Abstract
Background
Mesenchymal stromal cell (MSC) transplantation can improve the left ventricular ejection fraction (LVEF) after an acute myocardial infarction (AMI). Transplanted MSCs exert a paracrine effect, which might be augmented if repeated doses are administered. This study aimed to compare the effects of single versus double transplantation of Wharton’s jelly MSCs (WJ-MSCs) on LVEF post-AMI.
Methods
We conducted a single-blind, randomized, multicenter trial. After 3–7 days of an AMI treated successfully by primary PCI, 70 patients younger than 65 with LVEF < 40% on baseline echocardiography were randomized to receive conventional care, a single intracoronary infusion of WJ-MSCs, or a repeated infusion 10 days later. The primary endpoint was the 6-month LVEF improvement as per cardiac magnetic resonance (CMR) imaging.
Results
The mean baseline EF measured by CMR was similar (~ 40%) in all three groups. By the end of the trial, while all patients experienced a rise in EF, the most significant change was seen in the repeated intervention group. Compared to the control group (n = 25), single MSC transplantation (n = 20) improved the EF by 4.54 ± 2%, and repeated intervention (n = 20) did so by 7.45 ± 2% when measured by CMR imaging (P < 0.001); when evaluated by echocardiography, these values were 6.71 ± 2.4 and 10.71 ± 2.5%, respectively (P < 0.001).
Conclusions
Intracoronary transplantation of WJ-MSCs 3–7 days after AMI in selected patients significantly improves LVEF, with the infusion of a booster dose 10 days later augmenting this effect.
Trial registration: Trial registration: Iranian Registry of Clinical Trials, IRCT20201116049408N1. Retrospectively Registered 20 Nov. 2020, https://en.irct.ir/trial/52357
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Coronary artery disease can lead to a myocardial infarction (MI), representing one of the leading causes of death globally [1]. Although the recent decades have seen a decrease in post-MI mortality, this has been coupled with an increase in heart failure (HF) [2]. Roughly 14–36% of patients hospitalized due to an acute myocardial infarction (AMI) develop HF [3]. This chronic condition burdens the health system, accounting for annual healthcare costs of roughly 40 billion USD in America [4]. Furthermore, mortality and morbidity rates of post-MI HF remain high despite the therapies available [5,6,7], with current treatments not failing to regenerate the damaged cardiac tissues. Hence, the need for novel strategies is strongly felt [8], with stem-cell-based therapies presenting as a promising option [9].
Beginning in the late twentieth century, the possibility of regenerating cardiac tissue using stem cells was explored in pre-clinical studies [10,11,12,13] before swiftly transitioning to the clinical phase [14, 15]. One safe and highly available stem cell population for such applications is mesenchymal stromal cells (MSCs), which can be isolated from the bone marrow, heart, Wharton’s jelly, and adipose tissue [16, 17]. These cells are easy to isolate and grow ex vivo and have excellent characteristics for in vivo usage [18]. Furthermore, allogeneic and autologous MSCs reportedly offer equal safety and efficacy [19], with one trial on cardiomyopathy patients, indicating that MSCs have twice the efficacy of bone marrow-derived mononuclear cells (BM-MNCs) [20]. Accordingly, MSCs appear to be the optimal option for cardiac stem-cell-based therapies.
Studies on the use of MSCs in AMI have provided promising yet controversial results. The most extensive trial was conducted by Gao et al. on 116 patients, reporting that these stem cells augmented the LVEF by almost 5% [21]. A related meta-analysis noted a 3.84% rise in LVEF [22], with the treatment benefiting relatively younger patients with a reduced LVEF the most. It appears that transplanted MSCs exert an indirect paracrine effect, and direct differentiation to cardiomyocytes does not occur [23]. Hence, we hypothesized that the paracrine effect of MSCs would be augmented if repeated doses were transplanted. Accordingly, we conducted a randomized clinical trial to compare the efficacy of single vs. double injection of MSCs in treating post-AMI heart failure.
