Abstract
Mesenchymal stem/stromal cells (MSCs)‐based therapy brings the reassuring capability to regenerative medicine through their self‐renewal and multilineage potency. Also, they secret a diversity of mediators, which are complicated in moderation of deregulated immune responses, and yielding angiogenesis in vivo. Nonetheless, MSCs may lose biological performance after procurement and prolonged expansion in vitro. Also, following transplantation and migration to target tissue, they encounter a harsh milieu accompanied by death signals because of the lack of proper tensegrity structure between the cells and matrix. Accordingly, pre-conditioning of MSCs is strongly suggested to upgrade their performances in vivo, leading to more favored transplantation efficacy in regenerative medicine. Indeed, MSCs ex vivo pre-conditioning by hypoxia, inflammatory stimulus, or other factors/conditions may stimulate their survival, proliferation, migration, exosome secretion, and pro-angiogenic and anti-inflammatory characteristics in vivo. In this review, we deliver an overview of the pre-conditioning methods that are considered a strategy for improving the therapeutic efficacy of MSCs in organ failures, in particular, renal, heart, lung, and liver.
Similar content being viewed by others
Introduction
Researchers have focused on mesenchymal stem/stromal cells (MSCs) for the past 60 years because of their unique competencies, such as ease of isolation, lower immunogenicity, and immunoregulatory capacities [1]. These cells are highly amenable to cultivation in vitro; they can differentiate independently and secrete various growth factors and cytokines [2]. First, MSCs were procured from murine bone marrow (BM) by Friendenstein et al. and were called hematopoiesis-supporting cells in BM [3]. After that, Kaplan firstly proposes the term "mesenchymal stem cells," which are cells isolated from fully developed bone marrow (BM) that can usually differentiate into several types of mesenchymal origin cells [4]. Following the first successful human MSCs isolation from BM tissue [5], MSCs isolation from a diversity of adult tissues, such as the perivascular area, has been managed [6, 7]. Although there is no particular quantitative assay to provide MSCs identification in mixed cells population [8], the International Society for Cellular Therapy (ISCT) has provided minimum principles to determine MSCs. These criteria are the plastic adherence property, expressing CD73, D90, CD105 without CD14, CD34, CD45, and human leucocyte antigen-DR (HLA-DR) expression, and finally differentiation into adipocyte, chondrocyte, and osteoblast in vitro. The stromal vascular fraction of adipose tissue (AT) and BM are the two most common reservoirs of human MSCs [9]. However, the umbilical cord and the placenta, often discarded after delivery, are also excellent sources for human MSCs [10, 11]. Multiple types of cells, including adipose tissue, cartilage, bone, and even macrophages, have been shown to originate from MSCs [12, 13]. Importantly, MSCs have emerged as one of the most promising and vital potential sources for new clinical treatments for organ failure [14, 15].
Stem cell therapy has been the subject of many studies for its potential to cure many disorders. These include transplant infectious disease, progressive multiple sclerosis, diabetes, stroke, bronchopulmonary dysplasia, cardiomyopathy, and osteoarthritis [16]. Various in vivo reports indicated that MSCs could interfere with immune cells' infiltration, proliferation and activation post-transplantation [17, 18]. They also can inspire angiogenesis by direct differentiation, cell-to-cell interaction, or paracrine effects. Also, MSC-exosome contains cytokines, chemokines, microRNAs (miRNAs), growth factors, and proteins, making it an ideal therapeutic option [19]. According to these properties, they are an excellent candidate for treating organ failure, which is characterized by the inability of at least one of the body organs to conduct normal body functions [20]. However, natural MSCs in vivo survival and their biological effects on tissue recovery decrease with long-term cultivation called aging and also injected cells demonstrate poor targeted migration [21]. The harsh microenvironment with ischemia, inflammation, oxidative stress, and mechanical stress result in low survival rate of administrated cells [22]. Besides, MSC homing is inefficient, with only a small population of cells reaching the target tissue post systemic administration. These attritions signify a critical bottleneck in determining the full therapeutic competence of MSC-based therapies [23]. Thus, scientists have sought different modalities to bypass this drawback.
In recent years, researchers have focused on designing or developing novel approaches to expand the therapeutic merits of MSCs [24]. In this light, pre-conditioning has engendered significant interest. Pre-conditioning is a method depending on a diversity of means to improve the potential of MSCs during ex vivo growth [25, 26]. Universally, pre-conditioning strategies comprise hypoxia, cell exposure with pharmacological/chemical agents or trophic factors/cytokines, pre-conditioning with physical factors, and finally, gene modification [27]. The pre-conditioning strategies, in turn, promote the various attributes of the, including their proliferative, secretory, migratory, pro-angiogenic, and anti-inflammatory aptitudes. These properties may bring about more preferred beneficial outcomes in vivo. For example, low O2 levels decrease the prolyl hydroxylation under hypoxic conditions, leading to hypoxia-inducible factor 1-alpha (HIF-1α) accumulation and nuclear translocation. In the nucleus, HIF-1α creates a heterodimer with HIF-1β and subsequently binds to the hypoxia-response element (HRE) in the target genes, allied with CBP/p300 (Fig. 1) [28, 29]. This assemblage adjusts the transcription of more than 70 genes, primarily complicated in angiogenesis, invasion/metastasis, survival, and proliferation (Table 1). Also, MSCs pre-treatment with carboxyl‐terminated hyperbranched polyester (CHBP) supports the mitochondria membrane potential (MMP) and mitochondrial membrane integrity in MSCs and also induces the Nrf2/Sirt3/FoxO3a pathway, thereby offering more resistance to oxidative stress [30].
Hypoxia-inducible factor 1α (HIF-1α) signaling pathway. The figure depicts the action mechanism of HIF-1α in promoting the mesenchymal stem/stromal cells (MSCs)-mediated therapeutic influences. Hypoxia-response element (HRE), Hypoxia-inducible factor 1β (HIF-1β), CREB binding protein (CBP), HIF-1 prolyl hydroxylase (HPH), Indoleamine 2, 3-dioxygenase (IDO), Prostaglandin E2 (PGE2), Cyclin-dependent kinase (CDK), Vascular endothelial growth factor (VEGF), Hepatocyte growth factor (HGF), Fibroblast growth factor (FGF), Transforming growth factor beta (TGFβ), BCL-2 associated agonist of cell death (Bad), TNF-stimulated gene-6 (TSG-6)
Herein, we will look into the use of pre-conditioned MSCs in organ failure to deliver a unified and comprehensive view of the best approach to augment the therapeutic influences of MSCs in these conditions, with a particular focus on the recent preclinical reports.
The MSCs sources and their differences
Stem/progenitor cells with MSC-like biological features have been detected in different mature tissues in the past decade, including the BM, skin, placenta, umbilical cord blood (UCB), umbilical cord tissue, adipose tissue (AT), dental pulp, infant teeth, testicles, brain, etc. With mounting evidence that MSCs isolated from different sources form a diverse cell population, the development of uniform criteria for identifying MSCs became an urgent necessity [31].
Expression of CD73, CD90, and CD105 constitute the minimum criteria for identifying tissue-isolated MSCs [32]. The hBM-MSCs, hAT-MSCs, human adipose-derived stromal cells (hADSSCs), and human muscle-derived progenitor cells (hMDPCs) all express these markers at high levels [17, 33,34,35]. These cells also lack the hematopoietic markers CD34 and CD45. In this light, CD146 is a second MSC marker expressed by MSCs from different sources [17, 31]. Also, MSCs from various sources have varying degrees of paracrine potential, significantly impacting their aptitude to influence target cells and either dampen or amplify the immune response. MSCs derived from BM and secrete cytokines and growth factors such as interleukin (IL)-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), vascular endothelial growth factor (VEGF), osteoprotegerin, and tissue inhibitor of metalloproteinases 2 (TIMP2) [36]. Also, increased levels of the interferon-gamma (INF-γ), platelet-derived growth factor A (PDGFA), VEGF, IL-10, and stromal-derived factor (SDF) were found in human exfoliated deciduous teeth (SHED) in comparison to Wharton's jelly (WJ)- and BM-MSC [37]. Significantly, the microenvironment of MSCs affects the paracrine abilities of stem/progenitor cells. Interestingly, compared to other sources, skin-derived MSCs can secrete higher levels of trophic substances such as VEGF, granulocyte colony-stimulating factor (G-CSF), hepatocyte growth factor (HGF-1), and basic fibroblast growth factor (bFGF) [38]. Ribeiron et al. also found that AT-MSCs inhibited NK and B cells more effectively than BM- and UCB-MSCs [39]. Furthermore, compared to UC-MSCs, AT-MSCs showed more significant inhibitory effects on serum IL-1, IL-6, and IL-8 levels in lipopolysaccharide (LPS)-treated mice [40]. UC-MSCs also have demonstrated more evident proliferation and clonality due to the reduced expression of p53, p21, and p16 compared to cells derived from BM and AT [40]. In another study, BM-MSCs and WJ-MSC showed superiority over AT-MSCs in terms of proliferation and clonality potential [41]. In addition, AT-MSCs and UC-MSCs can demonstrate more prominent osteogenic potential compared to chorionic membrane (CM)- and decidua (DC)-MSCs [42].
Taken together, while MSCs from various tissues share many traits, their biological activity and some markers vary depending on the tissue from which they were derived. For researchers interested in the use of MSCs in clinical settings, understanding the biological principles underlying MSCs should be a key factor. For instance, higher CD146 expression promotes the cells migration capability in vitro and in vivo, and its down-regulation has correlation with higher osteogenic capacity [43]. These proofs verify differences between the MSCs from various sources, highlighting the importance of determining better sources respecting the conditions.
