Abstract
Background
The long-term survival after vascularized composite allotransplantation (VCA) is often limited by systemic rejection as well as the adverse effects of immunosuppressants. The stromal vascular fraction (SVF) can be expanded to produce adipose-derived stem cells (ADSC) which represents a combination of endothelial cells, preadipocytes, immune cells, and ADSC. It has been demonstrated that ADSC possess consistently reliable clinical results. However, literature is scarce regarding SVF in VCA. This study seeks to determine the impact of ex vivo allograft pretreatment in combination with SVF cells in the ability to promote composite tissue allotransplantation immunotolerance.
Methods
A rat hind limb allotransplant model was used to investigate the influence of ex vivo pretreatment of SVF and ADSC on VCA survival. Intravascular cell-free saline, ADSC, or SVF was infused into the models with immunosuppressants. The histopathological examination and duration that the allografts went without displaying symptoms of rejection was documented. Peripheral T lymphocytes and Tregs were quantified with flow cytometry while allotissue expressions of CD31 were quantified with immunohistochemical staining (IHC). ELISA was used to detect vascular endothelial growth factor (VEGF)-A as well as anti- and pro-inflammatory cytokines.
Results
We demonstrated that ex vivo treatment of allografts with SVF or ADSC prolonged allograft survival in contrast to medium control cohorts. There were also enhanced levels of immunomodulatory cytokines and increased VEGF-A and CD31 expression as well as reduced infiltration and proliferation of T lymphocytes along with raised Treg expressions.
Conclusion
These studies demonstrated that adipose-derived cellular therapies prolong graft survival in an allogenic hind limb transplantation model and have the potential to establish immunotolerance.
Similar content being viewed by others
Background
Vascularized composite allotransplantation (VCA) is an exciting modality for amputees and patients with large volume soft tissue loss that is not amenable to conventional reconstructive surgeries. It has the ability to restore function and appearance of damaged tissues in these patients [1, 2]. The ability of this modality of treatment is limited by the requirement of multiple immunosuppressant medications to allow for the grafts to survive in the recipient’s body [3, 4]. It is therefore essential for newer therapies that are able to effectively suppress the alloreactive immune response, thus opening up this treatment method to a larger population.
Several studies have attempted to promote peripheral tolerance and to modulate the local inflammatory immune responses within the graft using cellular therapy [5, 6]. Multiple cells types have been used for immune modulation or suppression to treat a range of maladies [7, 8]. It has been reported that bone marrow-derived stromal cells (BMSC) are immunosuppressive and inhibit the proliferation of alloreactive T cells [9]. BMSC have been intensely researched as a treatment modality in several animal and clinical studies across a myriad of diseases [10,11,12,13,14]. A previous study in a rat model hind limb transplantation demonstrated that infused BMSC into the allografts prolonged survival of transplanted hind limbs [15].
Another therapy that is derived from the nonadipocyte portion of adipose tissue is known as stromal vascular fraction (SVF). In 2001, a significant population of adipose-derived mesenchymal stem cells (ADSC) were extracted from a SVF isolate after being allowed to culture for several days [16]. Like BMSC, ADSC possess potent regenerative and immunomodulatory abilities. Their accessibility and excellent safety profile make them a promising alternative to BMSC-based treatments [17]. Our group previously demonstrated the important adjuvant role of ADSC when used as a pretreatment for allografts to induce VCA immunotolerance [18]. Freshly isolated SVF cells also possess substantial regenerative and immunosuppressive properties [19, 20]. This isolate is comprised of various immunomodulatory cells, which include ADSC, pre-adipocytes, pericytes, lymphocytes, smooth muscle cells, macrophages, endothelial cells, and endothelial precursor cells [16, 21, 22]. As these cells demonstrate equivalent regenerative and immunomodulatory abilities, and are logistically much easier to procure, they offer a viable alternative to ADSC treatments. However, evidence of SVF with immunomodulatory potential is lacking in the field of VCA.
In this study, we researched the prevention of allograft rejection through a unique perspective. We researched the ability and underlying mechanisms that allow ADSC and SVF to modulate the immune system to prevent allograft rejection in a VCA model.