Methods
Study design
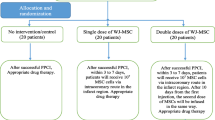
We conducted a randomized, single-blind, multicenter phase II trial to determine the effects of one or two intracoronary infusions of umbilical cord Wharton’s jelly tissue-derived MSCs on post-AMI LVEF when added to standard management. The Ethics Committee of Shiraz University of Medical Sciences approved the study protocol (IR.SUMS.REC.1399.406), which is registered with the Iranian Registry of Clinical Trials (IRCT20201116049408N1). Written informed consent was obtained from all patients before participation once they were in stable condition and recovered from the influence of sedatives. An independent Data and Safety and Monitoring Board (DSMB) monitored patient safety during the study. In-depth details regarding the study protocol are available in a previous publication [24] (Fig. 1).
Sample size determination
Based on the study objectives and related literature, the sample size for each group was formulated as \(n = \frac{{2s^{2} (z_{{1 - {\raise0.7ex\hbox{$\alpha $} \!\mathord{\left/ {\vphantom {\alpha 2}}\right.\kern-0pt} \!\lower0.7ex\hbox{$2$}}}} + z_{1 - \beta } )^{2} }}{{(\partial )^{2} }}\). The parameters used included 5% error, 90% power, 3% difference between placebo and single injection group, and 3% between single and double injection groups at the end of the 6 months of follow-up, with a standard deviation of 1.3 and a ratio of 1:1:1. Considering the study length and need for repeated measurements, the formula \(n^{\prime} = n \times \frac{1}{1 - p}\) was also used with a drop-out rate of 15%, resulting in a minimum of 16 participants in each group. Ultimately, to allow comparisons between all three groups, the formula \(n^{\prime} = n \times \sqrt k\) revealed the need for at least 20 subjects in each group.
Study participants
Seventy patients hospitalized at Al-Zahra, Nemazee, or Faghihi Hospital (Shiraz, Iran) following an anterior ST elevation myocardial infarction (STEMI) and treated with a successful primary percutaneous coronary intervention (PCI) were included in this study. Inclusion criteria consisted of: 1—patients were 20–60 years old, 2—had their MI 3–7 days earlier, 3—had an echocardiographic LVEF < 40%. Exclusion criteria were: 1—prior history of an anterior MI, 2—regional wall motion abnormalities in the non-infarct region, 3—history of coronary artery bypass graft (CABG) surgery, 4—significant valve disease (stenosis/regurgitation grade ≥ 2), 5—alternative etiology of LV dysfunction (non-ischemic cardiomyopathy, 6—anthracycline therapy, 7—regular ethanol abuse > 6 oz. ethanol/day), 8—poor echocardiography window, 9—active syphilis, 10—hepatitis B, hepatitis C, HIV or HTLV-1, 11—terminal disease or cancer, 12—history of a bone marrow transplant, or an autoimmune disease, 13—being pregnant.
Randomization and intervention
Patients were randomized using a web-based randomization service (https://www.sealedenvelope.com/simple-randomiser/v1/lists) into three groups with a 1:1:1 allocation ratio via permuted block randomization with a block size of 6. The outcome assessors were kept blinded to the group allocations. Following randomization, 30 patients received standard guideline-directed medical care, 20 received standard care plus a single intracoronary infusion of 107 hWJ-MSCs 3–7 days after the AMI and 20 received standard care, the initial infusion, plus a repeated infusion after 10 days.
According to previous clinical and animal model studies, among the various sources of MSCs including bone marrow, adipose tissue, cord blood, Wharton’s jelly, etc., WJ-MCS were more efficacious in myocardial infarction and showed lower immunogenicity, higher immunomodulatory effect, smaller cell sizes and higher replication rate [25]. Accordingly, we used MSCs as the stem cell source for this study.