MSCs' rationality for treatment of organ failure
In vitro, MSCs can differentiate into numerous mesoderm lineages and differentiated cells, such as osteoblast, fats, skeletal muscle myocytes/myotubes, pancreatic islet cells, and cardiomyocytes, when grown in a growth factor-rich culture environment [31]. However, small populations of MSCs differentiate into functional cells in vivo [44, 45]. To influence other cells, MSCs produce exosomes and micro-vesicles that carry potent angiogenic mediators, cytokines, or mRNA molecules [46]. The process by which MSCs are released from BM is critically vital to their regenerative function. These cells reside primarily in BM but can be found in other organs and tissues because of their mobile nature. Elm et al. detected the presence of MSCs in the PB of people who had suffered hip fractures [47]. Based on their observations, MSCs were found in peripheral blood (PB) from 22% of hip fracture patients, 46% of younger fracture patients, and in none of 63 pre- and postmenopausal women with hip OA [47]. Meanwhile, several lines of evidence suggest that MSCs are secreted from the BM in response to systemic cues; hypoxia recruits MSCs to the PB, triggering liver injury. Also, MSCs could be released from adipose tissue in response to inflammation and then collected in lymph nodes and blood arteries [48]. Recent research has demonstrated the importance of CCR9, CXCR4, and c-MET in guiding endogenous MSC migration to the damaged liver [49]. Further, MSCs have garnered interest as they could promote tissue regeneration and homeostasis in inflammatory conditions such as graft-versus-host disease (GVHD), multiple sclerosis (MS), lung inflammation, arthritis, and Crohn's disease (CD) [50, 51]. Exogenous MSCs are frequently applied to bring about tissue recovery in vivo due to their anti-inflammatory properties and their capacity to provoke angiogenesis and boost the proliferation of damaged cells [52,53,54,55].
Inhibition of inflammation
Recent years have seen remarkable progress in our knowledge of how MSCs modulate the immune system and reduce inflammation. MSC responses may vary with the intensity of environmental cues. In the earliest stages of inflammation, MSCs amplify the inflammatory response by sensing pro-inflammatory signals through IL-1-receptors (IL-1Rs), IFN-receptors (IFNRs), toll-like receptors (TLRs), and TNF- receptors (TNFRs) [56]. They increase T cell activation by secreting chemokines like C-X-C motif ligand (CXCL)-9, macrophage inflammatory protein-1 (MIP-1), CCL5, and CCL10. Low levels of inflammatory signals like TNF-ɑ and IFN-γ enhance the rise in chemokine secretion at this time [57, 58]. In later phases, when pro-inflammatory molecules like IL-1, IFN-γ, and TNF-α are present in more significant concentrations, MSCs are activated, and then secrete TGF-β and IL-10 to bypass inflammation and halt autoimmune responses [59]. Indoleamine 2,3-dioxygenase (IDO) and inducible nitric oxide synthase (iNOS) decrease the proliferation, migration, and maturation of dendritic cells (DC) and T cells and thus bargain their ability to deliver antigens. Therefore, IDO or iNOS levels may determine the pro-inflammatory or anti-inflammatory effects of MSCs [60]. Additional studies show that CD5 + regulatory B cells protect against colitis when treated with human MSCs, CD23 + CD43 + B cells and MSCs generated from human umbilical cords [61]. Each of these cells play a role in reducing intestinal inflammation [61]. Therefore, MSCs may suppress inflammation by enhancing anti-inflammatory factors and decreasing pro-inflammatory mediators [62, 63]. When MSCs come into direct contact with cells, they can dampen immune responses [61]. For instance, studies in rodents with GVHD exhibited that systemic injection of human MSC-exosome improved animals' survival by inhibiting CD4+ and CD8+ T cell performance and infiltration and increasing Treg cell activity [64]. In addition, TNF-α, NF-κB, IL-6, and IL-8 levels were reduced in the lung tissue of animals with acute lung injury upon MSCs systemic administration [65].
Induction of angiogenesis
Impaired angiogenesis and endothelial dysfunction are probably involved in the augmented prevalence of organ dysfunction. Angiogenesis is required for tissue repair, and a sufficient vascular network is paramount to supply blood and growth factors to damaged tissues [66]. Because of the marked positive effect on angiogenesis, MSCs have significant therapeutic power for treating organ failure such as heart failure (HF). Of course, the application of MSC-based therapies is confined by their low persistence level in targeted tissues and the low capabilities of transdifferentiation in vivo [67]. The most crucial property of MSCs for treating ischemic diseases is the secretion of pro-angiogenic mediators like VEGF, HGF, and FGF and their differentiation potential into vascular phenotypes in vitro [68]. They can promote endogenous angiogenesis via microenvironmental modulation and differentiating into various types of vascular cells [69]. Some proofs demonstrated that MSCs could be injected into injured areas and develop into the heart and endothelial cells [70]. Further, some clinical investigations indicate that MSCs can ameliorate key clinical parameters in patients suffering from organ failure [71]. MSCs can stimulate organ normal function by inducing angiogenesis through the secretion of VEGF, macrophage colony-stimulating factor (MCF), and IL-6. Meanwhile, VEGF serves essential roles in angiogenesis and microvascular permeability. Interaction between VEGF/VEGFR signaling in endothelial cells (EC) facilitates the production of cytokines and chemokines and up-regulates the cell adhesion molecules expression [72]. To promote local angiogenesis, MSCs can secrete both hepatocyte growth factor (HGF) and stromal cell-derived factor 1 (SDF-1) [73, 74]. SDF-1 is a critical chemokine that can regulate various physiological processes, including stimulating the proliferation of ECs and generating capillary tubes [75]. The ECs express the receptor c-Met, by which HGF exerts its angiogenic effect by tyrosine phosphorylation. The therapeutic efficacy of HGF was studied in a clinical experiment, offering some benefits in organ ischemia [76, 77].
Enhancing target cell proliferation and differentiation
Replacing damaged cells is needed for ameliorating organ dysfunction. Human MSCs are a great source of cells for cell transplantation and tissue engineering because of their capacity to stimulate target cell proliferation. For instance, MSCs secreted IGF-1 can promote primary hepatocyte proliferation [78]. In co-culture conditions, MSCs enhanced the numbers of the proliferating cell nuclear antigen (PCNA) expressing hepatocyte in vitro [79]. Likewise, MSCs-derived exosomes intensified cardiomyocyte proliferation by miR-210 delivery [80]. The miR-210 overexpressing MSC-exosomes also could improve myocyte protection in response to both in vitro and in vivo stress [80]. Exosomal miR-25-3p from MSCs was capable of decreasing cardiomyocytes apoptosis and sustaining their expansion by negative regulation of enhancer of zeste homolog 2 (EZH2) [81]. In addition, Yi et al. found that miR-30b-3p-overexpressing MSCs increased type II alveolar epithelial cells (AECs) growth and protected versus lipopolysaccharide-induced lung damages by inhibiting serum amyloid A 3 (Saa3) [82].
The rationality of MSCs pre-conditioning
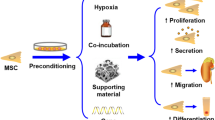
Cultural conditions are the most imperative factors influencing the functional potential of MSCs. Regeneration functions of MSCs and their clinical implementation for repairing and regenerating damaged and destroyed tissues are also hindered by "disease conditions" and the "age" of the donor. Accordingly, stem cells are suggested to be manipulated before their use in clinical settings to potentiate their survival, migration, and therapeutic competencies in vivo [83]. Pre-conditioning cells in a particular design/engineering with varied physical or chemical characteristics and variables under ex vivo settings has increased MSCs' capability to survive in hostile microenvironments and boost their immune responses [84]. Several methods, such as low-heat shock, glucose depletion, and pre-conditioning with growth factors, have been employed to accomplish this. Since oxygen levels are already low in stem cell niches compared to typical situations, hypoxic pre-conditioning may improve their natural capabilities [85]. Adapting cells to their external environment, reducing oxidative stress, switching metabolism to glycolysis, increasing cell proliferation, differentiation, and stemness maintenance, and increasing their movement to sustain hypoxic conditions after transplantation all suggest that hypoxia may be a valuable strategy for improving cell functions [83]. Hypoxic pre-condition up-regulates anti-apoptotic proteins expression in MSCs and thus promotes their survival in the hostile environment. Hypoxic pre-conditioning also reduces MSCs' glucose consumption, lactate release, and cytochrome c and heme oxygenase-1 (HO-1) levels [86]. Further, the human MSCs' exposure to IFN-γ could ease the inhibition of NK activation and improve the protection of MSCs from NK-induced cytotoxicity [87]. Besides, 3-dimensional cell culture could intensify the immunomodulatory aptitudes of human MSCs, as shown by reduced TNF-α, IL-6, IL-12p40, IL-23, and CXCL2 and improved IL-10 levels in conditioned media [88]. Recent reports also indicated that MSCs pre-treatment with angiotensin II enhances the outcome of MSC-based therapy for myocardial infarction (MI) in part via increasing the paracrine production of VEGF, and supporting gap junctions (GJs) [89]. The positive effects of the MSCs on angiogenesis could also be further heightened by hypoxia pre-treatment as a result of the increased secretion of VEGF [90]. Figure 2 depicts the effects of the pre-conditioning on MSCs' therapeutic benefits in vivo. As described, pre-conditioned MSCs show better therapeutic efficacy over naïve MSCs concerning the. They raise target cell growth, persuade angiogenesis and modify immune responses.
Pre-conditioned MSCs in lung failure
Lung failure is the most shared organ failure seen in the intensive care unit. The pathogenesis of acute respiratory failure (ARF) can be categorized as (1) neuromuscular in origin, (2) secondary to acute and chronic obstructive airway disorders, (3) alveolar procedures like cardiogenic and noncardiogenic pulmonary edema and pneumonia, and (4) finally vascular disorders such as acute or chronic pulmonary embolism [91, 92]. Based on the literature, MSCs and their secreted products can attenuate lung inflammation and support its structure and performance [93, 94]. The safety, feasibility and efficacy of MSCs administration is under-investigation in phase 1 and phases 2 trials in patients with lung failure and related conditions (NCT02112500, NCT04392778 and NCT04537351).
As previously described, a growing body of reports has signified that hypoxia, thermal shock, small-molecule medicines, cytokines and growth factors could increase the therapeutic merits of MSCs transplantation [96,97,98]. Genetic modification and overexpression of pro-survival genes, chemokine receptors, or anti-apoptotic proteins can also be used to perform cellular pre-conditioning before transplantation [99,100,101,102].
