;9(1):1–12.
Zeringer E, Barta T, Li M, Vlassov AV. Strategies for Isolation of Exosomes[J]. Cold Spring Harb Protoc. 2015;2015(4):074476.
Heo JS, Choi Y, Kim H-S, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue[J]. Int J Mol Med. 2016;37(1):115–25.
Kim YG, Choi J, Kim K. Mesenchymal stem cell-derived exosomes for effective cartilage tissue repair and treatment of osteoarthritis[J]. Biotechnol J. 2020;15(12):2000082.
Yeo RWY, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, et al. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery[J]. Adv Drug Deliv Rev. 2013;65(3):336–41.
Lanzoni G, Linetsky E, Correa D, MessingerCayetano S, Alvarez RA, Kouroupis D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial[J]. Stem Cells Transl Med. 2021;10(5):660–73.
Huang L, Zhang C, Gu J, Wu W, Shen Z, Zhou X, et al. A randomized, placebo-controlled trial of human umbilical cord blood mesenchymal stem cell infusion for children with cerebral palsy[J]. Cell Transplant. 2018;27(2):325–34.
Bolli R, Hare JM, March KL, Pepine CJ, Willerson JT, Perin EC, et al. Rationale and design of the CONCERT-HF trial (combination of mesenchymal and c-kit+ cardiac stem cells as regenerative therapy for heart failure)[J]. Circ Res. 2018;122(12):1703–15.
Komaki M, Numata Y, Morioka C, Honda I, Tooi M, Yokoyama N, et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis[J]. Stem Cell Res Ther. 2017;8(1):1–12.
Cai G, Cai G, Zhou H, Zhuang Z, Liu K, Pei S, et al. RETRACTED ARTICLE: Mesenchymal stem cell-derived exosome miR-542–3p suppresses inflammation and prevents cerebral infarction[J]. Stem Cell Res Ther. 2021;12(1):1–12.
He C, Shan N, Xu P, Ge H, Yuan Y, Liu Y, et al. Hypoxia-induced downregulation of SRC-3 suppresses trophoblastic invasion and migration through inhibition of the AKT/mTOR pathway: implications for the pathogenesis of preeclampsia[J]. Sci Rep. 2019;9(1):10349.
Xue P, Fan W, Diao Z, Li Y, Kong C, Dai X, et al. Up-regulation of PTEN via LPS/AP-1/NF-κB pathway inhibits trophoblast invasion contributing to preeclampsia[J]. Mol Immunol. 2020;118:182–90.
Zygmunt M, Herr F, Münstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy[J]. Eur J Obstetr Gynecol Reprod Biol. 2003;110:S10–8.
Chen Y, Ding H, Wei M, Zha W, Guan S, Liu N, et al. MSC-Secreted exosomal H19 promotes trophoblast cell invasion and migration by downregulating let-7b and upregulating FOXO1[J]. Mol Ther Nucleic Acids. 2020;19:1237–49.
Yang Z, Shan N, Deng Q, Wang Y, Hou Y, Mei J, et al. Extracellular vesicle-derived microRNA-18b ameliorates preeclampsia by enhancing trophoblast proliferation and migration via Notch2/TIM3/mTORC1 axis[J]. J Cell Mol Med. 2021;25(10):4583–95.
Cui J, Chen X, Lin S, Li L, Fan J, Hou H, et al. MiR-101-containing extracellular vesicles bind to BRD4 and enhance proliferation and migration of trophoblasts in preeclampsia[J]. Stem Cell Res Ther. 2020;11(1):1–15.
Jiang Y, Luo T, Xia Q, Tian J, Yang J. microRNA-140-5p from human umbilical cord mesenchymal stem cells-released exosomes suppresses preeclampsia development[J]. Funct Integr Genomics. 2022;22(5):813–24.
