Abstract
Hematopoietic stem cells (HSCs) with the ability to self-renew and differentiate are responsible for maintaining the supply of all types of blood cells. The complex and delicate microenvironment surrounding HSCs is called the HSC niche and can provide physical, chemical, and biological stimuli to regulate the survival, maintenance, proliferation, and differentiation of HSCs. Currently, the exploration of the biophysical regulation of HSCs remains in its infancy. There is evidence that HSCs are susceptible to biophysical stimuli, suggesting that the construction of engineered niche biophysical microenvironments is a promising way to regulate the fate of HSCs in vitro and ultimately contribute to clinical applications. In this review, we introduced the spatiotemporal heterogeneous biophysical microenvironment during HSC development, homeostasis, and malignancy. Furthermore, we illustrated how these biophysical cues contribute to HSC behaviors, as well as the possible mechanotransduction mechanisms from the extracellular microenvironment into cells. Comprehending the important functions of these biophysical regulatory factors will provide novel approaches to resolve clinical problems.
Similar content being viewed by others
Introduction
Hematopoietic stem cells (HSCs) are heterogeneous cells at the top of the hematopoietic system that exhibit self-renewal and differentiation abilities [1, 2]. The hematopoietic system uses billions of fresh cells every day to update the blood and immune cells in the body [3]. HSCs maintain hematopoietic homeostasis through self-renewal, maturation, apoptosis, resting mode, and trafficking in vivo, which are highly complex and controlled physiological features [4]. Since the 1960s, HSCs have been used in the clinical treatment of patients with hematological diseases such as leukemia and lymphoma [5]. The main sources of HSCs for clinical applications are cord blood (CB), adult bone marrow (BM), and mobilized peripheral blood stem cells. However, because of the low availability of matching donors and the number of HSCs per transplant, the supply of HSCs is very limited [6]. The clinical bottleneck of hematopoietic stem cell transplantation (HSCT) is how to efficiently proliferate functional HSCs in vitro. This challenge may be addressed by studying the growth environment of HSCs in vivo.
HSC niches are highly specialized microenvironments that provide HSCs with all the signals that regulate HSC survival, maintenance, proliferation, and differentiation [7]. Since the HSC niche hypothesis was proposed, researchers have continued to discover new cells, factors, and other parameters that play a role in these niches [8, 9]. Efforts have been made to understand the heterogeneity of these niches at different stages of development, homeostasis, and malignancy [10], enriching the complexity of niche regulation. The microenvironment surrounding HSCs can provide physical, chemical, and biological stimuli that modulate HSC activity and fate alone or in combination. Nevertheless, the exploration of the biophysical regulation of HSCs remains in its infancy [11,12,13]. This review discusses the heterogeneity and the important roles of these biophysical microenvironments in HSC fate regulation, which have led to exciting new ideas for the design of artificial niches for HSC engineering and clinical applications.
Complexity of HSC niches
Spatiotemporal heterogeneous niches during HSC development, homeostasis, and malignancy
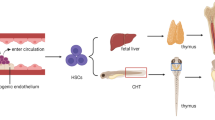
The abilities of HSC self-renewal and maintenance are strictly regulated throughout the human lifetime to maintain blood system homeostasis. Recent technological progress has shown that HSC and hematopoietic stem and progenitor cell (HSPC) groups are not discrete homogeneous populations, but rather heterogeneous populations. Evidence shows that there are HSCs with lineage bias and progenitor cells with lineage restriction in HSC niches [14]. Previous studies have drawn a single-cell transcription map of human hematopoietic stem cells from hematopoietic endothelium to birth and have found that HSC and progenitor cells can be distinguished based on their signature [2]. The HSC niche in development also shows heterogeneity. The anatomical location of HSPC niches changes with space and time (Fig. 1).
Primitive hematopoiesis begins in the yolk sac, and definitive hematopoiesis occurs in the aorta-gonad-mesonephros (AGM) region [15]. HSCs then undergo active expansion and specification in the fetal liver [16]. Afterward, HSCs emigrate from the fetal liver to the fetal spleen [17]. Finally, stromal cell-derived factor 1 generated by BM stromal cells induces the expression of CXCR4 from HSC, which mediates HSC reside into the BM [18].
Throughout adult life, HSCs are maintained and regulated in BM niches. According to different anatomical positions, niches close to the endosteum are currently defined as endothelial niches [19], while niches close to the BM sinusoids [20] or arterioles [21] are defined as perivascular niches. Obviously, each of these BM niches is produced by a variety of cell types and the constituent cells vary greatly [22]. These different microenvironments have different functions, and the HSCs hosted in them are also heterogeneous [23]. Many recent studies have confirmed that the phenotypes and functions of HSCs are heterogeneous in different niches [24, 25]. The use of new technologies has revealed that HSCs are not a pool with unified functions, but a heterogeneous pool composed of different HSC subgroups. HSCs comprise several HSC subgroups with different immunophenotypes. These subgroups have different self-renewal and regeneration abilities, and the selectivity of lineage differentiation is also biased [26]. In addition, there is reason to believe that the heterogeneity of HSCs is closely related to the heterogeneity of HSC niches.
Generally speaking, the main site of hematopoietic activity is the BM. However, when the BM microenvironment becomes unsatisfactory, extra-medullary hematopoiesis may occur in the liver or spleen [27]. When the blood system is stable, the HSCs are in a dormant state. When infection, acute blood cell loss, chemotherapy, radiation-induced cytotoxicity, and other forms of stress and injury occur, HSCs can reversibly switch from a dormant state to an active state to restore the stable state of hematopoiesis. Once the blood system regenerates and re-establishes its stable state, the activated HSCs will re-enter their dormant state [28, 29]. HSCs in a dormant state can minimize the accumulation of DNA damage, thus preventing HSC exhaustion and BM failure [30, 31].
Alterations to the hematopoietic microenvironment upon aging might lead to diseases such as hematologic malignancies [10]. Therefore, the compositional and functional heterogeneity of niches in various anatomical sites during different developmental stages should be emphasized when identifying their special contributions to the fate of HSCs. The aging of HSCs partially contributes to the impairments of an aged hematopoietic system. Research shows that in young- and middle-aged mice, there is a stable balance between myeloid-biased, lymphoid-biased, and balanced HSC subsets [32, 33]. However, this balance was found to be broken in older mice. Myeloid-biased HSCs increased and became the main type. The self-renewal and regeneration ability of these HSCs decreased, resulting in a decline in the production of mature blood cells [24, 34]. Aging leads to the reduction in comfort of the HSC niche, thus breaking the hematopoietic homeostasis. Some studies have changed the mitochondrial membrane potential of HSC through pharmacological operations and have found that it can have beneficial effects on the function of HSC [35].
Multiple components of HSC niches
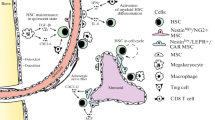
The communication between a variety of niche components, including niche cells, soluble components, and ECM molecular provides chemical signals regulating the fate decision of HSC. As shown in Fig. 2, the mechanosensors formed by cell–cell and cell–ECM interactions, such as adhesion receptor-ligand bonds, the cytoskeleton, mechanically gated ion channels, and primary cilia, enable HSCs to autonomously sense and react to mechanical cues in niches [36, 37]. Components of HSC niches that contribute to HSC fate regulation are shown in Table 1.