In 2019, Chen et al. found that BM-MSCs overexpressing heme oxygenase-1 (HO-1) could alleviate lipopolysaccharide (LPS)-induced acute lung injury (ALI) and resultant lung failure in rats [103]. The HO-1 has antioxidant, anti-inflammatory, and anti-apoptotic properties [103]. The release of HO-1 by MSCs post-transplantation has been shown to elicit protective effects against ALI. Compared to parental MSCs, MSCs-HO-1 transplantation showed significant improvements in cell survival, apoptosis, and paracrine activity in vivo [103]. Further, MSCs-HO-1 exhibited more evident pro-survival and anti-apoptotic impacts and paracrine activity in vitro. These findings shed light on the potential of genetic engineering of MSC for managing ALI [103]. Likewise, systemic injection of manganese superoxide dismutase (MnSOD)-overexpressing MSCs led to reduced lung inflammation, as shown by decreased IL-1, IL-6, and TNF-α levels [104]. Importantly, MnSOD-MSCs differentiated into epithelial-like cells in vivo [104], indicating the excellent capability of MnSOD-MSCs. Also, Liao et al. (2023) have found that administration of IL18-hUC-MSCs could drastically decrease viral load, fibrosis, and cell apoptosis in acute lung injuries [105]. Notably, T cell exudation and pro-inflammatory cytokine release in bronchoalveolar lavage fluid (BALF) were significantly inhibited by IL18-hUCMSC therapy [105].
Pre-conditioned MSCs in heart failure
Heart failure (HF) is a clinical syndrome characterized by structural and functional failings in the myocardium, eventually weakening ventricular filling or the ejection of blood. The HF often results from poor left ventricular function [106]. Decreased diastolic filling and ejection fraction can both result in less blood leaving the heart into systemic circulation [107]. Of course, deficits in the pericardium, myocardium, endocardium, heart valves, or great vessels alone or in combination are also allied with HF. Over the past two decades, numerous investigations have been carried out on the potential of MSCs for cardiac cell regeneration [108, 109]. Several MSCs-based strategies have been studied by employing the three ways of direct differentiation to heart cells, differentiation to vascular cells, and paracrine signaling [110]. The safety and modest efficacy of UCB-MSCs systemic administration has been verified in patients with HF [111]. Improvements in left ventricular function, functional status, and quality of life were detected in treated patients [111]. BM-MSCs transplantation by intra-myocardial [112] and intra-coronary route [113] also were safe and led to increased myocardial function in patients with HF.
In vitro, hypoxia pre-conditioning boosts hUC-MSCs proliferation and enhances their differentiation into cardiomyocyte-like cells (CLCs) [114]. Besides, it has previously been found that the growth arrest of particular gene 6 (Gas6) influences cell growth, adhesion, chemotaxis, mitogenesis, and cell survival because of the presence of gamma-linolenic acid-carboxyglutamic acid (Gla) [115]. Functional studies suggest that Gas6 overexpression could significantly reduce MSC apoptosis and increase MSC survival in vitro and in HF animal models compared to naïve MSCs. Also, Gas6 could enhance VEGF, bFGF, SDF, and IGF-1 secretion from MSCs [116]. Likewise, HIF-1-overexpressing MSCs were found to increase cardiac output and decrease the size of myocardial scars in HF in vivo models [117]. Further, HIF-1 overexpression significantly augmented the secretion of angiogenesis proteins like activin A, angiopoietin, artemin, endothelin-1, MCP-1, and remodeling factors ADAMTS1, FGFs, TGF-β, MMPs, and serpins in MSCs in vitro [117]. Genetically modified MSCs to overexpress VEGF in hypoxic conditions also increased myocardial neovascularization in ischemic heart disease [118]. These engineered MSCs also decreased the apoptotic cell numbers in the infarcted area and caused the reduction of left ventricular remodeling in vivo [118]. Besides, another study on a mouse model of heart failure demonstrated that overexpression of anti-fibrotic substances, adrenomedullin (ADM), dramatically improved heart function, decreased fibrotic area, and decreased MMP-2 expression [119,120,121,122]. The ADM-MSC-treated group also shows markedly higher MSCs survival after transplantation. These findings indicate that MSCs overexpressing ADM can potentially increase anti-fibrotic actions, improving heart function in animals with heart failure [119]. Finally, pre-conditioning MSCs with caspase inhibition and hyperoxia could boost their capacity to diminish left ventricular remodeling and sustain left ventricular activity [123]. Additionally, gene and protein expression of caspases 1, 3, 6, 7, and 9 were decreased drastically in MSCs pre-conditioned with hyperoxia, caspase inhibition, or both, while up-regulating Akt1, NF-κB, and Bcl-2 expression in pre-conditioned MSCs. These alterations ultimately led to a substantial increase in MSC proliferation in hypoxic environment in vivo [123].
Pre-conditioned MSCs in renal failure
The term renal failure means incapability of the kidneys to accomplish the excretory activity, driving retention of nitrogenous waste yields from the blood. Once a patient necessities renal replacement therapy, the ailment is named end-stage renal disease (ESRD) [124]. Although kidney transplantation is now the gold standard for treating ESRD, significant difficulties exist in this field, particularly in preventing transplant rejection and ensuring long-term organ acceptance. In recent years, the probability of acute rejection (AR) has been mitigated by using triple immunosuppressive medication [125]. Given their involvement in regulating the immune system, MSCs have emerged as a promising candidate in this context [85]. Recently, Shao et al. (2021) exhibited that intravenous administration of autologous BM-MSCs led to improvement in renal and systemic functional parameters from baseline in Chinese renal failure patients [126].
One of the most prevalent injuries sustained with a kidney transplant is ischemia/reperfusion (I/R) damage. As a result of their ability to heal cellular damage, reduce tissue rejection, and attain organ tolerance, MSCs are a promising cell therapy candidate for use in kidney transplantation [127]. The MSC infusion in kidney transplant recipients is feasible, permits enlargement of Treg in the peripheral blood, and regulates memory CD8 + T cell function [128]. The pre-conditioning of MSCs also is believed to potentiate parental MSCs capability to support successful kidney transplantation by increasing the survival of MSCs and potentiating their migration and protecting them from natural killer (NK)-mediated cytotoxicity [129]. In this light, MSCs treatment with melatonin prior administration was shown to boost the survival of MSCs, enhance cell proliferation and angiogenesis, and enable quicker recovery of the renal function [130].
A recent study in an animal model of gentamicin-induced acute renal failure (ARF) showed that MSCs pre-conditioned with hypoxia could induce a more suitable therapeutic effect than naïve MSCs [131]. Hypoxia-induced MSCs administration diminished blood urea nitrogen (BUN) and creatinine level, thus supporting renal function [131]. The histological analysis of renal tissue isolated from hypoxia-induced MSCs treated animals also verified these findings [131]. Additionally, miR-19a-3p and miR-20a-5p co-expressing human iPS-MSCs protected kidney function in rat models of chronic kidney disease following acute ischemia [132]. Further, genetically modified iPS-MSCs were capable of decreasing oxidative stress, inflammatory downstream signaling, and renal cell death in vitro [132]. Likewise, Cao et al. (2021) showed that miRNA-133b-overexpressing MSCs could attenuate renal fibrosis in an animal model of renal failure in part by inhibition of connective tissue growth factor (CTGF) expression in renal tissue [133]. Negative regulation of CTGF leads to the suppression of the TGF-β1-induced EMT of HK2 cells, a proximal tubular cell (PTC) line derived from normal kidney, in vitro [133]. Nonetheless, genetic modification of MSCs to overexpress CXCR4 and CXCR7 did not increase their homing therapeutic capacities in acute kidney injury in vivo models [134]. Also, scientists found that administration of neither native nor engineered MSCs amended renal failure in vivo [134]. In contrast, Liu et al. (2013) demonstrated that CXCR4 overexpression increased BM-MSCs migration to the kidney tissue in acute kidney injury [135]. The SDF-1/CXCR4 signaling plays a central role in this event by transducing the PI3K/AKT and MAPK in BM-MSCs [135]. Besides, it has been suggested that expanding MSCs in hollow fiber bioreactor-based 3D) culture systems could potentiate their ability to ameliorate renal function in vivo mainly by enhancing exosome secretion [136].
Pre-conditioned MSCs in liver failure
The liver is a crucial organ that aids in digestion, elimination of toxins, and immune system function. The liver can renew itself because it contains particular cells, including mature liver cells, intrahepatic stem cells, and extra stem cells [137]. Although endogenous regeneration is possible, it is not effective after severe damage. Infections, medicines, toxins, chemicals, autoimmune disease and metabolic diseases are the leading causes of acute liver failure (ALF), in which liver dysfunction produces severe damage and necrosis. Acute liver failure has a high fatality rate despite aggressive treatment [138]. Liver transplantation has become less effective as the primary treatment for liver illnesses due to a lack of organ donors, unfavorable effects of immunosuppressive medicines on recipients, and procedural issues [139]. A shortage of oxygen and the presence of radical oxygen species (ROS) cause the vast majority of transplanted stem cells to die just a few days after administration. Investigations suggest that MSCs have a higher capacity to restore damaged liver tissue due to their ability to develop into specialized cells when incubated with damaged liver cells. Recovery of liver enzymes and histological improvement due to central necrosis repair has been documented [140, 141].
Various clinical trials have evidenced the safety and feasibility of MSCs along with enhanced serum albumin, cholinesterase, and prothrombin activity in patients with liver failure [142,143,144,145].
Notwithstanding, because of the limited success of MSCs in liver diseases therapy, numerous studies have been done to address this issue [146,147,148,149].