Methods
Animals and immunosuppressive agents
A rodent hind limb allotransplant model was employed to investigate the influence of adipose-derived cellular therapies on VCA survival. Shanghai Sippr-BK Laboratory Animal Co. Ltd. (Shanghai, China) supplied 16 male Lewis and 8 male Brown Norway rats weighing 200–250 g for this study. Two different recipients received 1 hind limb each from the same donor. Group I (n = 4) was the media control group that did not receive cell therapy. Allografts of group II (n = 6) received ADSC ex vivo treatment. Allografts of group III (n = 6) received SVF ex vivo treatment. All rats received 5 days of post-operative oral cyclosporine (Sigma Aldrich, USA) at a dose of 16 mg/kg. All animal procedures were approved by the ethics committee of Shanghai Ninth People’s hospital Affiliated to Shanghai Jiao Tong University, School of Medicine. This work was carried out in strict compliance to the guidelines of the Laboratory Animal Manual of the NIH Guide to the Care and Use of Animals.
Stromal vascular fraction isolation
Recipient Lewis rats aged 4–6 weeks were subjected to extraction of armpit and inguinal subcutaneous adipose tissues. These samples were diced into tiny pieces before being exposed for 1 h in serum-free Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, Logan City, UT) supplemented by 0.1% collagenase (NB4, Serva, France) at 37°C. Tissue residues were removed by filtering the cell through a 40-mm nylon filter mesh (BD Falcon, Bedford, MA). The filtrate was centrifuged, and cell pellets were resuspended in phosphate buffered saline (PBS; Hyclone Laboratories, Inc., Logan, UT).
Isolation, expansion, and characterization of ADSC
Rat ADSC was extracted from inguinal adipose tissue of Lewis rats (4 to 6 weeks old). These samples were first digested by 0.1% collagenase (NB4, Serva, France) for 1 h under gentle agitation at 37 °C, before being filtered through a 40-μm nylon strainer (Falcon, Corning, USA). The resultant substrate was subjected to centrifugation before the cell pellets were resuspended in 10 cm2 culture plates at 37 °C, under a 5% CO2 atmosphere which contained low glucose complete Dulbecco’s Modified Eagle Medium supplemented with 100 U/mL penicillin and 100 mg/mL streptomycin (Gibco, Invitrogen, USA) and 10% fetal bovine serum (FBS; Sciencell, USA). Further studies utilized cell passages 2 through 4.
Flow cytometric analysis of the cell surface marker profile of ADSC was performed with a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA) using primary antibodies for CD 90, CD 45, and CD44 (BioLegend Inc., San Diego, USA). A differentiation medium (Cyagen Bioscience, Inc., Guangzhou, China) was used to induce adipogenesis, osteogenesis, and chondrogenesis. Oil red, alizarin red, and alcian blue stains were used to identify differentiated cells.
Procedure of hind limb transplantation, ex vivo allograft engineering, and general observations
The rat hind limb transplantation model described by previous study was used [23]. All mice were anesthetized with isoflurane. Dissection of the femoral artery was done on the donor rat prior to amputation mid-thigh of the hind limb. Sixty units/mL heparinized saline (Sigma Aldrich, St. Louis, MO, USA) was used to flush the donor’s osteomyocutaneous flap before limb vasculature was perfused with 1 million ADSC or SVF cells in cold saline or cell-free saline alone, as previously described [4]. The Lewis rat receiver received similar operation procedures prior to transplantation of the donor hind limb onto the recipient. An 18-gauge needle was used as intramedullary rod to perform femoral osteosynthesis. 12-0 nylon sutures were used to anastomose femoral vessels under an operating microscope. Muscle and skin were closed with intermittent sutures using 3-0 nylon thread.
Recipient rats were housed in a plastic cage where they could walk freely. Mice were monitored for symptoms of acute organ rejection daily. The clinical experimental end point of vascularized composite allotransplantation rejection was determined to be the formation of entire donor skin area epidermolysis, desquamation, exudation, eschar formation, and necrosis.
Histological analyses of transplant allografts
Skin and muscle biopsies were obtained from the transplanted hind limb on days 7 and 15 post-transplantation or upon the occurrence of clinical picture of rejection. All biopsies were first immersed for 24 h of 4% paraformaldehyde prior to being embedded in paraffin. Tissue blocks were then sectioned and stained with hematoxylin and eosin using standard procedures.