Hematopoietic stem cells (HSCs) are stimulated by the biochemical, biological, and physical parameters of the microenvironment in vivo. HSCs are subjected to biochemical and biological signals elicited by cell–cell interactions including direct contacts and communication through soluble factors as well as cell–matrix interactions. At the same time, cells are stimulated by the physical parameters of the environment. Intrinsic forces (Fi) are generated intracellularly and transferred to other cells through cell–cell junctions, such as cadherin receptors, or via traction on extracellular matrix (ECM) adhesion ligands those are bound to integrin receptors. Extrinsic forces (Fe) are externally applied by shear or tension and/or compression on cells, and they can be sensed by mechanically gated ion channels, changes in receptor-ligand binding, deformation of the cytoskeleton, and the primary cilium. Physical properties, for example, the elastic modulus and nanotopography of the ECM, govern how mechanical cues are transduced. The cytoskeleton generates and transfers forces from membrane proteins to intracellular structures, such as the nucleus
HSC niches are composed of multiple cell types with specific functions [38]. These different types of cells either directly or indirectly support the maintenance and regulation of HSCs in HSC niches [39]. Mesenchymal stromal cells (MSCs) provide niche factors such as CXCL 12, SCF, and interleukin 7 (IL 7) and play an important role in the regulation of HSCs [40]. Osteolineage cells (osteocytes, osteoblasts, and osteoclasts) are crucial for lymphopoiesis and have been implicated in HSPC regulation [41]. Adipocytes might inhibit HSC activity, but this conclusion is still controversial [42, 43]. The endothelium can regulate HSC maintenance and the activity of perivascular cells [44]. Adrenergic nerves can regulate HSC mobilization and hematopoietic recovery [45]. Schwann cells may promote HSC quiescence through TGF-β signal conduction [46]. Macrophages may directly participate in the maintenance of HSC, and indirectly regulate the retention of HSC through niche cells [47]. Megakaryocytes can promote HSC quiescence via applying a feedback loop [48, 49]. T cells and neutrophils might direct interact with HSCs or regulate HSC behaviors via other immune and stromal cells [50, 51]. Recent studies (Table 1) have continuously enriched the known functions of each cell and factor during HSC homeostasis and niche formation. Although many studies have been conducted to elucidate the role of cells in the niche, the regulation of HSC populations remains highly complex and elusive.
The ECM is a fine and intricate network composed of macromolecules synthesized and secreted by cells to the outside that are distributed on the cell surface or between cells. Its components are mainly collagen, elastin, non-collagen glycoprotein, and aminoglycan or proteoglycan. The ECM maintains the organizational structure of cells and provides anchorage sites for cell adhesion and migration [52, 53]. A variety of ECM proteins participate in the regulation of HSCs. For example, structural proteins such as collagen, laminin, and fibronectin provide anchorage sites for cells to support HSC retention and mobilization [52]. In addition, laminins and fibronectin have also been found to affect the regulation of HSC proliferation and differentiation, and affect the implantation ability of HSC [54,55,56]. Osteopontin has been found to have a negative effect on HSC proliferation [57, 58]. The ECM in the BM is heterogeneous. The endothelial region is rich in osteopontin and type I collagen, and the vascular region is rich in laminin [59,60,61]. The changes in the ECM composition in different regions may lead to different functions of BM niches. Variations in the ECM composition may contribute to differences in biological, chemical, and biophysical factors in BM regions.
Studies have revealed that HSCs have the ability to sense external biophysical cues, such as shear stress, matrix stiffness, and matrix nanotopography [13]. These biophysical stimuli, alone or coupled with biological stimuli, can modulate the activity and fate of HSCs. HSCs convert the macroscale biophysical inputs they sense into molecular signals with chemical activity to guide cell behavior [62]. However, the mechanotransduction mechanism through which HSCs sense and react to the mechanical signals remains unknown.
Heterogeneous biophysical microenvironment of HSCs
HSC maintenance and self-renewal are strictly regulated throughout the human lifetime to maintain blood system homeostasis. HSC niches are spatiotemporally heterogeneous during HSC development, homeostasis, and malignancy. In studying the specific contributions of the biophysical microenvironment to the fate of HSCs, the heterogeneity of the composition and function of niches in different anatomical sites at different developmental stages should be emphasized (Fig. 3).
Biophysical microenvironment of heterogeneous HSC niches. Bone marrow is the primary niche for adult HSC maintenance. When the individual is under severe stress or the BM microenvironment becomes suboptimal due to pathological conditions, extra-medullary hematopoiesis (EMH) can occur, mostly in the liver or spleen
Biophysical microenvironment of embryonic HSCs
Studies have shown that hematopoietic stem cell formation, development, and regulation are dependent on blood flow [63,64,65]. Blood flow and hematopoietic cells occur synchronously, and research also shows that blood flow is an important regulatory factor for HSC development [66]. Blood flow passing through a vessel generates three forces: hydrostatic pressure, shear stress, and circumferential strain. Shear stress is the frictional force tangential to endothelial cells (ECs), while circumferential strain refers to the force perpendicular to the flow direction [67, 68]. At the embryonic stage, HSCs are generated by the hematopoietic endothelium in the AGM. Previous studies have shown that shear stress caused by blood flow is a necessary condition for HSC generation in this process [37, 62]. Similarly, studies in vitro have shown that shear stress affects the proliferation and differentiation of HSCs in vitro. These studies provide a new idea for the expansion of HSCs in vitro and will have a beneficial impact on the possible clinical applications of HSCs [69, 70]. Large-scale cell expansion requires sufficient medium containing essential nutrients and growth factors for the growing cell population. Medium flow also induces shear stress, so it is necessary to study whether shear stress affects HSC behavior. In addition, stromal cells at different regions in AGM lead to changes in the maintenance and differentiation of embryonic HSCs [71]. The biophysical interactions between embryonic HSCs and stromal cells in the AGM compartment regulate the development of embryonic HSCs [72]. These indicate that the activity of embryonic HSC requires the coordinated regulation of chemical signals, biological signals and physical signals from the AGM microenvironment.
Biophysical microenvironment of BM niches
BM niches are the main site of adult HSC maintenance and regulation. Most adult HSCs are not directly exposed to the fluid environment in the BM, but some functional cells in the HSC niche may be in the fluid environment and will react the influence of fluid to HSCs through paracrine signals [41]. The fluid flow in the cavity of the bone produces shear stresses of 6–50 dynes/cm2. These shear stresses have been shown to affect bone cells and endothelial cells, thereby regulating the quiescence and circulation of HSC [73]. In BM, the fibronectin-rich endosteum region is stiff (40–50 kPa), while the laminin-rich perivascular region is soft (3 kPa) [74, 75]. Moreover, niche stiffness is not a static parameter, but rather a dynamical property during physiological processes. During the mobilization of HSCs from BM niches to the blood circulation, adrenaline stimulates osteoblasts to flatten and harden [45, 76]. The composition and molecular cross-linking in the ECM will change due to aging and disease, leading to matrix stiffening [77, 78]. Similarly, in the occurrence of diseases such as atherosclerosis and myelofibrosis, the hematopoietic tissue is hardened. Research has shown that both embryonic and adult stem cells are sensitive to substrate stiffness, including HSCs. By changing the E-modulus of the substrate, the differentiation of MSCs can be controlled [79, 80]. Our previous work has also shown that matrix stiffness regulates macrophage growth and development [81]. Besides matrix stiffness, matrix elasticity, and nanotopography of the matrix can also regulate the adhesion of HSCs [82]. In addition, mechanical loading is required for HSC differentiation. When organisms are exposed to some special environments, the biophysical microenvironments in vivo will also change [83].
