Abstract
Background
Regenerative techniques combined with core decompression (CD) are commonly used to treat osteonecrosis of the femoral head (ONFH). However, no consensus exists on regeneration therapy combined with CD that performs optimally. Therefore, we evaluated six regenerative therapies combined with CD treatment using a Bayesian network meta-analysis (NMA).
Methods
We searched PubMed, Embase, Cochrane Library, and Web of Science databases. Six common regeneration techniques were categorized into the following groups with CD as the control group: (1) autologous bone graft (ABG), (2) autologous bone graft combined with bone marrow aspirate concentrate (ABG + BMAC), (3) bone marrow aspirate concentrate (BMAC), (4) free vascular autologous bone graft (FVBG), (5) expanded mesenchymal stem cells (MSCs), and (6) platelet-rich plasma (PRP). The conversion rate to total hip arthroplasty (THA) and progression rate to femoral head necrosis were compared among the six treatments.
Result
A total of 17 literature were included in this study. In the NMA, two of the six treatment strategies demonstrated higher response in preventing the progression of ONFH than CD: MSCs (odds ratio [OR]: 0.098, 95% confidence interval [CI]: 0.0087–0.87) and BMAC (OR: 0.27, 95% CI: 0.073–0.73). Additionally, two of the six treatment strategies were effective techniques in preventing the conversion of ONFH to THA: MSCs (OR: 0.062, 95% CI: 0.0038–0.40) and BMAC (OR: 0.32, 95% CI: 0.1–0.074). No significant difference was found among FVBG, PRP, ABG + BMAC, ABG, and CD in preventing ONFH progression and conversion to THA (P > 0.05).
Conclusions
Our NMA found that MSCs and BMAC were effective in preventing ONFH progression and conversion to THA among the six regenerative therapies. According to the surface under the cumulative ranking value, MSCs ranked first, followed by BMAC. Additionally, based on our NMA results, MSCs and BMAC following CD may be necessary to prevent ONFH progression and conversion to THA. Therefore, these findings provide evidence for the use of regenerative therapy for ONFH.
Similar content being viewed by others
Background
Osteonecrosis of the femoral head (ONFH) is a common refractory disease in joint orthopedics. More than 10,000 new patients are affected with ONFH annually in the United States, accounting for approximately 10% of total hip arthroplasties (THAs) [1]. The cumulative number of patients with ONFH in China reached 8.12 million in 2013 [2]. According to statistics, the prevalence rate of ONFH is increasing yearly [3]. ONFH is a progressive disease typically caused by insufficient blood supply to the femoral head, which leads to increased pressure in it, eventually culminating in its collapse. The femoral head usually develops into secondary arthritis when it collapses [4]. Core decompression (CD) is a commonly used procedure for treating femoral head necrosis despite some controversy; it is a simple procedure that treats ONFH by drilling into the necrotic area of the femoral head [5,6,7,8]. The theoretical advantage of CD is in relieving the pain by reducing venous congestion and bone marrow pressure. Blood flow increases in the osteonecrosis area with the decrease in intraosseous pressure, thereby alleviating the pathology and promoting bone regeneration in the osteonecrosis area [9, 10]. CD combined with regeneration therapy appears to accelerate the healing of osteonecrosis and reduce the risk of femoral head collapse [11]. Recently, studies have shown that bone marrow aspirate concentrate (BMAC), expanded mesenchymal stem cells (MSCs), autologous bone graft (ABG), and other regenerative therapies show gratifying outcomes in the treatment of bone diseases [12,13,14,15,16,17]. In addition to BMAC, MSCs, and ABG, common regeneration therapies also include platelet-rich plasma (PRP), autologous bone graft combined with bone marrow aspirate concentrate (ABG + BMAC), and free vascular autologous bone graft (FVBG). Despite the promising results of these diferent methods, the best regeneration therapy for ONFH has not yet been determined.
Bayesian network meta-analysis (NMA), also known as multiple treatment comparison meta-analysis, can simultaneously analyze direct and indirect evidence from different studies, expand the scope of traditional conventional pairwise analysis, and subsequently estimate the relative effectiveness of all interventions and rank them [18]. To date, no comparison of the different regenerative therapies has been performed for ONFH using NMA. Herein, we used a Bayesian NMA to evaluate the efficacy of different regenerative therapies based on ONFH progression and conversion to THA.