IL-1 is a therapeutic option for sustaining MSCs to treat ALF by promoting the MSCs' capacity to regenerate damaged liver [150]. Through the increasing CXCR4 expression and ensuing enhancement in MSCs homing capacity, IL-1 pre-treatment can improve MSCs-mediated impacts on ALF [151]. Also, direct modification of MSCs to overexpress CXCR4 potentiates their potential to increase liver regeneration [152]. Also, sodium butyrate (NaB) treatment was supposed to improve the hepatic differentiation of BM-MSCs post-transplantation in vivo [153]. The NaB-MSCs transplantation also enhanced albumin (ALB), alpha 1-antitrypsin (AAT), and the serum total protein (TP), while reducing serum alanine transaminase (ALT) levels in vivo [153]. Further, umbilical cord blood (UCB)-MSCs engineered to overexpress the VEGF 165 gene could facilitate ALF treatment. VEGF165 overexpression promoted the multipotency of UCB-MSCs and increased their homing and colonization in the liver tissues of ALF rat [154]. VEGF165 –MSCs transplantation ameliorated liver damage and improved liver regeneration more evidently than native UCB-MSCs [154].
Interleukin-35 (IL-35) is an emerging cytokine critical for preventing autoimmune illnesses and responsible for the Treg's ability to moderate and decrease immunological responses [155]. The IL-35 gene-modified MSCs could migrate to the damaged liver tissues, reduce hepatocyte apoptosis, and down-regulate IFN-γ secretion by liver mononuclear cells mainly by negative regulation of JAK1-STAT1/STAT4 axis by IL-35 [156].
A summary of the studies investigating the therapeutic effects of genetically modified MSCs in organ failure disease is provided in Table 2. Figure 3 also depicts the impact of the HIF-1ɑ-, ADM-, miR-133-, IL-35-, VEGF165-, and HO-1-overexpressing MSCs in vivo.
Pre-conditioned MSCs in ovarian failure
One of the common disorders affecting women that contributes to 1% of female infertility is premature ovarian failure (POF) [157]. Hypoestrogenism, or a lack of estrogen, an elevated gonadotropin level, and, most significantly, amenorrhea are all clinical signs of POF. As the most popular hormone replacement therapy cannot successfully restore ovarian function [158], there is now a greater need for effective and novel POF therapeutics. Meanwhile, human MSCs therapy offers new opportunities for POF as regenerative medicine advances [159, 160]. In mice receiving chemotherapy, the MSCs therapy was discovered to diminish granulosa cell (GC) apoptosis and DNA damage [161] and can also promote the growth of primordial follicles and raise FSH levels to levels that are close to normal [162]. MSCs also promote reactivate folliculogenesis [163] and increase insulin-like growth factor-1 (IGF-1) in ovaries [164].
Recent studies demonstrated that overexpressing miR-21 in BM-MSCs could restore ovarian function in rats with chemotherapy-induced POF. This was associated with the inhibition of granulosa cell apoptosis by targeting recombinant human programmed cell death 4 (PDCD4) and phosphatase and tensin homolog deleted on chromosome 10 (PTEN) [165]. Numerous reports also have shown that heat shock (HS) pre-treatment can cause the production of heat shock transcription factor (HSF1), which activates particular signaling pathways (e. g., HSF1/miR-34a/HSP70) to create a number of HSPs [166]. HSPs play a role in the obstruction of various apoptotic pathways. Apoptosome formation and the mitochondrial apoptotic pathway, for instance, are blocked when HSP27 and HSP90 bind to Apaf-1 [167]. In order to prevent the caspase-mediated apoptotic pathway from being activated, HSP70 interacts with apoptosis inducing factor (AIF) [168]. By inhibiting granulosa cell apoptosis more effectively than with naive MSC therapy in the rat model of chemotherapy-induced POF, the HS pre-treatment of MSCs increased the repair effect of MSCs on chemotherapy-induced POF [169]. Additionally, in rats treated with HS-MSCs, levels of sex hormones tended to stabilize [169]. Additionally, low-intensity pulsed ultrasound (LIPUS) can stimulate the expression of a number of growth factors and anti-inflammatory molecules, both of which are important for maintaining follicle growth and preventing GCs apoptosis in the ovary [170, 171]. In a recent study, LIPUS-pretreated human MSCs were found to have additional benefits over naive MSC therapy in rats with chemotherapy-induced POI, including the ability to reduce inflammation, inhibiting granulosa cell apoptosis, repairing ovarian injury, and promoting ovarian function [172].
Conclusion
In spite of the encouraging outcomes of MSCs therapy in a diversity of diseases, dysfunction of MSCs in host tissue may help explain how some animal studies and clinical trials yield different results. MSCs are vulnerable to the internal environment after infusion, which reduces their survival and grafting to the target tissues [173]. In light of this problem, scientists are exploring different strategies to improve the therapeutic efficacy of MSCs. Recent reports have clarified that pre-conditioning, as a multi-technique approach, could improve MSCs' survival and migration to the target tissue and also could potentiate their immunoregulatory, differentiation, and pro-angiogenic competencies post-transplantation. Nonetheless, there still exist several difficulties in defining the optimal approaches for pre-conditioning in MSC‐based treatment. The compounds used may have negative effects on the cell. The optimal dose of these substances should be determined. It must be ensured that these cells do not undergo abnormal genetic changes. Further, it is also possible to increase the therapeutic effects of MSCs by using combined treatments. Lastly, detailed mechanisms are required to be studied as no simple regulative route protects MSCs from damage.
Availability of data and materials
Not applicable.
Abbreviations
- MSCs:
-
Mesenchymal stem/stromal cells
- BM:
-
Bone marrow
- UC:
-
Umbilical cord
- AT:
-
Adipose tissue
- IDO:
-
Indoleamine 2,3-dioxygenase
- LF:
-
Liver failure
- VEGF:
-
Vascular endothelial growth factor
- FGF:
-
Fibroblast growth factors
- TNF-α:
-
Tumor necrosis factor alpha
- IFN-γ:
-
Interferon gamma
- HIF-1α:
-
Hypoxia-inducible factor 1α
References
Marofi F, Hassanzadeh A, Solali S, Vahedi G, Mousavi Ardehaie R, Salarinasab S, Aliparasti MR, et al. Epigenetic mechanisms are behind the regulation of the key genes associated with the osteoblastic differentiation of the mesenchymal stem cells: The role of zoledronic acid on tuning the epigenetic changes. J Cell Physiol. 2019;234(9):15108–22.
Tavakoli S, Ghaderi Jafarbeigloo HR, Shariati A, Jahangiryan A, Jadidi F, Jadidi Kouhbanani MA, Hassanzadeh A, et al. Mesenchymal stromal cells; a new horizon in regenerative medicine. J Cell Physiol. 2020.
Friedenstein A, Piatetzky-Shapiro I, Petrakova K. Osteogenesis in transplants of bone marrow cells. Development. 1966;16(3):381–90.
Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–50.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, et al. Multilineage potential of adult human mesenchymal stem cells. science. 1999;284(5411):143–7.
Crisan M, Yap S, Casteilla L, Chen C-W, Corselli M, Park TS, Andriolo G, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13.
Kugo H, Moriyama T, Zaima N. The role of perivascular adipose tissue in the appearance of ectopic adipocytes in the abdominal aortic aneurysmal wall. Adipocyte. 2019;8(1):229–39.
Saalbach A, Anderegg U. Thy-1: more than a marker for mesenchymal stromal cells. FASEB J. 2019;33(6):6689–96.
Moghadasi S, Elveny M, Rahman HS, Suksatan W, Jalil AT, Abdelbasset WK, Yumashev AV, et al. A paradigm shift in cell-free approach: the emerging role of MSCs-derived exosomes in regenerative medicine. J Transl Med. 2021;19(1):302.
Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21(1):105–10.
In'tAnker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–45.
Reger RL, Tucker AH, Wolfe MR. Differentiation and characterization of human MSCs. Mesenchymal Stem Cells: Springer; 2008. p. 93–107.
Mazini L, Rochette L, Amine M, Malka G. Regenerative capacity of adipose derived stem cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int J Mol Sci. 2019;20(10).
Laroye C, Boufenzer A, Jolly L, Cunat L, Alauzet C, Merlin JL, Yguel C, et al. Bone marrow vs Wharton’s jelly mesenchymal stem cells in experimental sepsis: a comparative study. Stem Cell Res Ther. 2019;10(1):192.
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–9.
Strauer BE, Kornowski R. Stem cell therapy in perspective. Circulation. 2003;107(7):929–34.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7.
Laroye C, Gibot S, Reppel L, Bensoussan D. Concise review: mesenchymal stromal/stem cells: a new treatment for sepsis and septic shock? Stem Cells. 2017;35(12):2331–9.
Gnecchi M, Ciuffreda MC, Mura M. Mesenchymal Stromal Cell Secretome for Tissue Repair. Cell Engineering and Regeneration. 2020:641–66.
Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, Demarquay C, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5(12):1028–38.
Infante A, Rodríguez CI. Cell and cell-free therapies to counteract human premature and physiological aging: MSCs come to light. J Personal Med. 2021;11(10):1043.
Li L, Chen X, Wang WE, Zeng C. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells Int. 2016;2016:9682757.
Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. 2019;15:421–38.
Liu W, Li L, Rong Y, Qian D, Chen J, Zhou Z, Luo Y, et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196–212.
Nakao Y, Fukuda T, Zhang Q, Sanui T, Shinjo T, Kou X, Chen C, et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306–24.
Liu W, Tang P, Wang J, Ye W, Ge X, Rong Y, Ji C, et al. Extracellular vesicles derived from melatonin-preconditioned mesenchymal stem cells containing USP29 repair traumatic spinal cord injury by stabilizing NRF2. J Pineal Res. 2021;71(4): e12769.
Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Gonçalves RM. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol. 2018;9:2837.
Ishiuchi N, Nakashima A, Doi S, Yoshida K, Maeda S, Kanai R, Yamada Y, et al. Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem Cell Res Ther. 2020;11(1):130.
Gupta S, Rawat S, Krishnakumar V, Rao EP, Mohanty S. Hypoxia preconditioning elicit differential response in tissue-specific MSCs via immunomodulation and exosomal secretion. Cell Tissue Res. 2022;388(3):535–48.
Wang S, Zhang C, Niyazi S, Zheng L, Li J, Zhang W, Xu M, et al. A novel cytoprotective peptide protects mesenchymal stem cells against mitochondrial dysfunction and apoptosis induced by starvation via Nrf2/Sirt3/FoxO3a pathway. J Transl Med. 2017;15(1):33.