Chang X, He Q, Wei M, Jia L, Wei Y, Bian Y, et al. Human umbilical cord mesenchymal stem cell derived exosomes (HUCMSC-exos) recovery soluble fms-like tyrosine kinase-1 (sFlt-1)-induced endothelial dysfunction in preeclampsia[J]. Eur J Med Res. 2023;28(1):277.
Zhu LL, Huang X, Yu W, Chen H, Chen Y, Dai YT. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats[J]. Andrologia. 2018;50(2):e12871.
Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a[J]. J Cell Sci. 2016;129(11):2182–9.
Zhang L, Ouyang P, He G, Wang X, Song D, Yang Y, et al. Exosomes from microRNA-126 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway[J]. J Cell Mol Med. 2020;25(4):2148–62.
Gao Z, Wang N, Liu X. Human placenta mesenchymal stem cell-derived exosome shuttling microRNA-130b-3p from gestational diabetes mellitus patients targets ICAM-1 and perturbs human umbilical vein endothelial cell angiogenesis[J]. Acta Diabetol. 2022;59(8):1091–107.
Qu Q, Wang L, Bing W, Bi Y, Zhang C, Jing X, et al. miRNA-126-3p carried by human umbilical cord mesenchymal stem cell enhances endothelial function through exosome-mediated mechanisms in vitro and attenuates vein graft neointimal formation in vivo[J]. Stem Cell Res Ther. 2020;11(1):464.
Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway[J]. Stem Cells Transl Med. 2015;4(5):513–22.
Aneman I, Pienaar D, Suvakov S, Simic TP, Garovic VD, McClements L. Mechanisms of key innate immune cells in early- and late-onset preeclampsia[J]. Front Immunol. 2020;1864:11.
Yang C, Lim W, Park J, Park S, You S, Song G. Anti-inflammatory effects of mesenchymal stem cell-derived exosomal microRNA-146a-5p and microRNA-548e-5p on human trophoblast cells[J]. Mol Hum Reprod. 2019;25(11):755–71.
Taglauer ES, Fernandez-Gonzalez A, Willis GR, Reis M, Yeung V, Liu X, et al. Mesenchymal stromal cell-derived extracellular vesicle therapy prevents preeclamptic physiology through intrauterine immunomodulationdagger[J]. Biol Reprod. 2021;104(2):457–67.
Shahir M, Mahmoud Hashemi S, Asadirad A, Varahram M, Kazempour-Dizaji M, Folkerts G, et al. Effect of mesenchymal stem cell-derived exosomes on the induction of mouse tolerogenic dendritic cells[J]. J Cell Physiol. 2020;235(10):7043–55.
Xin L, Lin X, Zhou F, Li C, Wang X, Yu H, et al. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation[J]. Acta Biomater. 2020;113:252–66.
Yu H, Pan Y, Dai M, Wang X, Chen H. Mesenchymal stem cell-originated exosomal Lnc A2M-AS1 alleviates hypoxia/reperfusion-induced apoptosis and oxidative stress in cardiomyocytes[J]. Cardiovasc Drugs Ther. 2022;37(5):891–904.
Cao L, Xu H, Wang G, Liu M, Tian D, Yuan Z. Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization[J]. Int Immunopharmacol. 2019;72:264–74.
Chiarello DI, Abad C, Rojas D, Toledo F, Vázquez CM, Mate A, et al. Oxidative stress: Normal pregnancy versus preeclampsia[J]. Molecular Basis of Disease. 2020;1866(2):165354.
Li X, Wang Y, Cai Z, Zhou Q, Li L, Fu P. Exosomes from human umbilical cord mesenchymal stem cells inhibit ROS production and cell apoptosis in human articular chondrocytes via the miR-100-5p/NOX4 axis[J]. Cell Biol Int. 2021;45(10):2096–106.
Brown DI, Griendling KK. Nox proteins in signal transduction[J]. Free Radical Biol Med. 2009;47(9):1239–53.