The reason for transplanting 10 million cells goes back to the animal studies showing that a higher number of MSCs may cause microvascular obstruction [26, 27] and also results from clinical trials showing that higher numbers would cause lower improvements in LVEF [22].
On each day of infusion, fresh cGMP-certified clinical-grade hWJ-MSCs (Cell Tech Pharmed Co. Ltd., Tehran, Iran) were transported to the catheterization laboratory in 0.9% normal saline. Cells were provided at the same day that procedure was planned to be performed from a cell bank. Qualification was performed according to GMP grade qualification protocols, and viability was assessed with methylene blue before transplantation. More details are provided on the published protocol of study [24].
The patients were returned to the catheterization laboratory and received heparin if their activating clotting time was below 200 s. A therapeutic 6-Fr guiding catheter was inserted into the left main artery, and 200 μg of nitroglycerin was infused using the guiding catheter. Coronary angiography was done, and TIMI flow was documented. A 0.014-inch soft-tipped guidewire wire was entered into LAD distal to the stent. Vessel occlusion was achieved by inserting and inflating an over-the-wire balloon within the stented area. After removing the guidewire, an infusion syringe was connected to the infusion catheter. The micro-infusion of MSCs was initiated at 2.5 ml/min, and the cells were infused in three equal portions. Prior to the infusion of each portion, total arterial occlusion was confirmed through dye injection, and TIMI coronary flow was assessed. Intracoronary cell infusion complications were averted by the use of low balloon inflation pressure [2–4 bar] and divided infusion portions.
Safety monitoring
For monitoring the safety of treatment, a cardiologist visited the patients daily during hospitalization. A physical examination was conducted, and vital signs were noted. The patients were monitored for indicators of arrhythmia, pulmonary embolism, and coronary artery injury. Baseline tests including fasting blood sugar (FBS), complete cell blood count (CBC), urea and electrolytes, liver function tests (LFTs), creatine kinase, and cardiac troponin T and C reactive protein were also requested. A standard 12-lead electrocardiogram (ECG) and echocardiography were also taken. Safety measure was monitored using a standard questionnaire presented in Additional file 1. Subjects were examined for adverse events for 6 months after treatment. All adverse events graded 2 or higher in severity using the NCI Common Terminology Criteria for Adverse Events (version 4.0) were submitted by the clinical centers to the Data Coordinating Center safety team at UTHealth (Houston, Texas) for records review and sponsor assessment.
Outcome measures and follow-up
A cardiologist visited the patients daily during hospitalization. A physical examination was conducted, and vital signs were noted. The patients were monitored for indicators of arrhythmia, pulmonary embolism, and coronary artery injury. Baseline tests including fasting blood sugar (FBS), complete cell blood count (CBC), urea and electrolytes, liver function tests (LFTs), creatine kinase, and cardiac troponin T and C reactive protein were also requested. A standard 12-lead electrocardiogram (ECG) was also taken. LV function was first evaluated via echocardiography, with the EF being calculated using the motion score and Simpson’s rule. The global longitudinal strain (GLS) was also measured with automated formulas in standard views. Cardiac magnetic resonance (CMR) imaging was done 3 days after primary PCI. Cine-CMR evaluated ventricular function and volume, delayed enhancement (DE)-CMR determined microvascular obstruction and infarct size, and T2 imaging assessed myocardial salvage and infarct size. Myocardial nulling was optimized according to the inversion time. Scar and edema volumes were manually traced with endocardial and epicardial contours after the semi-automated selection of the normal remote myocardium per slice. Images were assessed by an expert operator who was blinded to the study group allocations.
All patients were discharged with a beta-blocker, angiotensin-converting enzyme (ACE) inhibitor, aldosterone antagonist, aspirin, ticagrelor, statin, nitrates (as needed), and a cardiac rehabilitation program. The physical examination, blood tests, and ECG were repeated on day 10 and after 3 and 6 months. Echocardiography and CMR were repeated after 6 months. Follow-up visits were at Imam Reza Clinic (Shiraz, Iran) or the outpatient cardiology clinic of Al-Zahra Heart Hospital (Shiraz, Iran).