Biophysical microenvironment of extra-medullary HSC niches
When the BM microenvironment becomes unsatisfactory, extra-medullary hematopoiesis may occur in the liver or spleen [27]. Clearly, the physical microenvironment provided by the liver or spleen for HSCs is completely different from the BM niche. Taking stiffness as an example, the Young's modulus of the liver and spleen ranges from 4 to 7 kPa, which is comparable to the stiffness of blood vessels, but far from the Young's modulus of the endosteal region. Most adult HSCs are located in BM, but a small number of HSCs circulate in the body [84]. Blood vessels provide another important extra-medullary HSC niche. Although blood vessels have no hematopoietic function, they are an important site for HSC activity. The biophysical characteristics of these extra-medullary niches are obviously different from those of the BM niches. For example, the shear force, which is generated by blood flow in the blood vessels, is an important stimulating factor that cannot be ignored. In mice, when HSCs circulate via blood flow, the shear stress in some areas exceeds 600 dyne/cm2 [85].
Changes in the biophysical microenvironmental caused by aging and disease
Aging will change the microenvironment of HSCs, resulting in the decline of HSC function. Individual HSCs can exhibit lineage bias, giving rise to myeloid-biased, lymphoid-biased, or more balanced differentiation, with the proportion of myeloid-biased HSCs increasing with age [86]. With the increase in age, the balance of long-term hematopoietic stem cells (LT-HSCs) in maintaining hematopoietic output is destroyed. LT-HSCs give rise to myeloid-biased, and myeloid leukemia eventually develops. Using single-cell RNA sequencing (scRNA-seq) [87], a previous study identified an age-related myeloid-biased subset and revealed important regulators of inflammatory myeloid bias, providing guidance for preventing aging-induced myeloid leukemia [88].
The vascular remodeling and changes in adrenergic signaling during aging influence the niche function. HSCs and their derivatives remodel the niche component. These events may cooperatively bias the fate of HSCs toward myeloid differentiation [89].
Mechanosensors and mechanotransduction in HSCs
The HSC senses the biomechanical signals from the HSC niche through mechanosensors, thereby generating intrinsic forces and triggering a series of mechanotransduction. Finally, biomechanical signals will affect the cytoskeleton and chromatin structure, thus guiding cell behavior (Fig. 4).
Mechanosensors and mechanotransduction of HSC. The drawing schematically depicts the mechanosensory units and molecules and highlights the molecules downstream of integrin that are expressed by HSPCs and/or play a role in HSPC biology. Biomechanical inputs from external loads directly stimulate mechanosensors such as mechanically gated ion channels, adhesion receptor-ligand bonds, cytoskeleton, and primary cilia. Intrinsic forces are generated under environmental mechanical constraints, and then transmit to neighboring cells through junctional interfaces, and consequently elicit cellular mechanoresponses. Besides, intrinsic forces can directly pass on to the nucleus through lamin A/C (LMNA), affecting chromatin structure and thereby controlling epigenetic processes. Biomechanical cues cooperate with biochemical signals in mechanotransduction
Mechanosensors
The signals generated by supporting cells and ECM are necessary for the maintenance and regulation of HSCs [90, 91]. The mechanosensors formed by cell–cell and cell–ECM interactions, such as adhesion receptor–ligand bonds, the cytoskeleton, mechanically gated ion channels, and primary cilia, enable HSCs to autonomously sense and react to mechanical cues in niches [36].
Integrin-mediated adhesion
Cell adhesion molecules (CAMs) mediate cell–cell and cell–ECM connections. The domain formed by the connection initiates intracellular signal transduction or interacts with the cell cytoskeleton. CAMs include integrins, cadherins, selectins, and the immunoglobulin superfamily [92, 93]. Cadherin mediates intercellular adhesion, while integrin mediates cell–ECM adhesion and finally uses intrinsic forces to form focal adhesions (FAs) [74, 94, 95]. ECM proteins with Arg-Gly-Asp integrin recognition motifs, such as fibronectin, laminin, and collagen, can connect with integrin, thus enabling cells to sense mechanical signals [73]. The activated integrin will bind and activate kindlins and talins. In addition, vinculin combines with talin to promote the enrichment of multiple activated integrins. Vinculin also transmits signals to the cytoskeleton by combining its tail domain with actin [96, 97].
In addition to transmitting the signal to the cytoskeleton, integrin also transmits the signal into the cell through the intracellular multi-protein complex [98]. In this process, paxillin, focal adhesion kinase (FAK), Pyk2, Crk, and P130CAS are induced to phosphorylate. These key elements play a role in the mechanotransduction of HSCs. FAK and Pyk2 interact with talin and paxillin and are involved in the activation of paxillin and guanine nucleotide exchange factors [99, 100]. When the adaptor molecule P130cas, as a mechanosensor located at the downstream of integrins, is phosphorylated, it becomes the substrate for the interaction of kinases of the Src family [101]. In addition, the Src family kinases (SFKs) are quickly activated. The SFKs can directly bind with integrin and can also connect with FAK. SFKs have an effect on the mobilization of HSCs from the BM to the circulation and play a role in HSCT [102, 103]. Phosphatidylinositol-4,5-bisphosphate3-kinase (PI3K) activated by SFKs can regulate HSC adherence and motility [76]. The HSCs can sense the force from the ECM and can apply traction force to the ECM in return. HSCs can secrete matrix components or proteases to regulate the ECM, thus enhancing or eliminating the adhesion interactions between HSCs and the ECM [104, 105]. ECM remodeling proteins change the niche microenvironment, thus regulating the quiescence, mobilization, and hematopoiesis of HSCs [106,107,108].
Intrinsic forces generated by the cytoskeleton
The cytoskeleton is the communication hub between the cell and the external biophysical microenvironment. Through the changes of the cytoskeleton, HSCs can sense, transmit, and generate force [109,110,111]. It has been shown that myosin IIA in HSCs is regulated by matrix stiffness. The activity of myosin IIA is enhanced on stiff matrices, whereas it is decreased on soft matrices [112, 113]. When the cytoskeleton or the transmembrane adhesion receptors connected with the cytoskeleton are stimulated by biomechanical stimuli, the cytoskeleton is remodeled and the cytoskeleton tension is rearranged, thus generating intrinsic forces [114, 115]. Through a component of nuclear lamina proteins, laminin A/C, the intrinsic force can be directly transmitted to the nucleus, thus modifying the chromatin structure and controlling epigenetic transcription [116]. In Ptpn21 deletion, HSCs, by dephosphorylating Spetin1 in cells, damages the stability of the cytoskeleton, reducing the decrease in HSC stiffness and increasing the physical deformation ability of HSC, thus weakening the quiescence and hematopoietic reconstitution capabilities of HSCs [117]. Ptpn21-deleted leukemic cells also showed a decrease in mechanical rigidity and an increase in cell deformability. These studies support the concept that the cytoskeleton is a hub of communication in mechanotransduction [118].