Methods
This systematic review and NMA adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [19]. Additionally, our review was registered on PROSPERO (http://www.crd.york.ac.uk/PROSPERO) under the registration number CRD42023412784.
Search strategy
All articles published between 2003 and 2023 in PubMed, EMBASE, Cochrane Library, and Web of Science databases were searched. We used the following keywords: “femur head” AND (“bone necrosis” OR “avascular necrosis” OR “osteonecrosis”) AND (“regenerative therapies” OR “stem cells” OR “bone marrow” OR “bone graft” OR “platelet rich plasma”). An additional file presents the details of the search process (see Additional file 1).
Study selection
The inclusion and exclusion process followed the PICOS (Participants, Intervention, Comparison, Outcome, and Study) principle. Additionally, the mean age of patients with ONFH was 18 years. Studies include at least two of the following treatments: ABG, ABG + BMAC, BMAC, CD, MSCs, FVBG, and PRP. The included studies reported at least one of the two outcomes as follows: the rate of THA requirement and that of ONFH stage progression after the intervention. Furthermore, the included studies were randomized controlled trials (RCTs) or retrospective cohort studies conducted in English, published from 2003 to 2023.
The exclusion criteria were as follows: Non-English text; literature with a low-quality treatment evaluation; and reviews, protocols, case reports, conference papers, and animal experiments.
All relevant studies were screened independently by two reviewers, and any disagreement between the two reviewers regarding a study’s eligibility was resolved through discussion with a third reviewer.
Data extraction
Two independent reviewers extracted the following information from each included study: the first author’s surname, year of publication, study types, follow-up time, average age, hip sample size, ONFH staging of patient, conversion to THA, and ONFH progression. Any differences were resolved through discussion with a third reviewer.
Quality assessment
Two independent reviewers assessed the literature quality. RCT and retrospective cohort studies were assessed for quality using the Cochrane Risk of Bias tool [20] and the Newcastle–Ottawa Scale (NOS), respectively. The following factors were assessed for each study: randomization sequence generation (selection bias), allocation concealment, subject blinding, outcome assessment, attrition bias (incomplete outcome data), reporting bias (selective reporting), and other biases. Studies with scores of 8 and 9, and 6 and 7 were considered high and medium quality studies, respectively [21]. Any differences were resolved through discussion with a third reviewer.
Statistical analysis
We analyzed the following two metrics: ONFH conversion rates to THA and its progression rates. The results are expressed as the odds ratio (OR) and 95% confidence interval (CI). A pairwise meta-analysis was performed using R software (version 5.35; Lucent Technologies, Paris, France).
Heterogeneity between comparable studies was examined using the chi-square (χ2) and I2 tests. Values < 25%, 25–75%, and > 75% for the I2 statistic represented mild, moderate, and severe heterogeneity, respectively [22]. Furthermore, node-splitting analysis was used to assess the inconsistency of a particular comparison based on direct and indirect evidence; statistical significance was considered at P < 0.05 [23]. Furthermore, funnel plots were used to test for publication bias.
We also calculated the surface under the cumulative ranking (SUCRA) value, a simple numerical summary to supplement the graphical display of cumulative ranking, which is used to estimate the SUCRA line for each treatment. The SUCRA values of 1 and 0 signify a treatment that is certain to be optimal and the worst, respectively [23].
Results
Study selection and characteristics
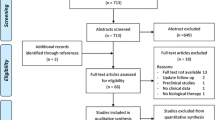
Figure 1 shows the study selection process. We retrieved 2591 articles, ultimately including 17 studies. A total of 1019 hips were included in our NMA groups, comprising 245, 80, 25, 177, 50, 151, and 291 hips in the BMAC, MSCs, PRP, ABG, ABG + BMAC, FVBG, and CD groups, respectively. Table 1 presents the basic characteristics of the included studies. The network structure of the analyzed comparisons for the primary outcomes is shown in Fig. 2.
Network plots of comparison-based network meta-analyses. Each circular node represents a type of intervention. The circle size is proportional to the total number of patients. The width of the lines is proportional to the number of studies performing head-to-head comparisons in the same study. a ONFH progression and b conversion to total hip arthroplasty (THA). ABG: autologous bone grafting; ABG + BMAC: autologous bone grafting and bone marrow aspirate concentrate; BMAC: bone marrow aspirate concentration; CD: core decompression; FVBG: free vascular vascularized bone grafting; MSCs: mesenchymal stem cells; PRP: platelet-rich plasma
For RCTs using the Cochrane Risk of Bias tool, the overall quality assessment showed a low or moderate risk of bias, with a higher risk observed in the blinded component, mainly because the procedure required informed consent and it was difficult for the operator and patient to be blinded; however, this does not imply that the study was meaningless (Fig. 3). Retrospective cohort studies were assessed using the NOS, revealing two medium-quality (score: 6 or 7) and two high-quality (score: 8 or 9) studies (Fig. 4). We assessed a funnel diagram of the included studies (Fig. 5), and the roughly symmetrical diagram suggests no publication bias.