The primary outcomes were both safety and the LVEF improvement after 6 months. Secondary outcomes included the 6-month CMR-recorded change in the infarct area and echographic changes in LV function, left ventricular mass (LVM), left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESD), and GLS.
Statistical analysis
An independent, blinded expert evaluated and judged all measurements and excluded those of inadequate quality from the analysis. Then, the data were analyzed with an intention-to-treat analysis. In line with the literature, we considered a 6-month 3% improvement in EF as significant [22]. Baseline data were compared between the study arms and reported as mean and standard deviation of continuous and as frequencies and percentages if categorical. Changes in study outcomes were assessed between groups using one-way analysis of variance (ANOVA) coupled with Tukey’s post hoc test. Only patients who completed the study were included in the final analysis. Within-group changes were assessed via the paired t-test. The estimated treatment effect was reported with its 95% confidence interval (CI). Two-sided P values were obtained. Major adverse cardiac events (MACEs) and serious adverse events (SAEs) were compared between the three groups, with Kaplan–Meier curves plotted to show these events' patterns during follow-up. The Cox’s proportional hazards model was used to determine the hazard ratios with 95% CIs. The group allocation codes were kept blinded until after the analysis.
Results
The baseline characteristics of patients are summarized in Table 1. As evident, the study groups were comparable in most baseline parameters, with a mean age of 55.46 ± 7.14 years. A male predominance was seen in all groups. Notably, most patients were smokers, and roughly a quarter had hypertension (Table 1).
The mean baseline EF measured by CMR was roughly 40% in all three groups (P = 0.392), as evident in Table 2. The final EF was significantly higher in the repeated intervention group relative to the control group (P = 0.003). By the end of the trial, while all patients experienced a rise in EF, the most significant change was in the repeated intervention group, where the EF increased on average by 10.24%—a 7.55% greater increase than that seen in the control group (P = 0.001) (Table 2).
Table 3 compares the diastolic function parameters measured by echocardiography between the groups. Pairwise comparisons revealed that the control group had a significantly lower E final than the intervention (P = 0.006) and repeated intervention (P = 0.013) groups. Regarding the e’ baseline, the single intervention group had a higher value than the control (P = 0.002) and repeated intervention (P = 0.008) groups. The control group had a significantly lower e’ final relative to the repeated (P = 0.019) and single intervention (P = 0.002) groups. The baseline E/e’ was lower in the single intervention group than control (P = 0.001) and repeated intervention (P = 0.004) groups. This is while the control group had a significantly higher final E/e’ than the intervention (P = 0.005) and repeated intervention groups (P = 0.014) (Table 3).
The mean baseline EF, as measured by echocardiography, was around 34%, with the repeated intervention group having a significantly lower baseline EF than the control group (P = 0.013) (Table 4). By the end of the trial, while the echocardiographic EF increased in all groups, this increase was more than twice greater in the repeated intervention group (19.25 ± 8.35%) relative to the control group (8.53 ± 9.39%) (P < 0.001). Also, the LVESD saw a 6.62 ± 7.9 mm increase in the control group compared with a 4.65 ± 14.55 mm decrease in the repeated intervention group (P = 0.008). While a decrement in GLS was seen in all groups, the single intervention (P = 0.015) and repeated intervention (P < 0.001) groups had roughly two and three times greater decrements in GLS than the control group, respectively (Table 4).
Table 5 displays the measurements of infarct size, indicating that the reduction in the amount of scared myocardium was more pronounced in the repeated intervention group.
Regarding safety outcomes, the intracoronary infusion of MSCs resulted in no adverse effects such as cardiac arrhythmias, re-flow phenomenon, or TIMI flow decrease in the intervention or repeated intervention groups. Furthermore, no intracardiac tumorigenic effects were noticed.