Yan C, Xv Y, Lin Z, Endo Y, Xue H, Hu Y, et al. Human umbilical cord mesenchymal stem cell-derived exosomes accelerate diabetic wound healing via ameliorating oxidative stress and promoting angiogenesis[J]. Front Bioeng Biotechnol. 2022;10: 829868.
Zheng S, Shi A, Hill S, Grant C, Kokkinos MI, Murthi P, et al. Decidual mesenchymal stem/stromal cell-derived extracellular vesicles ameliorate endothelial cell proliferation, inflammation, and oxidative stress in a cell culture model of preeclampsia[J]. Pregnancy Hypertension. 2020;22:37–46.
Xiang YJ, Hou YY, Yan HL, Liu H, Ge YX, Chen N, et al. Mesenchymal stem cells-derived exosomes improve pregnancy outcome through inducing maternal tolerance to the allogeneic fetus in abortion-prone mating mouse[J]. Kaohsiung J Med Sci. 2020;36(5):363–70.
Wan Z, Zhao L, Lu F, Gao X, Dong Y, Zhao Y, et al. Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium[J]. Theranostics. 2020;10(1):218–30.
Ishiuchi N, Nakashima A, Doi S, Yoshida K, Maeda S, Kanai R, et al. Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats[J]. Stem Cell Res Ther. 2020;11(1):1–15.
Wang JW, Qiu YR, Fu Y, Liu J, He ZJ, Huang ZT. Transplantation with hypoxia-preconditioned mesenchymal stem cells suppresses brain injury caused by cardiac arrest–induced global cerebral ischemia in rats[J]. J Neurosci Res. 2017;95(10):2059–70.
Tang YL, Hofmann NA, Ortner A, Jacamo RO, Reinisch A, Schallmoser K, et al. Oxygen sensing mesenchymal progenitors promote neo-vasculogenesis in a humanized mouse model in vivo[J]. PLoS ONE. 2012;7(9):e44468.
Ge L, Xun C, Li W, Jin S, Liu Z, Zhuo Y, et al. Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612[J]. J Nanobiotechnology. 2021;19(1):380.
Zhu J, Lu K, Zhang N, Zhao Y, Ma Q, Shen J, et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way[J]. Artif Cells Nanomed Biotechnol. 2017;46(8):1659–70.
Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R[J]. Int J Biochem Cell Biol. 2019;109:59–68.
Liu W, Li L, Rong Y, Qian D, Chen J, Zhou Z, et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126[J]. Acta Biomater. 2020;103:196–212.
Yuan N, Ge Z, Ji W, Li J. Exosomes secreted from hypoxia-preconditioned mesenchymal stem cells prevent steroid-induced osteonecrosis of the femoral head by promoting angiogenesis in rats[J]. Biomed Res Int. 2021;2021:6655225.
Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, et al. Exosomes derived from akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D[J]. Stem Cells Transl Med. 2017;6(1):51–9.
Sun J, Shen H, Shao L, Teng X, Chen Y, Liu X, et al. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis[J]. Stem Cell Res Ther. 2020;11(1):1–13.
Cui G-h, Guo H-D, Li H, Zhai Y, Gong Z-B, Wu J, et al. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease[J]. Immun Age. 2019;16(1):1–12.
Anna King CN, Lui S, Widdows K, Kotamraju VR, Agemy L, TambetTeesalu JDG, Cellesi F, Tirelli N, Aplin JD, ErkkiRuoslahti LKH. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta[J]. Sci Adv. 2016;2(5):e1600349.
Kim HY, Kumar H, Jo M-J, Kim J, Yoon J-K, Lee J-R, et al. Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord injury treatment[J]. Nano Lett. 2018;18(8):4965–75.
Dai G, Xu Q, Luo R, Gao J, Chen H, Deng Y, et al. Atorvastatin treatment improves effects of implanted mesenchymal stem cells: meta-analysis of animal models with acute myocardial infarction[J]. BMC Cardiovasc Disorders. 2015;15(1):1–16.