Heterogeneity was found among the comparisons of treatments on the rates of ONFH progression and conversion to THA (Tables 2, 3). We found that the Bayesian NMA results were reliable; the inconsistency between the direct and indirect effects of comparisons of different treatments on the two outcomes showed no significant differences (Tables 4, 5). The nodal split method for ONFH progression and conversion to THA showed no significant heterogeneity (Tables 6, 7).
Femoral head necrosis progress
All 17 articles reported ONFH progression. The NMA results showed that MSCs (OR: 0.098, 95% CI: 0.0087–0.87, SUCRA = 0.705) were the first effective technique for preventing ONFH progression, followed by BMAC (OR: 0.27, 95% CI: 0.073–0.73, SUCRA = 0.431). However, no significant difference was found among VBG, PRP, ABG + BMAC, ABG, or CD in preventing ONFH progression (P > 0.05) (Fig. 6).
Forest plots of osteonecrosis of the femoral head (ONFH) progression. ABG: autologous bone grafting; ABG + BMAC: autologous bone grafting and bone marrow aspirate concentrate; BMAC: bone marrow aspirate concentration; CD: core decompression; CI, confidence interval; FVBG: free vascular vascularized bone grafting; MSCs: mesenchymal stem cells; PRP: platelet-rich plasma
Conversion to total hip arthroplasty
A total of 16 articles reported ONFH conversion to THA. MSCs (OR: 0.062, 95% CI: 0.0038–0.40, SUCRA = 0.902) and BMAC (OR: 0.32, 95% CI: 0.1–0.074, SUCRA = 0.511) were the first and second effective techniques, respectively, for preventing ONFH conversion to THA. No significant difference was found among VBG, PRP, ABG + BMAC, ABG, or CD in preventing ONFH conversion to THA (P > 0.05) (Fig. 7).
Forest plots of conversion to total hip arthroplasty (THA). ABG: autologous bone grafting; ABG + BMAC: autologous bone grafting and bone marrow aspirate concentrate; BMAC: bone marrow aspirate concentration; CD: core decompression; CI, confidence interval; FVBG: free vascular vascularized bone grafting; MSCs: mesenchymal stem cells; PRP: platelet-rich plasma
Discussion
This NMA investigated regenerative therapy for nontraumatic femoral head necrosis and included data from 17 clinical trials, including 1019 hips assigned to 6 different treatment options. The quality of evidence was generally low or moderate risk of bias. Our NMA found that MSCs and BMAC can prevent ONFH progression and conversion to THA. When ranked according to the SUCRA value, MSCs were the first, followed by BMAC; therefore, we concluded that MSCs and BMAC transplantation may be necessary after CD. To our knowledge, this is the first NMA to compare these six regenerative therapies, and these findings provide evidence for regenerative therapy for ONFH.
Previous traditional meta-analyses, such as the study by Andriolo et al. [11], did not compare different regenerative therapies separately with CD but combined the data of different regenerative therapies, which may have led to biased results. Zhang et al. [49] found that the combination of bone marrow stem cells had better prognosis outcomes than CD alone, such as ONFH progression or Harris Hip Score. However, during their study search, they classified bone and PRP graftings as stem cells, which broadened the study’s scope but inevitably increased its bias. Our study categorized regenerative therapy into six categories, which improved the search accuracy and obtained reliable study results. Migliorini et al. [50] found that bone marrow-derived cells had a lower probability of THA than CD, whereas conventional meta-analyses only compared bone marrow-derived cells with CD alone and could not compare multiple regenerative therapies. Bayesian NMA was used to review the regenerative therapy for ONFH in the CD (control), ABG, ABG + BMAC, BMAC, FVBG, MSCs, and PRP groups. Based on our NMA SUCRA analysis, MSCs ranked as the first intervention among the six regenerative therapies for preventing ONFH progression and conversion to THA, presumably because they provide better repair capacity [17, 39, 47]. However, ABG, ABG + BMAC, FVBG, PRP, and CD showed no significant differences in preventing ONFH progression and conversion to THA.