Kozlowska U, Krawczenko A, Futoma K, Jurek T, Rorat M, Patrzalek D, Klimczak A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J Stem Cells. 2019;11(6):347–74.
Dominici M, LeBlanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–7.
Bogdanova A, Berzins U, Nikulshin S, Skrastina D, Ezerta A, Legzdina D, Kozlovska T. Characterization of human adipose-derived stem cells cultured in autologous serum after subsequent passaging and long term cryopreservation. J Stem Cells. 2014;9(3):135–48.
Vishnubalaji R, Manikandan M, Al-Nbaheen M, Kadalmani B, Aldahmash A, Alajez NM. In vitro differentiation of human skin-derived multipotent stromal cells into putative endothelial-like cells. BMC Dev Biol. 2012;12:7.
Chirieleison SM, Feduska JM, Schugar RC, Askew Y, Deasy BM. Human muscle-derived cell populations isolated by differential adhesion rates: phenotype and contribution to skeletal muscle regeneration in Mdx/SCID mice. Tissue Eng Part A. 2012;18(3–4):232–41.
Park CW, Kim KS, Bae S, Son HK, Myung PK, Hong HJ, Kim H. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells. 2009;2(1):59–68.
Konala VBR, Bhonde R, Pal R. Secretome studies of mesenchymal stromal cells (MSCs) isolated from three tissue sources reveal subtle differences in potency. In Vitro Cellular & Developmental Biology-Animal. 2020:1–12.
Shim JH, Park JY, Lee MG, Kang HH, Lee TR, Shin DW. Human dermal stem/progenitor cell-derived conditioned medium ameliorates ultraviolet a-induced damage of normal human dermal fibroblasts. PLoS ONE. 2013;8(7): e67604.
Ribeiro A, Laranjeira P, Mendes S, Velada I, Leite C, Andrade P, Santos F, et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther. 2013;4(5):125.
Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986–8001.
Wang Q, Yang Q, Wang Z, Tong H, Ma L, Zhang Y, Shan F, et al. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton’s jelly as sources of cell immunomodulatory therapy. Hum Vaccin Immunother. 2016;12(1):85–96.
Shen C, Yang C, Xu S, Zhao H. Comparison of osteogenic differentiation capacity in mesenchymal stem cells derived from human amniotic membrane (AM), umbilical cord (UC), chorionic membrane (CM), and decidua (DC). Cell Biosci. 2019;9(1):17.
Harkness L, Zaher W, Ditzel N, Isa A, Kassem M. CD146/MCAM defines functionality of human bone marrow stromal stem cell populations. Stem Cell Res Ther. 2016;7:4.
Rowland AL, Xu JJ, Joswig AJ, Gregory CA, Antczak DF, Cummings KJ, Watts AE. In vitro MSC function is related to clinical reaction in vivo. Stem Cell Res Ther. 2018;9(1):295.
Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. npj Regen Med. 2019;4(1):22.
Ward MR, Abadeh A, Connelly KA. Concise review: rational use of mesenchymal stem cells in the treatment of ischemic heart disease. Stem Cells Transl Med. 2018;7(7):543–50.
Alm JJ, Koivu HM, Heino TJ, Hentunen TA, Laitinen S, Aro HT. Circulating plastic adherent mesenchymal stem cells in aged hip fracture patients. J Orthop Res. 2010;28(12):1634–42.
Hassanzadeh A, Shamlou S, Yousefi N, Nikoo M, Verdi J. Genetically-modified stem cell in regenerative medicine and cancer therapy; a new era. Curr Gene Ther. 2022;22(1):23–39.
Rochefort GY, Delorme B, Lopez A, Hérault O, Bonnet P, Charbord P, Eder V, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24(10):2202–8.
Chen Y, Xiang LX, Shao JZ, Pan RL, Wang YX, Dong XJ, Zhang GR. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J Cell Mol Med. 2010;14(6b):1494–508.
Marofi F, Hassanzadeh A, Solali S, Vahedi G, Mousavi Ardehaie R, Salarinasab S, Aliparasti MR, et al. Epigenetic mechanisms are behind the regulation of the key genes associated with the osteoblastic differentiation of the mesenchymal stem cells: The role of zoledronic acid on tuning the epigenetic changes. J Cell Physiol. 2019.
Guo J, Lin G-s, Bao C-y, Hu Z-m, Hu M-y. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007;30:97–104.
Zhang R, Liu Y, Yan K, Chen L, Chen X-R, Li P, Chen F-F, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10(1):1–12.
Seo KW, Sohn SY, Bhang DH, Nam MJ, Lee HW, Youn HY. Therapeutic effects of hepatocyte growth factor-overexpressing human umbilical cord blood-derived mesenchymal stem cells on liver fibrosis in rats. Cell Biol Int. 2014;38(1):106–16.
Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang J-A, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135(4):799–808.
Hassanzadeh A, Altajer AH, Rahman HS, Saleh MM, Bokov DO, Abdelbasset WK, Marofi F, et al. Mesenchymal stem/stromal cell-based delivery: a rapidly evolving strategy for cancer therapy. Front Cell Dev Biol. 2021;9(1758).
Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53(1): e12712.
Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE. 2010;5(4): e10088.
López-García L, Castro-Manrreza ME. TNF-α and IFN-γ Participate in improving the immunoregulatory capacity of mesenchymal stem/stromal cells: importance of cell-cell contact and extracellular vesicles. Int J Mol Sci. 2021;22(17).
Salari V, Mengoni F, Del Gallo F, Bertini G, Fabene PF. The Anti-inflammatory properties of mesenchymal stem cells in epilepsy: possible treatments and future perspectives. Int J Mol Sci. 2020;21(24).
Chao K, Zhang S, Qiu Y, Chen X, Zhang X, Cai C, Peng Y, et al. Human umbilical cord-derived mesenchymal stem cells protect against experimental colitis via CD5(+) B regulatory cells. Stem Cell Res Ther. 2016;7(1):109.
Shrestha M, Nguyen TT, Park J, Choi JU, Yook S, Jeong J-H. Immunomodulation effect of mesenchymal stem cells in islet transplantation. Biomed Pharmacother. 2021;142: 112042.
Sun S-J, Lai W-H, Jiang Y, Zhen Z, Wei R, Lian Q, Liao S-Y, et al. Immunomodulation by systemic administration of human-induced pluripotent stem cell-derived mesenchymal stromal cells to enhance the therapeutic efficacy of cell-based therapy for treatment of myocardial infarction. Theranostics. 2021;11(4):1641.
Fujii S, Miura Y, Fujishiro A, Shindo T, Shimazu Y, Hirai H, Tahara H, et al. Graft-versus-host disease amelioration by human bone marrow mesenchymal stromal/stem cell-derived extracellular vesicles is associated with peripheral preservation of naive T cell populations. Stem Cells. 2018;36(3):434–45.
Zhou T, Chen YL. The functional mechanisms of miR-30b-5p in acute lung injury in children. Med Sci Monit. 2019;25:40–51.
Schwarz TM, Leicht SF, Radic T, Rodriguez-Arabaolaza I, Hermann PC, Berger F, Saif J, et al. Vascular incorporation of endothelial colony-forming cells is essential for functional recovery of murine ischemic tissue following cell therapy. Arterioscler Thromb Vasc Biol. 2012;32(2):e13-21.
Lin RZ, Moreno-Luna R, Zhou B, Pu WT, Melero-Martin JM. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis. 2012;15(3):443–55.
Xia X, Tao Q, Ma Q, Chen H, Wang J, Yu H. Growth hormone-releasing hormone and its analogues: significance for MSCs-mediated angiogenesis. Stem Cells Int. 2016;2016:8737589.
Das M, Sundell IB, Koka PS. Adult mesenchymal stem cells and their potency in the cell-based therapy. J Stem Cells. 2013;8(1):1–16.
Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22(3):377–84.
Tao H, Han Z, Han ZC, Li Z. Proangiogenic features of mesenchymal stem cells and their therapeutic applications. Stem Cells Int. 2016;2016:1314709.
Wada T, Jesmin S, Gando S, Yanagida Y, Mizugaki A, Sultana SN, Zaedi S, et al. Angiogenic factors and their soluble receptors predict organ dysfunction and mortality in post-cardiac arrest syndrome. Crit Care. 2012;16(5):R171.
Deshane J, Chen S, Caballero S, Grochot-Przeczek A, Was H, Li Calzi S, Lach R, et al. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med. 2007;204(3):605–18.
Marquez-Curtis LA, Janowska-Wieczorek A. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. BioMed research international. 2013;2013.
Qin H, Zhao X, Hu YJ, Wang S, Ma Y, He S, Shen K, et al. Inhibition of SDF-1/CXCR4 axis to alleviate abnormal bone formation and angiogenesis could improve the subchondral bone microenvironment in osteoarthritis. Biomed Res Int. 2021;2021:8852574.
Shigematsu H, Yasuda K, Iwai T, Sasajima T, Ishimaru S, Ohashi Y, Yamaguchi T, et al. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17(9):1152–61.
Pasquet M, Golzio M, Mery E, Rafii A, Benabbou N, Mirshahi P, Hennebelle I, et al. Hospicells (ascites-derived stromal cells) promote tumorigenicity and angiogenesis. Int J Cancer. 2010;126(9):2090–101.
Ayatollahi M, Soleimani M, Geramizadeh B, Imanieh MH. Insulin-like growth factor 1 (IGF-I) improves hepatic differentiation of human bone marrow-derived mesenchymal stem cells. Cell Biol Int. 2011;35(11):1169–76.
Shi XL, Gu JY, Zhang Y, Han B, Xiao JQ, Yuan XW, Zhang N, et al. Protective effects of ACLF sera on metabolic functions and proliferation of hepatocytes co-cultured with bone marrow MSCs in vitro. World J Gastroenterol. 2011;17(19):2397–406.
Cheng H, Chang S, Xu R, Chen L, Song X, Wu J, Qian J, et al. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res Ther. 2020;11(1):224.