Mechanically gated ion channel
Cationic stretch-activated channels can sense mechanical forces as well as intrinsic forces and are permeable to Ca2+ as the second messenger [134,135,136,137]. The blocker, activator, or modulator of Na+ /K+-channels can regulate the fate of HSCs [64]. Ca2+ can regulate the activity of eNOS and stimulate the release of nitric oxide (NO). NO is a necessary regulator for HSC functions [119]. The depletion of NO in HSCs leads to the transformation of HSCs from differentiation to proliferation [120].
Primary cilia
Increasing evidence indicates that the primary cilia in almost all human blood and BM cells (97–99%) may be a communication hub for signal transduction. Because of the abundant calcium channels and receptors in its membrane, cilia have the ability to sense and transmit microenvironmental mechanical and chemical stimuli [121, 122]. The mechanical signals transmitted by the primary cilia are required for the osteogenic response and proliferation of human MSCs, and thus contribute to the maintenance of the essential components of BM niches that support HSCs [123]. In addition, vascular ECs sense the fluid flow signals through the primary cilia and regulate the biosynthesis of NO [124, 125]. However, the further influence of the NO released mediated by the primary cilia on the outputs of HSCs within vascular niches remains unclear.
Mechanoresponsive transcription factor
On and inside the cell membrane, changes in cytoskeletal remodeling and protein recruitment are the first step of mechanical signal inputting. This step introduces the downstream mechanotransductive effects, thus stimulating the changes in the cytoplasmic localization of molecules and ultimately stimulating transcriptional effects. Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) are two transcriptional cofactors that shuttle between the nucleus and the cytoplasm. They can transmit signals triggered by biomechanics to the nucleus to affect gene transcription [109, 110]. The activity of YAP/TAZ is limited to cells experiencing biomechanical stresses, and its localization and degradation are regulated by the Hippo pathway [110]. YAP is detectable at low levels only in murine long-term HSCs, but not in murine short-term HSCs or Lin+ hematopoietic lineages. In addition, the nuclear skeleton proteins laminin A and laminin B are important elements involved in biomechanical signal transmission to the nucleus. These proteins have been confirmed to be biomechanosensitive and play an important role in HSC transmigration [126]. KLF2 is an important biomechanically activated transcription factor and a key medium for HSC production induced by blood flow [127, 128]. cAMP response element-binding protein (CREB) is a downstream effector of fluid shear stress that has been demonstrated to affect the emergence of HSCs [129, 130].
Complicated crosstalk under mechanical conditions
The above describes the mechanosensors and mechanotransduction in HSCs. Mechanosensors and mechanotransduction work together to determine the fate of HSCs. The cytoskeleton is closely related to YAP/TAZ. Studies have shown that the F-actin related protein can enhance the YAP nuclear translocation and can abrogate YAP/TAZ activity [131, 132]. Cdc42-Rho-GTPase promotes F-actin polymerization to enhance the nuclear retention of YAP [133]. Ciliary bending caused by biomechanical stimuli can induce cytoskeletal deformation and membrane stretching, thus initiating extracellular Ca2+ influx through calcium channels in the ciliary membrane [134, 135]. In short, these mechanosensors and mechanotransduction are highly interconnected rather than mutually exclusive.
Currently, previous studies have shown that HSC is mechanically sensitive, and some biomechanical sensing elements have been proposed, but the molecular mechanism of mechanotransduction needs further clarification.
Engineering the biophysical niches for clinical applications
Clinical significance of mimicking niche biophysical signals
Stem cell therapy, such as transplantation and tumor purging, is used to treat hematological diseases and malignant tumors [136]. HSCT was achieved in the 1950s [137]. The BM or HSCs extracted from autogenous or allogeneic grafts can be infused into patients after myeloablative treatment [74]. The main bottleneck of this treatment is the lack of sufficient HSC supply, because the number of stem cells from common sources such as the BM and umbilical cord blood is scarce [138]. In addition, there are also obstacles to the function of transplanted HSCs, which are mainly manifested in the low homing efficiency of HSCs transplanted into the BM cavity [139]. Therefore, how to effectively amplify HSCs in vitro is of great significance for clinical treatment.
The method of amplifying HSCs in vitro by referring to the natural microenvironment of HSCs is emerging. However, the common HSCs culture system only focuses on the provision of growth factors and cytokines, and rarely pays attention to the influence of biomechanical clues. Such systems can enhance the proliferation of HSPCs, but the proliferating HSCs have differentiated and lost their self-renewal ability, which has no clinical significance [140, 141]. Therefore, in order to reproduce the natural microenvironment of HSC, physical factors must be considered. Some designs with biomechanical clues help to realize the continuous expansion of HSCs while maintaining their ability to self-renew and differentiate [142, 143].
Engineering HSC niches in vitro
How to reconstruct the unique and intricate microenvironment architecture and nanotopography of HSC niches in vivo and provide a variety of biophysical cues for HSCs and HSC-related accessory cells are important research directions of HSC culture in vitro [13, 37]. Through biomaterial technology, scaffolds with complex structures can be manufactured to provide biophysical clues for cells [144]. The methods of mimicking the BM niche in vitro and the evaluation of their advantages and disadvantages were summarized in our previous investigation [11].
BM bionic three-dimensional (3D) scaffold can better maintain and expand HSCs than traditional two-dimensional (2D) culture system [142]. Human umbilical cord blood HSCs proliferate more strongly in 3D scaffolds than in 2D conditions [145,146,147,148,149]. Similarly, compared with the standard 2D culture system, MSCs have a more significant positive effect on the proliferation of HSPC in the 3D PEG co-culture system [150]. This method of combining stromal cells with bioscaffolds mimics the BM microenvironment more effectively. Our group has investigated the regulation of HSC by matrix dimensionality. Compared to 2D cultures, HPCs within 3D systems generate a cluster of “3D-macrophages,” and 3D matrices enhance the communications between such 3D macrophages and other hematopoietic clusters based on bioinformatic analyses [151]. The proportion of Lin− c-kit+ Sca1+ (LSK) cells in BM cells can be significantly increased by culture on stiff matrix [36]. In addition, although the adhesion of HSCs to material surfaces is not strong enough [152], many studies have found that nanotopography can indeed affect the behavior of HSCs. Since research found that nanotopography can affect the behavior of HSPC [152], the regulatory mechanism of nanotopography on HSCs has received extensive attention.
Conclusion and perspective
The clinical bottleneck of HSCT is how to efficiently proliferate functional HSCs in vitro. Identifying the influencing factors during HSC development and grasping the underlying mechanisms are the key to understanding why HSCs have the abilities of self-renewal and versatility. The complex and exquisite HSC niche can provide physical, chemical, and biological stimuli to regulate HSC survival, maintenance, proliferation, and differentiation. When identifying the specific contribution of the microenvironment to HSC fate, all types of environmental stimuli acting on cells must be considered.