Discussion
The present study revealed that two post-AMI intracoronary MSC infusions could significantly improve LVEF on both CMR imaging and echocardiography. Compared to the control group, single MSC transplantation improved the EF by 4.54 ± 2%, and repeated intervention did so by 7.45 ± 2% when measured by CMR imaging (P < 0.001); when evaluated by echocardiography, these values were 6.71 ± 2.4 and 10.71 ± 2.5%, respectively (P < 0.001).
Currently, post-AMI therapeutic measures seek to avert cardiac remodeling and myocyte loss [5]. This is while stem-cell-based therapies go one step further and attempt to reverse the damage through the provision of fresh, functional cells [21]. While early studies on this topic were not very promising [9], later meta-analyses showed that this therapy might be effective in certain populations [22, 24, 28,29,30,31,32]. Hence, in the present study, we selected patients below 65 with an echocardiographic LVEF < 40%, yielding promising results.
One issue with cardiac stem cell therapy is the type of cell used. A Cochrane meta-analysis showed that treating AMI patients with a reduced LVEF with BM-MNCs effectively increases LVEF, yielding survival and functional benefits in patients below 55 with an EF below 37% [33]. On the other hand, two more recent meta-analyses found that while post-AMI therapy with BM-MNCs can decrease hospitalization for CHF and re-infarction, their effect on all-cause mortality and stroke rate is questionable [29, 30]. Another cell type is MSCs, which were about twice as effective as BM-MNCs in the TAC-HFT trial [20]. Meta-analyses indicate that MSCs can improve LVEF by 3.84% [22], compared with 2.72% in BM-MNCs [33]. In the only meta-analysis (36 trials; 2,489 patients) to directly compare MSCs with BM-MNCs, the former performed superiorly than the latter (3.67% vs. 2.13%), particularly when therapy was delivered within the first 10 days of an AMI (5.65% vs. 3.07%) [28]. Furthermore, the POSEIDON trial indicated that allogeneic MSCs are as safe and effective as autologous MSCs [34]. Hence, MSCs are more accessible and effective in regenerative cardiology than BM-MNCs, and our preference for MSCs in the present trial is vehemently justified.
The route of stem cell injection has also been a subject of study. In an animal study, Gong et al. showed that a repeated intravenous dose of human umbilical cord-derived MSCs had a superior therapeutic effect than single-dose treatment in improving the LV function of rats with dilated cardiomyopathy [35]. In a groundbreaking phase 1 trial, Hsiao et al. found that combined intracoronary and intravenous (2 days apart) umbilical cord-derived MSCs appeared to be safe, feasible, and effective (9.80 ± 7.56% rise in EF after 12 months), though confirmatory phase 2 studies are needed [36]. On the other hand, we delivered the stem cells via an intracoronary micro-infusion, which a meta-analysis revealed to have similar efficacy as a transendocardial injection [22].
The timing of delivery of stem cells for following an AMI is a crucial therapeutic parameter. Numerous trials [37,38,39] and meta-analyses [40, 41] have examined this matter on BM-MNCs, revealing an optimal transplantation time of 3–7 days after an AMI. If done sooner, the transplanted cells might be lost, considering the myocardium’s highly inflammatory state; if delayed, it may be less effective due to myocyte loss and fibrosis. While this issue has not been directly evaluated in trials on MSCs, meta-analyses indicate that the first dose would be more effective if transplanted within 7 to 10 days of an AMI [22, 28].
Regarding the number of MSCs used, the current understanding is that the optimal number is 107 cells, with fewer or more cells not altering the outcomes [22]. Studies on pigs and sheep indicate that transplanting excessive MSCs into the heart can induce microvascular obstruction and myocardial injury [26, 27]. Hence, we used 107 cells.