We derive the rationale behind this conclusion from the premise that regeneration therapy operates on the ability of cells and molecules to induce and promote tissue repair of ONFH. For example, BMAC contains many growth factors and non-mesenchymal cells, including endothelial, hematopoietic, and inflammatory cells, in which growth factors can induce stem cells to migrate to the injured site [40,41,42]. Many hematopoietic stem cells can provide vascular support and drive MSCs toward osteogenic differentiation [43]. With the progress in BMAC research, researchers have found that the non-progenitor cell component of BMAC may negatively affect its regeneration characteristics, limiting BMAC’s repair ability [44]. Generally, MSCs account for only 0.001–0.01% of the number of nucleated cells in BMAC [45, 46]. Studies have shown that high concentrations of MSCs can promote cartilage healing more than low concentrations of MSCs without causing adverse reactions [47]. Additionally, MSCs can differentiate into several cell types (including fibroblasts, chondroblasts, and other forms of tissue regeneration cells), thereby promoting tissue repair [48].
Although our study is the first Bayesian NMA to compare traditional CD with other regenerative therapies, it has limitations. First, it included only 17 related articles; therefore, the scale of direct comparison was limited. For example, only 80 and 245 hips were in the MSCs and BMAC groups, respectively; therefore, a limited sample size may increase statistical dispersion. Second, the study’s sample size was not large enough, potentially reducing the credibility of the results. Moreover, including patients with different Association Research Circulation Osseous (ARCO) stages leads to heterogeneity, and the prognoses of patients with different stages may differ. Considering these limitations, we recommend caution in our conclusions. Therefore, future research studies should include larger sample sizes covering the various treatments and ARCO/Ficat stages.
Conclusion
Our NMA found that MSCs and BMAC were effective in preventing ONFH progression and conversion to THA among the six regenerative therapies. MSCs ranked first, followed by BMAC according to the SUCRA value. Based on our NMA results, MSCs and BMAC after CD may be necessary to prevent ONFH progression and conversion to THA. Furthermore, these findings provide evidence for regenerative therapy for ONFH.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Abbreviations
- ABG:
-
Autologous bone grafting
- ABG + BMAC:
-
Autologous bone grafting and bone marrow aspirate concentrate
- ARCO:
-
Association research circulation osseous
- BMAC:
-
Bone marrow aspirate concentration
- CI:
-
Confidence interval
- CD:
-
Core decompression
- FVBG:
-
Free vascular vascularized bone grafting
- MD:
-
Mean difference
- NMA:
-
Network meta-analysis
- OR:
-
Odds ratio
- ONFH:
-
Osteonecrosis of the femoral head
- PRP:
-
Platelet-rich plasma
- RCT:
-
Randomized controlled trial
- SUCRA:
-
Surface under the cumulative ranking
- THA:
-
Total hip arthroplasty
References
Mont MA, Cherian JJ, Sierra RJ, Jones LC, Lieberman JR. Nontraumatic osteonecrosis of the femoral head: where do we stand today? A ten-year update. J Bone Joint Surg Am. 2015;97:1604–27.
Zhao DW, Yu M, Hu K, Wang W, Yang L, Wang BJ, et al. prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the Chinese population: results from a nationally representative survey. Chin Med J (Engl). 2015;128:2843–50.
Mont MA, Salem HS, Piuzzi NS, Goodman SB, Jones LC. Nontraumatic osteonecrosis of the femoral head: where do we stand today?: a 5-year update. J Bone Joint Surg Am. 2020;102:1084–99.
Migliorini F, Maffulli N, Baroncini A, Eschweiler J, Tingart M, Betsch M. Failure and progression to total hip arthroplasty among the treatments for femoral head osteonecrosis: a Bayesian network meta-analysis. Br Med Bull. 2021;138:112–25.
Andronic O, Hincapie CA, Burkhard MD, Loucas R, Loucas M, Ried E, et al. Lack of conclusive evidence of the benefit of biologic augmentation in core decompression for nontraumatic osteonecrosis of the femoral head: a systematic review. Arthroscopy. 2021;37:3537-51.e3.
Learmonth ID, Maloon S, Dall G. Core decompression for early atraumatic osteonecrosis of the femoral head. J Bone Joint Surg Br. 1990;72:387–90.