The study of biomechanical signals has been underestimated in research on HSC niches. This is a breakthrough complementary theory that improves and expands the currently known types of HSC regulatory signals. This review introduced the spatiotemporal heterogeneous biophysical microenvironment during HSC development, homeostasis, and malignancy, illustrated how these biophysical cues contribute to HSC behaviors, and discussed the possible mechanotransduction mechanisms from the extracellular microenvironment into cells. Comprehending the important functions of these biophysical regulatory factors will provide novel approaches for resolving clinical problems.
HSC maintenance and self-renewal are strictly regulated throughout the human lifetime to maintain blood system homeostasis. HSC niches are spatiotemporally heterogeneous during HSC development, homeostasis, and malignancy. In studying the specific contribution of the biophysical microenvironment to HSC fates, the heterogeneity of the composition and function of niches in different anatomical sites at different developmental stages should be emphasized. In addition, under some special environments such as weightlessness and high-altitude hypoxia, abnormal physiological processes such as obesity and malignancy will cause changes in the microenvironment of the niche, which in turn affects the fate of HSCs. This is also a direction for future research.
HSC can sense and transmit the biophysical signals from HSC niche, so as to guide the behavior of HSC. However, most studies only describe the phenomenon that HSC has biomechanical sensitivity, without clarifying the intrinsic molecular mechanism, and some key problems have not been solved: Whether HSC has the same sense and transmission mode for different biomechanical signals, whether the feedback of HSC to biomechanical signals is short term or long term, and whether the complex process of HSCs regulated by biomechanical signal, biological signal, and chemical signal has series connection. These are still hot topics to be studied in the future.
Burgeoning experimental techniques have also facilitated HSC research, such as single-cell sequencing [87], high spatiotemporal resolution imaging, CyTOF [153], and bioengineering. Advances in these technologies will allow researchers to elucidate the mechanisms through which the physical microenvironment regulates HSC fate, and thus it is conceivable that harnessing these biophysical cues as master regulators for HSC fate regulation could be exploited for artificial niches and therapeutic gain.
Abbreviations
- AGM:
-
Aorto-gonad-mesonephros
- BM:
-
Bone marrow
- CB:
-
Cord blood
- CyTOF:
-
Cytometry by time of flight
- ECM:
-
Extracellular matrix
- ECs:
-
Endothelial cells
- EHT:
-
Endothelial-to-hematopoietic transition
- EMH:
-
Extra-medullary hematopoiesis
- FAK:
-
Focal adhesion kinase
- FAs:
-
Focal adhesions
- HSC:
-
Hematopoietic stem cell
- HSCT:
-
Hematopoietic stem cell transplantation
- HSPC:
-
Hematopoietic stem and progenitor cell
- LINC:
-
Linker of nucleoskeleton and cytoskeleton
- LMNA:
-
Lamin A/C
- LSK:
-
Lin− c-kit+ Sca1+
- LT-HSC:
-
Long-term hematopoietic stem cell
- MSC:
-
Mesenchymal stromal cells
- NK:
-
Natural killer
- NO:
-
Nitric oxide
- PAM:
-
Polyacrylamide
- PI3K:
-
Phosphatidylinositol-4,5-bisphosphate3-kinase
- scRNA-seq:
-
Single-cell RNA sequencing
- SFKs:
-
Src family kinases
- TAZ:
-
Transcriptional co-activator with PDZ-binding motif
- TF:
-
Transcription factors
- UCB:
-
Umbilical cord blood
- WSS:
-
Wall shear stress
- YAP:
-
Transcriptional cofactors Yes-associated protein
References
Laurenti E, Göttgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature. 2018;553(7689):418–26.
Calvanese V, Capellera-Garcia S, Ma F, Fares I, Liebscher S, Ng ES, Ekstrand S, Aguade-Gorgorio J, Vavilina A, Lefaudeux D: Mapping human haematopoietic stem cells from haemogenic endothelium to birth. Nature 2022(604-Apr.21 TN.7906).
Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81(11):2844–53.
Cheng T. Toward “SMART” stem cells. Gene Ther. 2008;15(2):67–73.
Thomas ED, Blume KG. Historical markers in the development of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transplant. 1999;5(6):341–6.
Dahlberg A, Delaney C, Bernstein ID. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011.
Schofield R. The relationship between the spleen colony-forming cell and the haematopoietic stem cell. Blood Cells. 1978;4(1–2):7–25.
Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014(7483).
Crane GM, Jeffery E, Morrison SJ. Adult haematopoietic stem cell niches. Nat Rev Immunol. 2017.
Gao X, Xu C, Asada N, Frenette PS. The hematopoietic stem cell niche: from embryo to adult. Development 2018;145(2):dev139691.
Zhang P, Zhang C, Li J, Han J, Liu X, Yang H: The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res Ther. 2019;10.
Li H, Luo Q, Shan W, Cai S, Tie R, Xu Y, Lin Y, Qian P, Huang H. Biomechanical cues as master regulators of hematopoietic stem cell fate. Cell Mol Life Sci. 2021;78(16):5881–902.
Lee-Thedieck C, Spatz JP. Biophysical regulation of hematopoietic stem cells. Biomater Sci. 2014;2(11):1548–61.
Haas S, Trumpp A, Milsom MD. Causes and consequences of hematopoietic stem cell heterogeneity. Cell Stem Cell. 2018;22(5):627–38.
Kumar A, D 'Souza SS, Thakur AS. Understanding the journey of human hematopoietic stem cell development. Stem Cells Int. 2019;2019(3):1–13.
Rybtsov SA, Lagarkova MA. Development of hematopoietic stem cells in the early mammalian embryo. Biochem Mosc. 2019;84(3):190–204.
Zhao M, Tao F, Venkatraman A, Li Z, Smith SE, Unruh J, Chen S, Ward C, Qian P, Perry JM. N-cadherin-expressing bone and marrow stromal progenitor cells maintain reserve hematopoietic stem cells. Cell Rep. 2019;26(3):652–69.
Singh P, Pelus LM. CXCR4-SDF-1 signaling in Nestin+ mesenchymal stem cell is required for HSC maintenance during homeostasis and regeneration after irradiation. Blood. 2016;128(22):3883–3883.
Zhang J, Niu C, Ye L, Huang H, He X, Tong W-G, Ross J, Haug J, Johnson T, Feng JQ. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41.
Kiel MJ, Yilmaz ÖH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005; 121(7):1109–21.
Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–43.
Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208(3):421–8.
Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5.
Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, Rossi DJ. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci. 2010;107(12):5465–70.
Oguro H, Ding L, Morrison Sean J. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13(1):102–16.
Jurecic R. Hematopoietic stem cell heterogeneity. In: Birbrair A, editor. Stem Cells heterogeneity in different organs. Cham: Springer; 2019. p. 195–211.
Morita Y, Iseki A, Okamura S, Suzuki S, Nakauchi H, Ema H. Functional characterization of hematopoietic stem cells in the spleen. Exp Hematol. 2011;39(3):351–59.
Baumgartner C, Toifl S, Farlik M, Halbritter F, Scheicher R, Fischer I, Sexl V, Bock C, Baccarini M. An ERK-dependent feedback mechanism prevents hematopoietic stem cell exhaustion. Cell Stem Cell. 2018;22(6):879-892.e876.
Cabezas-Wallscheid N, Buettner F, Sommerkamp P, Klimmeck D, Ladel L, Thalheimer FB, Pastor-Flores D, Roma LP, Renders S, Zeisberger P, et al. Vitamin A-retinoic acid signaling regulates hematopoietic stem cell dormancy. Cell. 2017;169(5):807-823.e819.