The literature does not support the theory of differentiation into cardiomyocytes as the mechanisms behind the therapeutic effects of stem cells on post-AMI heart, as the number of cells required to account for such effects is immense [42]. Differentiation into vessels is also possible [43], where vasculogenesis would rescue hypoxic cardiomyocytes. However, this is probably not the main mechanism, as patients in all related trials (including the present study) had undergone revascularization or had received thrombolytics [22]. The paracrine effect theory represents the current paradigm, where paracrine cytokine signaling by transplanted cells affects neighboring cells in the recipient’s heart [23]. Hence, we hypothesized that a repeated dose would induce a better therapeutic effect, which was confirmed by our findings. Similarly, Yao et al. found that a repeated dose of BM-MNCs was more effective than a single dose, though their second dose was delivered after 3 months [44]. In the rat study by Gong et al., the repeated intravenous administration of MSCs reduced myocardial inflammation while upregulating indoleamine 2,3-dioxygenase, which offers anti-inflammatory properties [35].
This study had some limitations. Firstly, while we originally planned to conduct a sham procedure as a placebo treatment for the control group [24], this did not receive ethical clearance from our institute. Hence, the control group was restricted to standard guideline-directed medical therapy and cardiac rehabilitation after the successful primary PCI. Secondly, the duration of follow-up was 6 months, so the long-term effects could not be established. In addition, the patients could not be blinded regarding the group to which they were randomly allocated. Finally, we failed to assess the liquid levels of inflammatory markers before and after stem cell transplantation. Nonetheless, our study had some major strengths, including its robust protocol, use of both CMR and echocardiographic imaging, and blinding of the outcome assessors.
Conclusion
Intracoronary transplantation of WJ-MSCs 3–7 days after AMI in patients younger than 65 with a baseline LVEF below 40% significantly improves LVEF, with the infusion of a booster dose 10 days later augmenting this effect.
Availability of data and materials
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Change history
14 February 2024
A Correction to this paper has been published: https://doi.org/10.1186/s13287-024-03663-x
Abbreviations
- MSCs:
-
Mesenchymal stromal cells
- LVEF:
-
Left ventricular ejection fraction
- AMI:
-
Acute myocardial infarction
- WJ-MSCs:
-
Wharton’s jelly mesenchymal stromal cells
- MI:
-
Myocardial infarction
- BM:
-
Bone marrow
- HF:
-
Heart failure
- BM-MNCs:
-
Bone marrow-derived mononuclear cells
- EF:
-
Ejection fraction
- CMR:
-
Cardiac magnetic resonance
- GLS:
-
Global longitudinal strain
- DE:
-
Delayed enhancement
- MACE:
-
Major adverse cardiac events
- SAE:
-
Serious adverse events
- DSMB:
-
Data and Safety and Monitoring Board
References
Organization WH. The global burden of disease: 2004 update. Geneva: World Health Organization; 2008.
Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D’Agostino RB, et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118(20):2057–62.
Hellermann JP, Jacobsen SJ, Gersh BJ, Rodeheffer RJ, Reeder GS. Heart failure after myocardial infarction: a review. Am J Med. 2002;113(4):324–30.
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–220.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2016;134(13):e282–93.
Juillière Y, Cambou JP, Bataille V, Mulak G, Galinier M, Gibelin P, et al. Heart failure in acute myocardial infarction: a comparison between patients with or without heart failure criteria from the FAST-MI registry. Rev Espan Cardiol (Engl Ed). 2012;65(4):326–33.
Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW, et al. Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol. 2003;42(8):1446–53.
Juillière Y, Cambou JP, Bataille V, Mulak G, Galinier M, Gibelin P, et al. Heart failure in acute myocardial infarction: a comparison between patients with or without heart failure criteria from the FAST-MI registry. Rev Españ Cardiol (Engl Ed). 2012;65(4):326–33.
Braunwald E. Cell-based therapy in cardiac regeneration: an overview. Circ Res. 2018;123(2):132–7.
Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4(8):929–33.
Leor J, Patterson M, Quinones MJ, Kedes LH, Kloner RA. Transplantation of fetal myocardial tissue into the infarcted myocardium of rat. A potential method for repair of infarcted myocardium? Circulation. 1996;94(Suppl 9):Ii332–6.
Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–5.
Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7(4):430–6.
Menasché P, Hagège AA, Scorsin M, Pouzet B, Desnos M, Duboc D, et al. Myoblast transplantation for heart failure. Lancet (London, England). 2001;357(9252):279–80.
Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation. 2002;106(24):3009–17.
Williams A, Hare J. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–40.
Houtgraaf JH, den Dekker WK, van Dalen BM, Springeling T, de Jong R, van Geuns RJ, et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with st-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59(5):539–40.
Karantalis V, Schulman I, Balkan W, Hare J. Allogeneic cell therapy a new paradigm in therapeutics. Circ Res. 2015;116:12–5.
Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369–79.
Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311(1):62–73.
Gao LR, Chen Y, Zhang NK, Yang XL, Liu HL, Wang ZG, et al. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015;13:162.
Attar A, Bahmanzadegan Jahromi F, Kavousi S, Monabati A, Kazemi A. Mesenchymal stem cell transplantation after acute myocardial infarction: a meta-analysis of clinical trials. Stem Cell Res Ther. 2021;12(1):600.
Sanganalmath S, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–34.
Attar A, Nouri F, Yazdanshenas A, Hessami K, Vosough M, Abdi-Ardekani A, et al. Single vs. double intracoronary injection of mesenchymal stromal cell after acute myocardial infarction: the study protocol from a randomized clinical trial: BOOSTER-TAHA7 trial. Trials. 2022;23(1):293.
Raposo L, Lourenço AP, Nascimento DS, Cerqueira R, Cardim N, Leite-Moreira A. Human umbilical cord tissue-derived mesenchymal stromal cells as adjuvant therapy for myocardial infarction: a review of current evidence focusing on pre-clinical large animal models and early human trials. Cytotherapy. 2021;23(11):974–9.
Fiarresga A, Mata MF, Cavaco-Gonçalves S, Selas M, Simões IN, Oliveira E, et al. Intracoronary delivery of human mesenchymal/stromal stem cells: insights from coronary microcirculation invasive assessment in a swine model. PLoS ONE. 2015;10(10):e0139870.
Grieve SM, Bhindi R, Seow J, Doyle A, Turner AJ, Tomka J, et al. Microvascular obstruction by intracoronary delivery of mesenchymal stem cells and quantification of resulting myocardial infarction by cardiac magnetic resonance. Circ Heart Fail. 2010;3(3):e5-6.
Hosseinpour A, Kheshti F, Kazemi A, Attar A. Comparing the effect of bone marrow mono-nuclear cells with mesenchymal stem cells after acute myocardial infarction on improvement of left ventricular function: a meta-analysis of clinical trials. Stem Cell Res Ther. 2022;13(1):1–16.
Hosseinpour A, Hosseinpour H, Attar A. Preventive effect of bone marrow mononuclear cell transplantation on acute myocardial infarction-induced heart failure: a meta-analysis of randomized controlled trials. Cardiovasc Drugs Ther. 2022:1–11. https://doi.org/10.1007/s10557-022-07359-3.
Attar A, Hosseinpour A, Hosseinpour H, Kazemi A. Major cardiovascular events after bone marrow mononuclear cell transplantation following acute myocardial infarction: an updated post-BAMI meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2022;22(1):1–12.
Jeong H, Yim HW, Park HJ, Cho Y, Hong H, Kim NJ, et al. Mesenchymal stem cell therapy for ischemic heart disease: systematic review and meta-analysis. Int J Stem Cells. 2018;11(1):1–12.
Wu H, Cao H. Efficacy and safety of mesenchymal stromal cells on left ventricular function after acute myocardial infarction: a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2017;10(4):5871–82.