Peng Y, Zhao JL, Peng ZY, Xu WF, Yu GL. Exosomal miR-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis. 2020;11(5):317.
Yi X, Wei X, Lv H, An Y, Li L, Lu P, Yang Y, et al. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp Cell Res. 2019;383(2): 111454.
Gharibi B, Hughes FJ. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med. 2012;1(11):771–82.
Saparov A, Ogay V, Nurgozhin T, Jumabay M, Chen WC. Preconditioning of human mesenchymal stem cells to enhance their regulation of the immune response. Stem Cells Int. 2016;2016:3924858.
Zhao L, Hu C, Han F, Cai F, Wang J, Chen J. Preconditioning is an effective strategy for improving the efficiency of mesenchymal stem cells in kidney transplantation. Stem Cell Res Ther. 2020;11(1):197.
Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA, Fierro FA. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells. 2015;33(6):1818–28.
Noone C, Kihm A, English K, O’Dea S, Mahon BP. IFN-γ stimulated human umbilical-tissue-derived cells potently suppress NK activation and resist NK-mediated cytotoxicity in vitro. Stem Cells Dev. 2013;22(22):3003–14.
Ylöstalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30(10):2283–96.
Liu C, Fan Y, Zhou L, Zhu HY, Song YC, Hu L, Wang Y, et al. Pretreatment of mesenchymal stem cells with angiotensin II enhances paracrine effects, angiogenesis, gap junction formation and therapeutic efficacy for myocardial infarction. Int J Cardiol. 2015;188:22–32.
Liu J, Hao H, Xia L, Ti D, Huang H, Dong L, Tong C, et al. Hypoxia pretreatment of bone marrow mesenchymal stem cells facilitates angiogenesis by improving the function of endothelial cells in diabetic rats with lower ischemia. PLoS ONE. 2015;10(5): e0126715.
Schmid C, Philipp A, Hilker M, Rupprecht L, Arlt M, Keyser A, Lubnow M, et al. Venovenous extracorporeal membrane oxygenation for acute lung failure in adults. J Heart Lung Transplant. 2012;31(1):9–15.
Enger TB, Philipp A, Videm V, Lubnow M, Wahba A, Fischer M, Schmid C, et al. Prediction of mortality in adult patients with severe acute lung failure receiving veno-venous extracorporeal membrane oxygenation: a prospective observational study. Crit Care. 2014;18(2):1–10.
Zhang L-B, He M. Effect of mesenchymal stromal (stem) cell (MSC) transplantation in asthmatic animal models: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2019;54:39–52.
Wang X. MSCs transplantation may be a potential therapeutic strategy for COVID-19 treatment. Eur Rev Med Pharmacol Sci. 2020;24(8):4537–8.
Khosravipour A, Amini A, Farahani RM, Zare F, Mostafavinia A, Fallahnezhad S, Akbarzade S, et al. Preconditioning adipose-derived stem cells with photobiomodulation significantly increased bone healing in a critical size femoral defect in rats. Biochem Biophys Res Commun. 2020;531(2):105–11.
Inamdar AC, Inamdar AA. Mesenchymal stem cell therapy in lung disorders: pathogenesis of lung diseases and mechanism of action of mesenchymal stem cell. Exp Lung Res. 2013;39(8):315–27.
Wang Y, Li H, Li X, Su X, Xiao H, Yang J. Hypoxic preconditioning of human umbilical cord mesenchymal stem cells is an effective strategy for treating acute lung injury. Stem Cells Dev. 2021;30(3):128–34.
Esmaeilzade B, Artimani T, Amiri I, Najafi R, Shahidi S, Sabec M, Farzadinia P, et al. Dimethyloxalylglycine preconditioning enhances protective effects of bone marrow-derived mesenchymal stem cells in Aβ-induced Alzheimer disease. Physiol Behav. 2019;199:265–72.
Ogle ME, Yu SP, Wei L. Primed for lethal battle: a step forward to enhance the efficacy and efficiency of stem cell transplantation therapy. J Thorac Cardiovasc Surg. 2009;138(3):527.
Lan YW, Choo KB, Chen CM, Hung TH, Chen YB, Hsieh CH, Kuo HP, et al. Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther. 2015;6(1):97.
Noort WA, Feye D, Van Den Akker F, Stecher D, Chamuleau SA, Sluijter JP, Doevendans PA. Mesenchymal stromal cells to treat cardiovascular disease: strategies to improve survival and therapeutic results. Panminerva Med. 2010;52(1):27–40.
Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10(3):244–58.
Chen X, Wu S, Tang L, Ma L, Wang F, Feng H, Meng J, et al. Mesenchymal stem cells overexpressing heme oxygenase-1 ameliorate lipopolysaccharide-induced acute lung injury in rats. J Cell Physiol. 2019;234(5):7301–19.
Chen HX, Xiang H, Xu WH, Li M, Yuan J, Liu J, Sun WJ, et al. Manganese superoxide dismutase gene-modified mesenchymal stem cells attenuate acute radiation-induced lung injury. Hum Gene Ther. 2017;28(6):523–32.
Liao Y, Fu Z, Huang Y, Wu S, Wang Z, Ye S, Zeng W, et al. Interleukin-18-primed human umbilical cord-mesenchymal stem cells achieve superior therapeutic efficacy for severe viral pneumonia via enhancing T-cell immunosuppression. Cell Death Dis. 2023;14(1):66.
Rossignol P, Hernandez AF, Solomon SD, Zannad F. Heart failure drug treatment. The Lancet. 2019;393(10175):1034–44.
Capasso JM, Fitzpatrick D, Anversa P. Cellular mechanisms of ventricular failure: myocyte kinetics and geometry with age. Am J Physiol. 1992;262(6 Pt 2):H1770–81.
Müller P, Lemcke H, David R. Stem cell therapy in heart diseases - cell types, mechanisms and improvement strategies. Cell Physiol Biochem. 2018;48(6):2607–55.
Smagul S, Kim Y, Smagulova A, Raziyeva K, Nurkesh A, Saparov A. Biomaterials loaded with growth factors/cytokines and stem cells for cardiac tissue regeneration. Int J Mol Sci. 2020;21(17).
Wu R, Hu X, Wang J. Concise review: optimized strategies for stem cell-based therapy in myocardial repair: clinical translatability and potential limitation. Stem Cells. 2018;36(4):482–500.
Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, Rojo P, et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circ Res. 2017;121(10):1192–204.
Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF, Haack-Sørensen M, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur Heart J. 2015;36(27):1744–53.
Gao LR, Tang CS, Zhu ZM, Wang ZG, Fei YX, Tian HT, Zhu JR, et al. The autologous bone marrow mononuclear cell transplantation by intracoronary route treat patients with severe heart failure after myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34(7):582–6.
Zhang L, Yang J, Tian YM, Guo H, Zhang Y. Beneficial effects of hypoxic preconditioning on human umbilical cord mesenchymal stem cells. Chin J Physiol. 2015;58(5):343–53.
Fernández-Fernández L, Bellido-Martín L, García de Frutos P. Growth arrest-specific gene 6 (GAS6) An outline of its role in haemostasis and inflammation. Thromb Haemost. 2008;100(4):604–10.
Shan S, Liu Z, Guo T, Wang M, Tian S, Zhang Y, Wang K, et al. Growth arrest-specific gene 6 transfer promotes mesenchymal stem cell survival and cardiac repair under hypoxia and ischemia via enhanced autocrine signaling and paracrine action. Arch Biochem Biophys. 2018;660:108–20.
Gara E, Ong SG, Winkler J, Zlabinger K, Lukovic D, Merkely B, Emmert MY, et al. Cell-Based HIF1α gene therapy reduces myocardial scar and enhances angiopoietic proteome, transcriptomic and miRNA expression in experimental chronic left ventricular dysfunction. Front Bioeng Biotechnol. 2022;10: 767985.
Kim SH, Moon HH, Kim HA, Hwang KC, Lee M, Choi D. Hypoxia-inducible vascular endothelial growth factor-engineered mesenchymal stem cells prevent myocardial ischemic injury. Mol Ther. 2011;19(4):741–50.
Li LL, Peng C, Zhang M, Liu Y, Li H, Chen H, Sun Y, et al. Mesenchymal stem cells overexpressing adrenomedullin improve heart function through antifibrotic action in rats experiencing heart failure. Mol Med Rep. 2018;17(1):1437–44.
Esfini-Farahani M, Farshdousti-Hagh M, Bashash D, Esmaeili S, Dehghan-Nayeri N, Yazdanpanah S, Gharehbaghian A. Analysis of cytotoxic activity and synergistic effect of curcuma longa extract in combination with prednisolone on acute lymphoblastic leukemia cell lines. International Journal of Cancer Management. 2017;10(11).
Yazdanpanah S, Esmaeili S, Bashash D, Nayeri ND, Farahani ME, Gharehbaghian A. Cytotoxic and apoptogenic activity of Bryonia aspera extract on pre-B acute lymphoblastic leukemia cell lines. Int J Hematol Oncol Stem Cell Res. 2018;12(3):204.
Zadi Heydarabad M, Baharaghdam S, Azimi A, Mohammadi H, Eivazi Ziaei J, Yazdanpanah B, Zak MS, et al. The role of tumor suppressor of resveratrol and prednisolone by downregulation of YKL-40 expression in CCRF-CEM cell line. J Cell Biochem. 2019;120(3):3773–9.
Saini U, Gumina RJ, Wolfe B, Kuppusamy ML, Kuppusamy P, Boudoulas KD. Preconditioning mesenchymal stem cells with caspase inhibition and hyperoxia prior to hypoxia exposure increases cell proliferation. J Cell Biochem. 2013;114(11):2612–23.
Missoum A. Recent updates on mesenchymal stem cell based therapy for acute renal failure. Current urology. 2019;13(4):189–99.
Raza A, Estepa A, Chan V, Jafar MS. Acute renal failure in critically ill COVID-19 patients with a focus on the role of renal replacement therapy: a review of what we know so far. Cureus. 2020;12(6).