Rackwitz L, Eden L, Reppenhagen S, Reichert JC, Jakob F, Walles H, et al. Stem cell- and growth factor-based regenerative therapies for avascular necrosis of the femoral head. Stem Cell Res Ther. 2012;3:7.
Yoon BH, Lee YK, Kim KC, Ha YC, Koo KH, et al. No differences in the efficacy among various core decompression modalities and non-operative treatment: a network meta-analysis. Int Orthop. 2018;42:2737–43.
Chughtai M, Piuzzi NS, Khlopas A, Jones LC, Goodman SB, Mont MA. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J. 2017;99-B:1267–79.
Tripathy SK, Goyal T, Sen RK. Management of femoral head osteonecrosis: current concepts. Indian J Orthop. 2015;49:28–45.
Andriolo L, Merli G, Tobar C, Altamura SA, Kon E, Filardo G. Regenerative therapies increase survivorship of avascular necrosis of the femoral head: A systematic review and meta-analysis. Int Orthop. 2018;42:1689–704.
Purita J, Lana JFSD, Kolber M, Rodrigues BL, Mosaner T, Santos GS, et al. Bone marrow-derived products: a classification proposal - bone marrow aspirate, bone marrow aspirate concentrate or hybrid? World J Stem Cells. 2020;12:241–50.
Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res. 2015;8:269–76.
Dallari D, Stagni C, Rani N, Sabbioni G, Pelotti P, Torricelli P, et al. Ultrasound-guided injection of platelet-rich plasma and hyaluronic acid, separately and in combination, for hip osteoarthritis: a randomized controlled study. Am J Sports Med. 2016;44:664–71.
Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32:1254–66.
Lana JFSD, Weglein A, Sampson SE, Vicente EF, Huber SC, Souza CV, et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med. 2016;12:69–78.
Li M, Ma Y, Fu G, Zhang R, Li Q, Deng Z, et al. 10-year follow-up results of the prospective, double-blinded, randomized, controlled study on autologous bone marrow buffy coat grafting combined with core decompression in patients with avascular necrosis of the femoral head. Stem Cell Res Ther. 2020;11:287.
Bafeta A, Trinquart L, Seror R, Ravaud P. Reporting of results from network meta-analyses: methodological systematic review. BMJ. 2014;348: g1741.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84.
Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928.
Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal. 2017;5:80–4.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–71.
Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49:1005–9.
Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplasty. 2012;27:679–86.
Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50:325–30.
Rastogi S, Sankineani SR, Nag HL, Mohanty S, Shivanand G, Marimuthu K, et al. Intralesional autologous mesenchymal stem cells in management of osteonecrosis of femur: a preliminary study. Musculoskelet Surg. 2013;97:223–8.
Ma Y, Wang T, Liao J, Gu H, Lin X, Jiang Q, et al. Efficacy of autologous bone marrow buffy coat grafting combined with core decompression in patients with avascular necrosis of femoral head: a prospective, double-blinded, randomized, controlled study. Stem Cell Res Ther. 2014;5:115.
Tabatabaee RM, Saberi S, Parvizi J, Mortazavi SMJ, Farzan M. Combining concentrated autologous bone marrow stem cells injection with core decompression improves outcome for patients with early-stage osteonecrosis of the femoral head: a comparative study. J Arthroplasty. 2015;30(9 Suppl):11–5.
Cruz-Pardos A, Garcia-Rey E, Ortega-Chamarro JA, Duran-Manrique D, Gomez-Barrena E. Mid-term comparative outcomes of autologous bone-marrow concentration to treat osteonecrosis of the femoral head in standard practice. Hip Int. 2016;26:432–7.
Pepke W, Kasten P, Beckmann NA, Janicki P, Egermann M, et al. Core decompression and autologous bone marrow concentrate for treatment of femoral head osteonecrosis: a randomized prospective study. Orthop Rev (Pavia). 2016;8:6162.
Sallam AA, Imam MA, Salama KS, Mohamed OA. Inverted femoral head graft versus standard core decompression in nontraumatic hip osteonecrosis at minimum 3 years follow-up. Hip Int. 2017;27:74–81.
Hauzeur JP, De Maertelaer V, Baudoux E, Malaise M, Beguin Y, Gangji V. Inefficacy of autologous bone marrow concentrate in stage three osteonecrosis: a randomized controlled double-blind trial. Int Orthop. 2018;42:1429–35.