Nakamura-Ishizu A, Takizawa H, Suda T. The analysis, roles and regulation of quiescence in hematopoietic stem cells. Development. 2014;141(24):4656–66.
Walter D, Lier A, Geiselhart A, Thalheimer FB, Huntscha S, Sobotta MC, Moehrle B, Brocks D, Bayindir I, Kaschutnig P, et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520(7548):549–52.
Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee S-J, Brinkman R, Eaves C. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1(2):218–29.
Sanjuan-Pla A, Macaulay IC, Jensen CT, Woll PS, Luis TC, Mead A, Moore S, Carella C, Matsuoka S, Jones TB, et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502(7470):232–6.
Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med. 2011;208(13):2691–703.
Mansell E, Sigurdsson V, Deltcheva E, Brown J, James C, Miharada K, Soneji S, Larsson J, Enver T. Mitochondrial potentiation ameliorates age-related heterogeneity in hematopoietic stem cell function. Cell Stem Cell. 2021;28(2):241–56.
Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, Kondyurin A, Ma L, Oberhauser AF, Weiss AS. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotechnol. 2010;28(10):1123–8.
Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017;18(12):728–42.
Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–34.
Wei Q, Frenette PS. Niches for hematopoietic stem cells and their progeny. Immunity. 2018;48(4):632–48.
Gomes AC, Hara T, Lim VY, Herndler-Brandstetter D, Nevius E, Sugiyama T, Tani-Ichi S, Schlenner S, Richie E, Rodewald H-R. Hematopoietic stem cell niches produce lineage-instructive signals to control multipotent progenitor differentiation. Immunity. 2016;45(6):1219–31.
Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Ann N Y Acad Sci. 2016;1370(1):82–96.
Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank A-M, Bocian C, Woelk L, Fan H, Logan DW, Schürmann A. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 2017;20(6):771–84.
Mattiucci D, Maurizi G, Izzi V, Cenci L, Ciarlantini M, Mancini S, Mensà E, Pascarella R, Vivarelli M, Olivieri A. Bone marrow adipocytes support hematopoietic stem cell survival. J Cell Physiol. 2018;233(2):1500–11.
Langen UH, Pitulescu ME, Kim JM, Enriquez-Gasca R, Sivaraj KK, Kusumbe AP, Singh A, Di Russo J, Bixel MG, Zhou B. Cell–matrix signals specify bone endothelial cells during developmental osteogenesis. Nat Cell Biol. 2017;19(3):189–201.
Katayama Y, Battista M, Kao W-M, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–21.
Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147(5):1146–58.
Albiero M, Poncina N, Ciciliot S, Cappellari R, Menegazzo L, Ferraro F, Bolego C, Cignarella A, Avogaro A, Fadini GP. Bone marrow macrophages contribute to diabetic stem cell mobilopathy by producing oncostatin M. Diabetes. 2015;64(8):2957–68.
Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, Scheiermann C, Schiff L, Poncz M, Bergman A. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20(11):1315–20.
Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, Ahamed J, Li L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20(11):1321–6.
Bonomo A, Monteiro AC, Gonçalves-Silva T, Cordeiro-Spinetti E, Galvani RG, Balduino A. AT cell view of the bone marrow. Front Immunol. 2016:184.
Kawano Y, Fukui C, Shinohara M, Wakahashi K, Ishii S, Suzuki T, Sato M, Asada N, Kawano H, Minagawa K. G-CSF-induced sympathetic tone provokes fever and primes antimobilizing functions of neutrophils via PGE2. Blood J Am Soc Hematol. 2017;129(5):587–97.
Peerani R, Zandstra PW. Enabling stem cell therapies through synthetic stem cell–niche engineering. J Clin Investig. 2010;120(1):60–70.
Votteler M, Kluger PJ, Walles H, Schenke-Layland K. Stem cell microenvironments-unveiling the secret of how stem cell fate is defined. Macromol Biosci. 2010;10(11):1302–15.
Sagar BMM, Rentala S, Gopal P, Sharma S, Mukhopadhyay A. Fibronectin and laminin enhance engraftibility of cultured hematopoietic stem cells. Biochem Biophys Res Commun. 2006;350(4):1000–5.
Siler U, Seiffert M, Puch S, Richards A, Torok-Storb B, Müller CA, Sorokin L, Klein G. Characterization and functional analysis of laminin isoforms in human bone marrow. Blood J Am Soc Hematol. 2000;96(13):4194–203.
Yokota T, Oritani K, Mitsui H, Aoyama K, Ishikawa J, Sugahara H, Matsumura I, Tsai S, Tomiyama Y, Kanakura Y. Growth-supporting activities of fibronectin on hematopoietic stem/progenitor cells in vitro and in vivo: structural requirement for fibronectin activities of CS1 and cell-binding domains. Blood J Am Soc Hematol. 1998;91(9):3263–72.
Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ, Simmons PJ, Haylock DN. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106(4):1232–9.
Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grünewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201(11):1781–91.
Klein G. The extracellular matrix of the hematopoietic microenvironment. Experientia. 1995;51(9):914–26.
Brouty-Boyé D, Doucet C, Clay D, Le Bousse-Kerdiles MC, Lampidis TJ, Azzarone B. Phenotypic diversity in human fibroblasts from myelometaplasic and non-myelometaplasic hematopoietic tissues. Int J Cancer. 1998;76(5):767–73.
Nilsson SK, Debatis ME, Dooner MS, Madri J, Becker PS. Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J Histochem Cytochem. 1998;46(3):371–7.
Yang Y, Wang K, Gu X, Leong KW. Biophysical regulation of cell behavior—cross talk between substrate stiffness and nanotopography. Engineering. 2017;3(1):36–54.
Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, Yoder MC. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459(7250):1131–5.
North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, Weber GJ, Harris J, Cutting CC, Huang P. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137(4):736–48.
Lundin V, Sugden WW, Theodore LN, Sousa PM, Han A, Chou S, Wrighton PJ, Cox AG, Ingber DE, Goessling W. YAP regulates hematopoietic stem cell formation in response to the biomechanical forces of blood flow. Dev Cell. 2020, 52(4):446–60.
Ji RP, Phoon CK, Aristizábal O, McGrath KE, Palis J, Turnbull DH. Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ Res. 2003;92(2):133–5.
Davies PF, Barbee KA, Volin MV, Robotewskyj A, Chen J, Joseph L, Griem ML, Wernick MN, Jacobs E, Polacek DC. Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu Rev Physiol. 1997;59(1):527–49.
Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10(1):53–62.
Liu Y, Liu T, Fan X, Ma X, Cui Z. Ex vivo expansion of hematopoietic stem cells derived from umbilical cord blood in rotating wall vessel. J Biotechnol. 2006;124(3):592–601.
Nielsen LK. Bioreactors for hematopoietic cell culture. Annu Rev Biomed Eng. 1999;1(1):129–52.
Oostendorp RA, Robin C, Steinhoff C, Marz S, Bräuer R, Nuber UA, Dzierzak EA, Peschel C. Long-term maintenance of hematopoietic stem cells does not require contact with embryo-derived stromal cells in cocultures. Stem Cells. 2005;23(6):842–51.