Shao Z, Meng X, Meng F. Efficacy and safety of mesenchymal stem cell in Chinese patients with chronic renal failure: A pilot study in Shandong province, China. Pak J Pharm Sci. 2021;34(3(Special)):1227–31.
Erpicum P, Weekers L, Detry O, Bonvoisin C, Delbouille M-H, Grégoire C, Baudoux E, et al. Infusion of third-party mesenchymal stromal cells after kidney transplantation: a phase I-II, open-label, clinical study. Kidney Int. 2019;95(3):693–707.
Perico N, Casiraghi F, Introna M, Gotti E, Todeschini M, Cavinato RA, Capelli C, et al. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6(2):412–22.
Xinaris C, Morigi M, Benedetti V, Imberti B, Fabricio AS, Squarcina E, Benigni A, et al. A novel strategy to enhance mesenchymal stem cell migration capacity and promote tissue repair in an injury specific fashion. Cell Transplant. 2013;22(3):423–36.
Mias C, Trouche E, Seguelas MH, Calcagno F, Dignat-George F, Sabatier F, Piercecchi-Marti MD, et al. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26(7):1749–57.
Putra A, Pertiwi D, Milla MN, Indrayani UD, Jannah D, Sahariyani M, Trisnadi S, et al. Hypoxia-preconditioned MSCs have superior effect in ameliorating renal function on acute renal failure animal model. Open Access Maced J Med Sci. 2019;7(3):305–10.
Lee MS, Yip HK, Yang CC, Chiang JY, Huang TH, Li YC, Chen KH, et al. Overexpression of miR-19a and miR-20a in iPS-MSCs preserves renal function of chronic kidney disease with acute ischaemia-reperfusion injury in rat. J Cell Mol Med. 2021;25(16):7675–89.
Cao D, Wang Y, Zhang Y, Zhang Y, Huang Q, Yin Z, Cai G, et al. Regulation of connective tissue growth factor expression by miR-133b for the treatment of renal interstitial fibrosis in aged mice with unilateral ureteral obstruction. Stem Cell Res Ther. 2021;12(1):171.
Gheisari Y, Azadmanesh K, Ahmadbeigi N, Nassiri SM, Golestaneh AF, Naderi M, Vasei M, et al. Genetic modification of mesenchymal stem cells to overexpress CXCR4 and CXCR7 does not improve the homing and therapeutic potentials of these cells in experimental acute kidney injury. Stem Cells Dev. 2012;21(16):2969–80.
Liu N, Tian J, Cheng J, Zhang J. Migration of CXCR4 gene-modified bone marrow-derived mesenchymal stem cells to the acute injured kidney. J Cell Biochem. 2013;114(12):2677–89.
Cao J, Wang B, Tang T, Lv L, Ding Z, Li Z, Hu R, et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res Ther. 2020;11(1):206.
Zhang J, Gao J, Lin D, Xiong J, Wang J, Chen J, Lin B, et al. Potential networks regulated by MSCs in acute-on-chronic liver failure: exosomal miRNAs and intracellular target genes. Front Genet. 2021;12: 650536.
Sundaram V, Jalan R, Wu T, Volk ML, Asrani SK, Klein AS, Wong RJ. Factors associated with survival of patients with severe acute-on-chronic liver failure before and after liver transplantation. Gastroenterology. 2019;156(5):1381–91.
O’Leary JG, Bajaj JS, Tandon P, Biggins SW, Wong F, Kamath PS, Garcia-Tsao G, et al. Outcomes after listing for liver transplant in patients with Acute-on-Chronic liver failure: the multicenter North American Consortium for the study of End-Stage liver disease experience. Liver Transpl. 2019;25(4):571–9.
Cho KA, Ju SY, Cho SJ, Jung YJ, Woo SY, Seoh JY, Han HS, et al. Mesenchymal stem cells showed the highest potential for the regeneration of injured liver tissue compared with other subpopulations of the bone marrow. Cell Biol Int. 2009;33(7):772–7.
Amiri F, Jahanian-Najafabadi A, Roudkenar MH. In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments : In vitro augmentation of mesenchymal stem cells viability. Cell Stress Chaperones. 2015;20(2):237–51.
Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1(10):725–31.
Schacher FC, Martins Pezzi da Silva A, Silla L, Álvares-da-Silva MR. Bone Marrow Mesenchymal Stem Cells in Acute-on-Chronic Liver Failure Grades 2 and 3: A Phase I-II Randomized Clinical Trial. Can J Gastroenterol Hepatol. 2021;2021:3662776.
Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, Liu QL, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66(1):209–19.
Tsuchiya A, Kojima Y, Ikarashi S, Seino S, Watanabe Y, Kawata Y, Terai S. Clinical trials using mesenchymal stem cells in liver diseases and inflammatory bowel diseases. Inflammation and Regeneration. 2017;37(1):16.
Boyer TD, Lindor KD. Zakim and Boyer's hepatology: A textbook of liver disease e-book: Elsevier Health Sciences; 2016.
Prasajak P, Leeanansaksiri W. Mesenchymal stem cells: current clinical applications and therapeutic potential in liver diseases. J Bone Marrow Res. 2014;2(137):2.
Wei H, Li Z, Hu S, Chen X, Cong X. Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J Cell Biochem. 2010;111(4):967–78.
Amiri F, Jahanian-Najafabadi A, Roudkenar MH. In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments. Cell Stress Chaperones. 2015;20(2):237–51.
Wang Y-H, Wu D-B, Chen B, Chen E-Q, Tang H. Progress in mesenchymal stem cell–based therapy for acute liver failure. Stem Cell Res Ther. 2018;9(1):1–9.
Nie H, An F, Mei J, Yang C, Zhan Q, Zhang Q. IL-1β Pretreatment improves the efficacy of mesenchymal stem cells on acute liver failure by enhancing CXCR4 expression. Stem Cells Int. 2020;2020:1498315.
Ma HC, Shi XL, Ren HZ, Yuan XW, Ding YT. Targeted migration of mesenchymal stem cells modified with CXCR4 to acute failing liver improves liver regeneration. World J Gastroenterol. 2014;20(40):14884–94.
Li QY, Chen J, Luo YH, Zhang W, Xiao EH. Sodium butyrate pre-treatment enhance differentiation of bone marrow mesenchymal stem cells (BM-MSCs) into hepatocytes and improve liver injury. Curr Mol Med. 2022;22(7):663–74.
Chen H, Tang S, Liao J, Liu M, Lin Y. VEGF(165) gene-modified human umbilical cord blood mesenchymal stem cells protect against acute liver failure in rats. J Gene Med. 2021;23(10): e3369.
Yang L, Zhang Q, Song J, Wang W, Jin Z. Interleukin-35 suppresses CD8+ T cell activity in patients with viral hepatitis-induced acute-on-chronic liver failure. Dig Dis Sci. 2020;65(12):3614–23.
Wang W, Guo H, Li H, Yan Y, Wu C, Wang X, He X, et al. Interleukin-35 gene-modified mesenchymal stem cells protect concanavalin a-induced fulminant hepatitis by decreasing the interferon gamma level. Hum Gene Ther. 2018;29(2):234–41.
Beck-Peccoz P, Persani L. Premature ovarian failure. Orphanet J Rare Dis. 2006;1:1–5.
Liu W, Nguyen T-N, Tran Thi T-V, Zhou S. Kuntai capsule plus hormone therapy vs. hormone therapy alone in patients with premature ovarian failure: a systematic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine. 2019;2019.
Shareghi-Oskoue O, Aghebati-Maleki L, Yousefi M. Transplantation of human umbilical cord mesenchymal stem cells to treat premature ovarian failure. Stem Cell Res Ther. 2021;12(1):1–13.
Bahrehbar K, Valojerdi MR, Esfandiari F, Fathi R, Hassani S-N, Baharvand H. Human embryonic stem cell-derived mesenchymal stem cells improved premature ovarian failure. World J Stem Cells. 2020;12(8):857.
Kilic S, Pinarli F, Ozogul C, Tasdemir N, Naz Sarac G, Delibasi T. Protection from cyclophosphamide-induced ovarian damage with bone marrow-derived mesenchymal stem cells during puberty. Gynecol Endocrinol. 2014;30(2):135–40.
Badawy A, Sobh MA, Ahdy M, Abdelhafez MS. Bone marrow mesenchymal stem cell repair of cyclophosphamide-induced ovarian insufficiency in a mouse model. Int J Womens Health. 2017;9:441–7.
Mohamed SA, Shalaby SM, Abdelaziz M, Brakta S, Hill WD, Ismail N, Al-Hendy A. Human mesenchymal stem cells partially reverse infertility in chemotherapy-induced ovarian failure. Reprod Sci. 2018;25(1):51–63.
Gabr H, Rateb MA, El Sissy MH, Ahmed Seddiek H, Ali Abdelhameed Gouda S. The effect of bone marrow-derived mesenchymal stem cells on chemotherapy induced ovarian failure in albino rats. Microsc Res Tech. 2016;79(10):938–47.
Fu X, He Y, Wang X, Peng D, Chen X, Li X, Wang Q. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res Ther. 2017;8(1):187.
Feng Y, Huang W, Meng W, Jegga AG, Wang Y, Cai W, Kim HW, et al. Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: a critical role for HSF1/miR-34a/HSP70 pathway. Stem cells. 2014;32(2):462–72.
Pires ES, Khole VV. A block in the road to fertility: autoantibodies to heat-shock protein 90-β in human ovarian autoimmunity. Fertil Steril. 2009;92(4):1395–409.
Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3(9):839–43.
Chen X, Wang Q, Li X, Wang Q, Xie J, Fu X. Heat shock pretreatment of mesenchymal stem cells for inhibiting the apoptosis of ovarian granulosa cells enhanced the repair effect on chemotherapy-induced premature ovarian failure. Stem Cell Res Ther. 2018;9(1):240.
Qin J, Chen J, Xu H, Xia Y, Tang W, Wang W, Li C, et al. Low-intensity pulsed ultrasound promotes repair of 4-vinylcyclohexene diepoxide-induced premature ovarian insufficiency in SD rats. J Gerontol Ser A. 2022;77(2):221–7.