Fisher SA, Zhang H, Doree C, Mathur A, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2015;9:Cd006536.
Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369–79.
Gong C, Chang L, Sun X, Qi Y, Huang R, Chen K, et al. Infusion of two-dose mesenchymal stem cells is more effective than a single dose in a dilated cardiomyopathy rat model by upregulating indoleamine 2, 3-dioxygenase expression. Stem Cell Res Ther. 2022;13(1):1–16.
Hsiao L-C, Lin Y-N, Shyu W-C, Ho M, Lu C-R, Chang S-S, et al. First-in-human pilot trial of combined intracoronary and intravenous mesenchymal stem cell therapy in acute myocardial infarction. Front Cardiovasc Med. 2022;9:961920.
Choudry F, Hamshere S, Saunders N, Veerapen J, Bavnbek K, Knight C, et al. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: the REGENERATE-AMI clinical trial†. Eur Heart J. 2016;37(3):256–63.
Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308(22):2380–9.
Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306(19):2110–9.
Zhang S, Sun A, Xu D, Yao K, Huang Z, Jin H, et al. Impact of timing on efficacy and safetyof intracoronary autologous bone marrow stem cells transplantation in acute myocardial infarction: a pooled subgroup analysis of randomized controlled trials. Clin Cardiol. 2009;32(8):458–66.
Delewi R, Hirsch A, Tijssen JG, Schächinger V, Wojakowski W, Roncalli J, et al. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST-segment elevation myocardial infarction: a collaborative meta-analysis. Eur Heart J. 2014;35(15):989–98.
Hong K, Guo Y, Li Q-H, Cao P, Al-Maqtari T, Vajravelu B, et al. c-kit+ cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS ONE. 2014;9:e96725.
Cai L, Johnstone B, Cook T, Tan J, Fishbein M, Chen P-S, et al. IFATS collection: human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem cells (Dayton, Ohio). 2008;27:230–7.
Yao K, Huang R, Sun A, Qian J, Liu X, Ge L, et al. Repeated autologous bone marrow mononuclear cell therapy in patients with large myocardial infarction. Eur J Heart Fail. 2009;11(7):691–8.
Acknowledgements
The authors would like to thank Shiraz University of Medical Sciences, Shiraz, Iran.
Funding
This study was supported by the clinician-scientist program of Iran’s Ministry of Health and Medical Education and by the Vice-Chancellory for Research of Shiraz University of Medical Sciences (Grants No. SG-98-5, SG-98-94, and SG-96-86). CellTech Pharmed Co. provided the investigators with the stem cells used. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
AA and AM designed and supervised the study. AA obtained funding and registration. AA, MFK, KH, JK, and MR collected the data. MF analyzed the data. AA, MFK, MV, and SAH interpreted the data. MFK, KH, MV, and MF drafted the manuscript. AA, JK, MR, AM, and SAH critically revised the manuscript. All authors read and approved the final manuscript and accept accountability for all aspects of this work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of Shiraz University of Medical Sciences approved the study protocol titled “Comparing the effects of once versus twice intracoronary injection of allogeneic-derived mesenchymal stem cells in improving ejection fraction of patients with post-myocardial infarction moderate to severe reduced ejection fraction” on 20/06/2020 under Approval Number IR.SUMS.REC.1399.406. All participants provided written informed consent, and all study procedures were carried out in line with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
MV is the regulatory affairs manager at CellTech Pharmed. He has no shares in the company. All other authors have nothing to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Treatment toxicity.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Attar, A., Farjoud Kouhanjani, M., Hessami, K. et al. Effect of once versus twice intracoronary injection of allogeneic-derived mesenchymal stromal cells after acute myocardial infarction: BOOSTER-TAHA7 randomized clinical trial. Stem Cell Res Ther 14, 264 (2023). https://doi.org/10.1186/s13287-023-03495-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-023-03495-1