Hu Y, Tao R, Chen L, Xiong Y, Xue H, Hu L, et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis[J]. J Nanobiotechnology. 2021;19(1):150.
Zhao S, Huang M, Yan L, Zhang H, Shi C, Liu J, et al. Exosomes derived from baicalin-pretreated mesenchymal stem cells alleviate hepatocyte ferroptosis after acute liver injury via the keap1-NRF2 pathway[J]. Oxid Med Cell Longev. 2022;2022:8287227.
Kim YS, Ahn Y, Kwon JS, Cho YK, Jeong MH, Cho JG, et al. Priming of mesenchymal stem cells with oxytocin enhances the cardiac repair in ischemia/reperfusion injury[J]. Cells Tissues Organs. 2012;195(5):428–42.
Liu J, Zhu P, Song P, Xiong W, Chen H, Peng W, et al. Pretreatment of adipose derived stem cells with curcumin facilitates myocardial recovery via antiapoptosis and angiogenesis[J]. Stem Cells International. 2015;2015:1–12.
Deng R, Liu Y, He H, Zhang H, Zhao C, Cui Z, et al. Haemin pre-treatment augments the cardiac protection of mesenchymal stem cells by inhibiting mitochondrial fission and improving survival[J]. J Cell Mol Med. 2019;24(1):431–40.
Huang P, Wang L, Li Q, Tian X, Xu J, Xu J, et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19[J]. Cardiovasc Res. 2020;116(2):353–67.
Sakowicz A, Bralewska M, Rybak-Krzyszkowska M, Grzesiak M, Pietrucha T. New ideas for the prevention and treatment of preeclampsia and their molecular inspirations[J]. Int J Mol Sci. 2023;24(15):12100.
Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, et al. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper[J]. J Extracell Vesicles. 2015;4(1):30087.
Sun L, Xu R, Sun X, Duan Y, Han Y, Zhao Y, et al. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell[J]. Cytotherapy. 2016;18(3):413–22.
Meng H, Cheng W, Wang L, Chen S, Teng Y, Lu Z, et al. Mesenchymal stem cell exosomes in the treatment of myocardial infarction: a systematic review of preclinical in vivo studies[J]. J Cardiovasc Transl Res. 2021;15(2):317–39.
Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation[J]. Cell Physiol Biochem. 2015;37(6):2415–24.
Reza-Zaldivar EE, Hernández-Sapiéns MA, Minjarez B, Gutiérrez-Mercado YK, Márquez-Aguirre AL, Canales-Aguirre AA. Potential effects of MSC-derived exosomes in neuroplasticity in Alzheimer’s disease[J]. Front Cell Neurosci. 2018;12:317.
Gandham S, Su X, Wood J, Nocera AL, Alli SC, Milane L, et al. Technologies and standardization in research on extracellular vesicles[J]. Trends Biotechnol. 2020;38(10):1066–98.
Mayr M, Zampetaki A. Analytical challenges and technical limitations in assessing circulating MiRNAs[J]. Thromb Haemost. 2017;108(10):592–8.
Kawikova I, Askenase PW. Diagnostic and therapeutic potentials of exosomes in CNS diseases[J]. Brain Res. 2015;1617:63–71.
Guo M, Yin Z, Chen F, Lei P. Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of Alzheimer’s disease[J]. Alzheimer’s Res Ther. 2020;12(1):1–14.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (General Program, NO. 82171678), Shenzhen Science and Technology Programe (NO.JCYJ20230807143504009), and Science, Technology and Innovation Commission of Shenzhen Municipality (NO.JCYJ20200109140614667).The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
SHR and YZJ drafted the manuscript and reviewed relevant literature. CJJ, TH, and MRL participated in the discussions. The corresponding author ZY guided manuscript preparation. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, H., Yang, Z., Cui, J. et al. Mesenchymal stem cell-derived exosomes: a promising alternative in the therapy of preeclampsia. Stem Cell Res Ther 15, 30 (2024). https://doi.org/10.1186/s13287-024-03652-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03652-0