Cao L, Guo C, Chen J, Chen Z, Yan Z. Free vascularized fibular grafting improves vascularity compared with core decompression in femoral head osteonecrosis: a randomized clinical trial. Clin Orthop Relat Res. 2017;475:2230–40.
Feng W, Chen J, Wu K, Lu L, Deng P, Ye P, et al. A comparative study of cortico-cancellous iliac bone graft with or without the combination of vascularized greater trochanter flap for the management of femoral head osteonecrosis: a minimum 6 years follow-up. BMC Musculoskelet Disord. 2019;20:298.
Hauzeur JP, Lechanteur C, Baudoux E, De Maertelaer V, Pather S, Katz R, et al. Did osteoblastic cell therapy improve the prognosis of pre-fracture osteonecrosis of the femoral head? A randomized, controlled trial. Clin Orthop Relat Res. 2020;478:1307–15.
Aggarwal AK, Poornalingam K, Jain A, Prakash M. Combining platelet-rich plasma instillation with core decompression improves functional outcome and delays progression in early-stage avascular necrosis of femoral head: A 4.5- to 6-year prospective randomized comparative study. J Arthroplasty. 2021;36:54–61.
Hoogervorst P, Campbell JC, Scholz N, Cheng EY. Core decompression and bone marrow aspiration concentrate grafting for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2022;104(Suppl 2):54–60.
Wan J, Hu Y, Li J, Zeng Y, Ren H. Comparison of the outcome of different bone grafts combined with modified core decompression for the treatment of ARCO II stage femoral head necrosis. Int Orthop. 2022;46:1955–62.
Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, et al. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320:914–9.
McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27:1033–42.
Kim GB, Seo MS, Park WT, Lee GW. Bone marrow aspirate concentrate: its uses in osteoarthritis. Int J Mol Sci. 2020;21:3224.
Holton J, Imam MA, Snow M. Bone marrow aspirate in the treatment of chondral injuries. Front Surg. 2016;3:33.
LaPrade RF, Murray IR. Editorial commentary: Bone marrow aspirate concentrate: time to harvest locally? Arthroscopy. 2020;36:2412–4.
Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Cruz RS, LaPrade RF. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4:2325967115625481.
Martin DR, Cox NR, Hathcock TL, Niemeyer GP, Baker HJ. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol. 2002;30:879–86.
Bartlett RS, Guille JT, Chen X, Christensen MB, Wang SF, Thibeault SL. Mesenchymal stromal cell injection promotes vocal fold scar repair without long-term engraftment. Cytotherapy. 2016;18:1284–96.
Jeyaraman M, Bingi SK, Muthu S, Jeyaraman N, Packkyarathinam RP, Ranjan R, et al. Impact of the process variables on the yield of mesenchymal stromal cells from bone marrow aspirate concentrate. Bioengineering (Basel). 2022;9:57.
Zhang C, Fang X, Huang Z, Li W, Zhang W, Lee GC. Addition of bone marrow stem cells therapy achieves better clinical outcomes and lower rates of disease progression compared with core decompression alone for early stage osteonecrosis of the femoral head: a systematic review and meta-analysis. J Am Acad Orthop Surg. 2020;28:973–9.
Migliorini F, Maffulli N, Eschweiler J, Tingart M, Baroncini A. Core decompression isolated or combined with bone marrow-derived cell therapies for femoral head osteonecrosis. Expert Opin Biol Ther. 2021;21:423–30.
Acknowledgements
We thank Editage (www.editage.cn) for English language editing.
Research registration unique identifying number (UIN)
(1) Name of the registry: PROSPERO. (2) Unique Identifying number or registration ID: CRD42023412784. (3) Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=412784
Funding
This study was supported by funding from the Science and Technology Plan of Liaoning Province of China (no.: 2023JH2/101600029) and the Shenyang City and Technology Plan Project (no.: 21-174-9-03).
Author information
Authors and Affiliations
Contributions
XD outlined the study concept and design and obtained funding. XW and LH drafted the manuscript. BW and JW performed statistical analysis. DH performs administration and is responsible for resolving differences. Data collection, analysis, and interpretation were completed by all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable; ethical approval was not required because this study retrieved and synthesized data from previously published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search Terms.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Hu, L., Wei, B. et al. Regenerative therapies for femoral head necrosis in the past two decades: a systematic review and network meta-analysis. Stem Cell Res Ther 15, 21 (2024). https://doi.org/10.1186/s13287-024-03635-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03635-1