Oostendorp RA, Harvey KN, Kusadasi N, De Bruijn MF, Saris C, Ploemacher RE, Medvinsky AL, Dzierzak EA. Stromal cell lines from mouse aorta-gonads-mesonephros subregions are potent supporters of hematopoietic stem cell activity. Blood J Am Soc Hematol. 2002;99(4):1183–9.
Lee HJ, Li N, Evans SM, Diaz MF, Wenzel PL. Biomechanical force in blood development: extrinsic physical cues drive pro-hematopoietic signaling. Differentiation. 2013;86(3):92–103.
Choi JS, Mahadik BP, Harley BA. Engineering the hematopoietic stem cell niche: Frontiers in biomaterial science. Biotechnol J. 2015;10(10):1529–45.
Kopp H-G, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology. 2005;20(5):349–56.
Lee-Thedieck C, Rauch N, Fiammengo R, Klein G, Spatz JP. Impact of substrate elasticity on human hematopoietic stem and progenitor cell adhesion and motility. J Cell Sci. 2012;125(16):3765–75.
Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9(2):108–22.
Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(24):4195–200.
Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–81.
Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9(6):518–26.
Li J, Li Y, Gao B, Qin C, He Y, Xu F, Yang H, Lin M. Engineering mechanical microenvironment of macrophage and its biomedical applications. Nanomedicine. 2018;13(5):555–76.
Altrock E, Muth CA, Klein G, Spatz JP, Lee-Thedieck C. The significance of integrin ligand nanopatterning on lipid raft clustering in hematopoietic stem cells. Biomaterials. 2012;33(11):3107–18.
Blaber E, Dvorochkin N, Torres M, Yousuf R, Burns B, Globus R, Almeida E. Mechanical unloading of bone in microgravity reduces mesenchymal and hematopoietic stem cell-mediated tissue regeneration. Stem Cell Res. 2014;13(2):181–201.
Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30(9):973–81.
Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler Thromb Vasc Biol. 2007;27(2):346–51.
Elias HK, Bryder D, Park CY. Molecular mechanisms underlying lineage bias in aging hematopoiesis. Semin Hematol. 2017;54(1):4–11.
Zhang P, Li X, Pan C, Zheng X, Hu B, Xie R, Hu J, Shang X, Yang H. Single-cell RNA sequencing to track novel perspectives in HSC heterogeneity. Stem Cell Res Ther. 2022;13(1):39.
Mann M, Mehta A, de Boer CG, Kowalczyk MS, Lee K, Haldeman P, Rogel N, Knecht AR, Farouq D, Regev A, et al. Heterogeneous responses of hematopoietic stem cells to inflammatory stimuli are altered with age. Cell Rep. 2018;25(11):2992-3005.e2995.
Ya-Hsuan H, Simón M-F. Microenvironmental contributions to hematopoietic stem cell aging. Haematologica. 2020;105(1):38–46.
Sugiyama D, Kulkeaw K, Mizuochi C. TGF-beta-1 up-regulates extra-cellular matrix production in mouse hepatoblasts. Mech Dev. 2013;130(2–3):195–206.
Tamplin OJ, Durand EM, Carr LA, Childs SJ, Hagedorn EJ, Li P, Yzaguirre AD, Speck NA, Zon LI. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell. 2015;160(1–2):241–52.
Weng S, Shao Y, Chen W, Fu J. Mechanosensitive subcellular rheostasis drives emergent single-cell mechanical homeostasis. Nat Mater. 2016;15(9):961–7.
Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10(1):21–33.
Choi JS, Harley BA. Challenges and opportunities to harnessing the (hematopoietic) stem cell niche. Curr Stem Cell Rep. 2016;2(1):85–94.
Klamer S, Voermans C. The role of novel and known extracellular matrix and adhesion molecules in the homeostatic and regenerative bone marrow microenvironment. Cell Adh Migr. 2014;8(6):563–77.
Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159(4):695–705.
Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007;179(5):1043–57.
Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9(8):858–67.
Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68.
Tilghman RW, Parsons JT. Focal adhesion kinase as a regulator of cell tension in the progression of cancer. In: Seminars in cancer biology: 2008; Elsevier: 45–52.
Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127(5):1015–26.
Borneo J, Munugalavadla V, Sims EC, Vemula S, Orschell CM, Yoder M, Kapur R. Src family kinase–mediated negative regulation of hematopoietic stem cell mobilization involves both intrinsic and microenvironmental factors. Exp Hematol. 2007;35(7):1026–37.
Orschell CM, Borneo J, Munugalavadla V, Ma P, Sims E, Ramdas B, Yoder MC, Kapur R. Deficiency of Src family kinases compromises the repopulating ability of hematopoietic stem cells. Exp Hematol. 2008;36(5):655–66.
Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12(5):458–65.
Kasper G, Glaeser JD, Geissler S, Ode A, Tuischer J, Matziolis G, Perka C, Duda GN. Matrix metalloprotease activity is an essential link between mechanical stimulus and mesenchymal stem cell behavior. Stem Cells. 2007;25(8):1985–94.
Theodore L, Cortes M, Natsuhara K, Liu S, Esain V, North TE. Distinct roles for matrix metalloproteinases 2 and 9 in embryonic hematopoietic stem cell production. Exp Hematol. 2016;44(9):S103.
Hoggatt J, Singh P, Tate TA, Chou B-K, Datari SR, Fukuda S, Liu L, Kharchenko PV, Schajnovitz A, Baryawno N: Rapid mobilization reveals a highly engraftable hematopoietic stem cell. Cell 2018;172(1–2):191–204.
Yahata T, Ibrahim AA, Muguruma Y, Eren M, Shaffer AM, Watanabe N, Kaneko S, Nakabayashi T, Dan T, Hirayama N. TGF-β–induced intracellular PAI-1 is responsible for retaining hematopoietic stem cells in the niche. Blood J Am Soc Hematol. 2017;130(21):2283–94.
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83.
Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013(5).
Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15(6):637–46.
Shin J-W, Buxboim A, Spinler KR, Swift J, Christian DA, Hunter CA, Léon C, Gachet C, Dingal PDP, Ivanovska IL. Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. Cell Stem Cell. 2014;14(1):81–93.
Raab M, Swift J, P. Dingal PD, Shah P, Shin J-W, Discher DE: Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J Cell Biol. 2012;199(4):669–83.
Svitkina T. The actin cytoskeleton and actin-based motility. Cold Spring Harb Perspect Biol. 2018;10(1):a018267.
Berrier AL, Yamada KM. Cell–matrix adhesion. J Cell Physiol. 2007;213(3):565–73.
Tajik A, Zhang Y, Wei F, Sun J, Jia Q, Zhou W, Singh R, Khanna N, Belmont AS, Wang N. Transcription upregulation via force-induced direct stretching of chromatin. Nat Mater. 2016;15(12):1287–96.
Ni F, Yu W-M, Wang X, Fay ME, Young KM, Qiu Y, Lam WA, Sulchek TA, Cheng T, Scadden DT: Ptpn21 controls hematopoietic stem cell homeostasis and biomechanics. Cell Stem Cell 2019; 24(4):608–20.
Hu L, Ni F, Wang X, Fay ME, Young KM, Lam WA, Sulchek TA, Qu C-K. Decreased cell stiffness enhances leukemia development and progression. Leukemia. 2020;34(9):2493–7.
Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–37.
Tiribuzi R, Crispoltoni L, Tartacca F, Orlacchio A, Martino S, Palmerini CA, Orlacchio A: Nitric oxide depletion alters hematopoietic stem cell commitment toward immunogenic dendritic cells. Biochimica et Biophysica Acta (BBA) - General Subjects 2013; 1830(3):2830–38.
Liu Z, Tu H, Kang Y, Xue Y, Ma D, Zhao C, Li H, Wang L, Liu F. Primary cilia regulate hematopoietic stem and progenitor cell specification through Notch signaling in zebrafish. Nat Commun. 2019;10(1):1–11.
Pala R, Alomari N, Nauli SM. Primary cilium-dependent signaling mechanisms. Int J Mol Sci. 2017;18(11):2272.
Lucas D: The bone marrow microenvironment for hematopoietic stem cells. In: Stem Cell Microenvironments and Beyond. Springer; 2017: 5–18.
AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res. 2009;104(7):860–9.
Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117(9):1161–71.
Shin J-W, Spinler KR, Swift J, Chasis JA, Mohandas N, Discher DE. Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. Proc Natl Acad Sci. 2013;110(47):18892–7.
Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell. 2006;11(6):845–57.
Wolfe RP, Ahsan T. Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes. Biotechnol Bioeng. 2013;110(4):1231–42.
Yamamizu K, Matsunaga T, Katayama S, Kataoka H, Takayama N, Eto K, Nishikawa SI, Yamashita JK. PKA/CREB signaling triggers initiation of endothelial and hematopoietic cell differentiation via Etv2 induction. Stem Cells. 2012;30(4):687–96.
Kim PG, Nakano H, Das PP, Chen MJ, Rowe RG, Chou SS, Ross SJ, Sakamoto KM, Zon LI, Schlaeger TM. Flow-induced protein kinase A-CREB pathway acts via BMP signaling to promote HSC emergence. J Exp Med. 2015;212(5):633–48.
Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154(5):1047–59.
Nakajima H, Yamamoto K, Agarwala S, Terai K, Fukui H, Fukuhara S, Ando K, Miyazaki T, Yokota Y, Schmelzer E. Flow-dependent endothelial YAP regulation contributes to vessel maintenance. Dev Cell. 2017;40(6):523–36.
Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, Lim D-S, Pawson T, Wrana J, McNeill H. Yap-and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 2013;9(3):e1003380.
Rydholm S, Zwartz G, Kowalewski JM, Kamali-Zare P, Frisk T, Brismar H. Mechanical properties of primary cilia regulate the response to fluid flow. Am J Physiol-Renal Physiol. 2010;298(5):F1096–102.
Jin X, Mohieldin AM, Muntean BS, Green JA, Shah JV, Mykytyn K, Nauli SM. Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell Mol Life Sci. 2014;71(11):2165–78.
Aggarwal R, Lu J, J Pompili V, Das H. Hematopoietic stem cells: transcriptional regulation, ex vivo expansion and clinical application. Curr Mol Med 2012;12(1):34–49
Thomas ED, Lochte HL Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491–6.
Körbling M, Anderlini P. Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood J Am Soc Hematol. 2001;98(10):2900–8.
Ng AP, Alexander WS. Haematopoietic stem cells: past, present and future. Cell Death Discov. 2017;3(1):1–4.
Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324(5935):1673–7.
Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–80.
Xu Y, Shan W, Li X, Wang B, Liu S, Wang Y, Long Y, Tie R, Wang L, Cai S. A synthetic three-dimensional niche system facilitates generation of functional hematopoietic cells from human-induced pluripotent stem cells. J Hematol Oncol. 2016;9(1):1–16.
Mousavi SH, Abroun S, Soleimani M, Mowla SJ. 3-Dimensional nano-fibre scaffold for ex vivo expansion of cord blood haematopoietic stem cells. Artif Cells Nanomed Biotechnol. 2018;46(4):740–8.
Lin X, Shi Y, Cao Y, Liu W. Recent progress in stem cell differentiation directed by material and mechanical cues. Biomed Mater. 2016;11(1):014109.
Ehring B, Biber K, Upton TM, Plosky D, Pykett M, Rosenzweig M. Expansion of HPCs from cord blood in a novel 3D matrix. Cytotherapy. 2003;5(6):490–9.
Chua K-N, Chai C, Lee P-C, Tang Y-N, Ramakrishna S, Leong KW, Mao H-Q. Surface-aminated electrospun nanofibers enhance adhesion and expansion of human umbilical cord blood hematopoietic stem/progenitor cells. Biomaterials. 2006;27(36):6043–51.
Chua K-N, Chai C, Lee P-C, Ramakrishna S, Leong KW, Mao H-Q. Functional nanofiber scaffolds with different spacers modulate adhesion and expansion of cryopreserved umbilical cord blood hematopoietic stem/progenitor cells. Exp Hematol. 2007;35(5):771–81.
Das H, Abdulhameed N, Joseph M, Sakthivel R, Mao H-Q, Pompili VJ. Ex vivo nanofiber expansion and genetic modification of human cord blood-derived progenitor/stem cells enhances vasculogenesis. Cell Transpl. 2009;18(3):305–18.
Arabkari V, Amirizadeh N, Nikougoftar M, Soleimani M. microRNA expression profiles in two-and three-dimensional culture conditions of human-umbilical-cord blood-derived CD34+ cells. J Cell Physiol. 2019;234(11):20072–84.
Raic A, Rödling L, Kalbacher H, Lee-Thedieck C. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials. 2014;35(3):929–40.
Zhang P, Xu L, Gao J, Xu G, Song Y, Li G, Ren J, Zhang Y, Yang C, Zhang Y. 3D collagen matrices modulate the transcriptional trajectory of bone marrow hematopoietic progenitors into macrophage lineage commitment. Bioactive Mater. 2022;10:255–68.
Jiang J, Papoutsakis ET. Stem-cell niche based comparative analysis of chemical and nano-mechanical material properties impacting ex vivo expansion and differentiation of hematopoietic and mesenchymal stem cells. Adv Healthcare Mater. 2013;2(1):25–42.
Tracey LJ, An Y, Justice MJ. CyTOF: an emerging technology for single-cell proteomics in the mouse. Curr Protocols. 2021;1(4):e118.
Acknowledgements
We would like to thank for all the authors for their participation and helpful discussions. Due to space constraints, we are aware that there is far more research associated with this field and regret that we could not cite every available report.
Funding
This work was supported by grants from the National Natural Science Foundation of China (12002285), the Natural Science Foundation of Shaanxi (2020JZ-11, 2022JQ-059) and the Fundamental Research Funds for the Central Universities (D5000220026). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
HY and NZ outlined the review and designed the figures. GS and PZ collected the literature and wrote the manuscript. XZ1 and JL drew the pictures in the manuscript. XZ2 and JY reviewed the grammatical and structural errors throughout the entire manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, G., Zhang, P., Zhang, X. et al. The spatiotemporal heterogeneity of the biophysical microenvironment during hematopoietic stem cell development: from embryo to adult. Stem Cell Res Ther 14, 251 (2023). https://doi.org/10.1186/s13287-023-03464-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-023-03464-8