Xu H, Xia Y, Qin J, Xu J, Li C, Wang Y. Effects of low intensity pulsed ultrasound on expression of B-cell lymphoma-2 and BCL2-Associated X in premature ovarian failure mice induced by 4-vinylcyclohexene diepoxide. Reprod Biol Endocrinol. 2021;19(1):1–11.
Ling L, Feng X, Wei T, Wang Y, Wang Y, Zhang W, He L, et al. Effects of low-intensity pulsed ultrasound (LIPUS)-pretreated human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation on primary ovarian insufficiency in rats. Stem Cell Res Ther. 2017;8(1):283.
Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. Faseb j. 2007;21(12):3197–207.
Yang DC, Yang MH, Tsai CC, Huang TF, Chen YH, Hung SC. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS ONE. 2011;6(9): e23965.
Liu J, He J, Huang Y, Ge L, Xiao H, Zeng L, Jiang Z, et al. Hypoxia-preconditioned mesenchymal stem cells attenuate microglial pyroptosis after intracerebral hemorrhage. Ann Transl Med. 2021;9(17):1362.
Abu-El-Rub E, Sequiera GL, Sareen N, Yan W, Moudgil M, Sabbir MG, Dhingra S. Hypoxia-induced 26S proteasome dysfunction increases immunogenicity of mesenchymal stem cells. Cell Death Dis. 2019;10(2):90.
Zhang W, Liu L, Huo Y, Yang Y, Wang Y. Hypoxia-pretreated human MSCs attenuate acute kidney injury through enhanced angiogenic and antioxidative capacities. Biomed Res Int. 2014;2014: 462472.
Antebi B, Rodriguez LA 2nd, Walker KP 3rd, Asher AM, Kamucheka RM, Alvarado L, Mohammadipoor A, et al. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res Ther. 2018;9(1):265.
Wagegg M, Gaber T, Lohanatha FL, Hahne M, Strehl C, Fangradt M, Tran CL, et al. Hypoxia promotes osteogenesis but suppresses adipogenesis of human mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent manner. PLoS ONE. 2012;7(9): e46483.
Feng XD, Zhu JQ, Zhou JH, Lin FY, Feng B, Shi XW, Pan QL, et al. Hypoxia-inducible factor-1α-mediated upregulation of CD99 promotes the proliferation of placental mesenchymal stem cells by regulating ERK1/2. World J Stem Cells. 2021;13(4):317–30.
Xu W, Xu R, Li Z, Wang Y, Hu R. Hypoxia changes chemotaxis behaviour of mesenchymal stem cells via HIF-1α signalling. J Cell Mol Med. 2019;23(3):1899–907.
Archacka K, Grabowska I, Mierzejewski B, Graffstein J, Górzyńska A, Krawczyk M, Różycka AM, et al. Hypoxia preconditioned bone marrow-derived mesenchymal stromal/stem cells enhance myoblast fusion and skeletal muscle regeneration. Stem Cell Res Ther. 2021;12(1):448.
Sareen N, Abu-El-Rub E, Ammar HI, Yan W, Sequiera GL, ShamsEldeen AM, Moudgil M, et al. Hypoxia-induced downregulation of cyclooxygenase 2 leads to the loss of immunoprivilege of allogeneic mesenchymal stem cells. Faseb j. 2020;34(11):15236–51.
Li L, Jaiswal PK, Makhoul G, Jurakhan R, Selvasandran K, Ridwan K, Cecere R. Hypoxia modulates cell migration and proliferation in placenta-derived mesenchymal stem cells. J Thorac Cardiovasc Surg. 2017;154(2):543-52.e3.
Abu-El-Rub E, Sareen N, Lester Sequiera G, Ammar HI, Yan W, ShamsEldeen AM, Rubinchik I, et al. Hypoxia-induced increase in Sug1 leads to poor post-transplantation survival of allogeneic mesenchymal stem cells. Faseb j. 2020;34(9):12860–76.
Liu J, Hao H, Huang H, Tong C, Ti D, Dong L, Chen D, et al. Hypoxia regulates the therapeutic potential of mesenchymal stem cells through enhanced autophagy. Int J Low Extrem Wounds. 2015;14(1):63–72.
Kim H, Kwon S. Dual effects of hypoxia on proliferation and osteogenic differentiation of mouse clonal mesenchymal stem cells. Bioprocess Biosyst Eng. 2021;44(9):1831–9.
Li B, Li C, Zhu M, Zhang Y, Du J, Xu Y, Liu B, et al. Hypoxia-induced mesenchymal stromal cells exhibit an enhanced therapeutic effect on radiation-induced lung injury in mice due to an increased proliferation potential and enhanced antioxidant ability. Cell Physiol Biochem. 2017;44(4):1295–310.
Abu-El-Rub E, Sareen N, Yan W, Alagarsamy KN, Rafieerad A, Srivastava A, Desiderio V, et al. Hypoxia-induced shift in the phenotype of proteasome from 26S toward immunoproteasome triggers loss of immunoprivilege of mesenchymal stem cells. Cell Death Dis. 2020;11(6):419.
Lee HH, Chang CC, Shieh MJ, Wang JP, Chen YT, Young TH, Hung SC. Hypoxia enhances chondrogenesis and prevents terminal differentiation through PI3K/Akt/FoxO dependent anti-apoptotic effect. Sci Rep. 2013;3:2683.
Lee SM, Jun DW, Kang HT, Oh JH, Saeed WK, Ahn SB. Optimal hypoxic preconditioning of human embryonic stem cell-derived mesenchymal stem cells (hES-MSCs) and their characteristics. Int J Stem Cells. 2021;14(2):221–8.
Xue C, Shen Y, Li X, Li B, Zhao S, Gu J, Chen Y, et al. Exosomes derived from hypoxia-treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. Stem Cells Dev. 2018;27(7):456–65.
Griffon DJ, Cho J, Wagner JR, Charavaryamath C, Wei J, Wagoner JA. Effects of hypoxia and chitosan on equine umbilical cord-derived mesenchymal stem cells. Stem Cells Int. 2016;2016:2987140.
Sugrue T, Lowndes NF, Ceredig R. Hypoxia enhances the radioresistance of mouse mesenchymal stromal cells. Stem Cells. 2014;32(8):2188–200.
Peng L, Shu X, Lang C, Yu X. Effects of hypoxia on proliferation of human cord blood-derived mesenchymal stem cells. Cytotechnology. 2016;68(4):1615–22.
Liu J, He J, Ge L, Xiao H, Huang Y, Zeng L, Jiang Z, et al. Hypoxic preconditioning rejuvenates mesenchymal stem cells and enhances neuroprotection following intracerebral hemorrhage via the miR-326-mediated autophagy. Stem Cell Res Ther. 2021;12(1):413.
Guo Y, He J, Wu J, Yang L, Dai S, Tan X, Liang L. Locally overexpressing hepatocyte growth factor prevents post-ischemic heart failure by inhibition of apoptosis via calcineurin-mediated pathway and angiogenesis. Arch Med Res. 2008;39(2):179–88.
Wang X, Hu Q, Mansoor A, Lee J, Wang Z, Lee T, From AH, et al. Bioenergetic and functional consequences of stem cell-based VEGF delivery in pressure-overloaded swine hearts. Am J Physiol Heart Circ Physiol. 2006;290(4):H1393–405.
Xie X, Yang X, Wu J, Tang S, Yang L, Fei X, Wang M. Exosome from indoleamine 2,3-dioxygenase-overexpressing bone marrow mesenchymal stem cells accelerates repair process of ischemia/reperfusion-induced acute kidney injury by regulating macrophages polarization. Stem Cell Res Ther. 2022;13(1):367.
Kong D, Xu H, Chen M, Yu Y, Qian Y, Qin T, Tong Y, et al. Co-encapsulation of HNF4α overexpressing UMSCs and human primary hepatocytes ameliorates mouse acute liver failure. Stem Cell Res Ther. 2020;11(1):449.
Mao Q, Lin C, Gao J, Liang X, Gao W, Shen L, Kang L, et al. Mesenchymal stem cells overexpressing integrin-linked kinase attenuate left ventricular remodeling and improve cardiac function after myocardial infarction. Mol Cell Biochem. 2014;397(1–2):203–14.
Madonna R, Taylor DA, Geng YJ, De Caterina R, Shelat H, Perin EC, Willerson JT. Transplantation of mesenchymal cells rejuvenated by the overexpression of telomerase and myocardin promotes revascularization and tissue repair in a murine model of hindlimb ischemia. Circ Res. 2013;113(7):902–14.
Liu P, Feng Y, Dong D, Liu X, Chen Y, Wang Y, Zhou Y. Enhanced renoprotective effect of IGF-1 modified human umbilical cord-derived mesenchymal stem cells on gentamicin-induced acute kidney injury. Sci Rep. 2016;6:20287.
Si X, Liu X, Li J, Wu X. Transforming growth factor-β1 promotes homing of bone marrow mesenchymal stem cells in renal ischemia-reperfusion injury. Int J Clin Exp Pathol. 2015;8(10):12368–78.
Zheng YB, Zhang XH, Huang ZL, Lin CS, Lai J, Gu YR, Lin BL, et al. Amniotic-fluid-derived mesenchymal stem cells overexpressing interleukin-1 receptor antagonist improve fulminant hepatic failure. PLoS ONE. 2012;7(7): e41392.
Acknowledgements
Not applicable
Funding
No funders.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and the main idea of the work. M.S.K, E.M, A.K, S.R, A.S, N.N. F.E, and S.J drafted the main text, figures, and tables. F.E and S.J supervised the work and provided the comments and additional scientific information. All authors read and approved the final version of the work to be published.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kahrizi, M.S., Mousavi, E., Khosravi, A. et al. Recent advances in pre-conditioned mesenchymal stem/stromal cell (MSCs) therapy in organ failure; a comprehensive review of preclinical studies. Stem Cell Res Ther 14, 155 (2023). https://doi.org/10.1186/s13287-023-03374-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-023-03374-9