In order to label cells for CD31, tissue sections were first processes for heat-induced antigen retrieval in a 10 mM citrate buffer (pH 7.3) prior to incubation with anti-rat CD31 (cat no. GB13063; 1:200 dilution; Servicebio, Wuhan, China). A chromogenic substrate solution (Beyotime Institute of Biotechnology, Haimen, China) was used to visualize samples. A final staining with hematoxylin was then done. Measurements of the integrated optical density (IOD) of CD31-stained area and blood vessel numbers were done using Image-Pro Plus version 6.0 (Media Cybernetics, Silver Spring, MD) with 3 randomly selected areas used to calculate the IOD/area and the numbers of blood vessel for each sample.
Flow cytometry analysis of peripheral T cells
Tail veins of the recipient rats were cannulated to extract 50 μL peripheral blood that was then incubated for 15 min in heparinized Eppendorf tubes with red blood cell lysate (Beyotime, Shanghai, China). Then, cells were stained in the dark for 30 min at 4°C with the following antibodies: PerCP-Cy5.5-CD45RA, APC-CD3, PE/Cy7-CD4, PE-CD8a, and V500-CD25 (BD Pharmingen, Franklin Lake, WI, USA). For Treg analysis, Foxp3/Transcription Factor Staining Buffer (eBioscience, San Diego, CA, USA) was first used to fix and permeabilize the cells before they were subjected to incubation with the anti-FoxP3 monoclonal antibody (eBioscience). After being processed, PBS was used to rinse samples twice before being resuspended. Flow cytometry (BD FACSCanto II), BD Diva software, and FlowJo software were used to analyze resultant data.
Detection of cytokine levels in peripheral blood
Lewis rats recipients were subjected to withdrawal of serum samples which were then maintained at − 80 °C, until further experiments were carried out. These samples were measured for levels of vascular endothelial growth factor (VEGF)-A, tumor necrosis factor (TNF)-α, transforming growth factor-β (TGF-β), and serum interleukin-10 (IL-10) with an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA) in compliance to protocols stipulated by the manufacturer.
Statistical analysis
Data analysis was carried out with the GraphPad Prism 6 software (La Jolla, CA). The log-rank test was used to assess survival rates. Analysis of the single measurable parameters was assessed with the one-way ANOVA test with post hoc analysis conducted using Tukey’s multiple comparison test. For the flow cytometric and anti- and pro-inflammatory cytokines analyses, statistical analysis of all groups was performed using two-way ANOVA with post hoc analysis conducted using Tukey’s multiple comparison test. A p value of < 0.05 was considered to reflect statistical significance.
Result
Adipose-derived cellular therapies prolong allograft survival
Lewis rat-derived inguinal adipose tissue was used for SVF and ADSC isolation. ADSC were multiplied using protocols that were previously reported [24]. The presence of negatively staining CD45 and positively staining mesenchymal stem cell surface markers CD90 and CD44 confirmed ADSC. ADSC were then stimulated to differentiate into adipogenic, osteogenic, and chondrogenesis lineages (Figure S1).
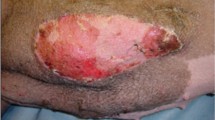
The results showed that hind limb allografts demonstrated obvious symptoms of grade III rejection, including necrosis, exudation, epidermolysis, and histological desquamation at a mean of 15.5 days in the control group. The average survival time of the allografts was 25.7 days in the ADSC treatment group. Allograft recipients treated with SVF ex vivo perfusion survived for a mean of 35 days, indicating a statistically significant increased survival time between the three groups (Fig. 1). Animal models that suffered from surgical failure, such as venous thrombosis, were excluded from statistical analysis.
Adipose-derived cells alleviate the rejection response of allotransplants
Daily observations of the transplanted limb were done to watch out for signs of rejection. At 7 and 15 days post-operatively, full-thickness biopsies of donor skin and muscle were obtained. Figure 2 demonstrates that no notably differences between all three groups at 7 days post-operatively. However, those in the control group began to demonstrate deranged muscle and skin tissue architecture and diffuse mononuclear cells infiltration at 15 days post-operatively. In the ADSC group, these changes were present but to a lesser degree. None of these features were noted in the SVF group.
Robust augmentation of anti-inflammatory cytokine expression modulates immune cell frequencies in blood with adipose-derived cells treatment
The concentrations of pro-inflammatory cytokine TNF-α and anti-inflammatory cytokines IL-10 and TGF-β in peripheral blood were determined by ELISA on postoperative day 7 and day 12. Those in the ADSC group had raised IL-10 only on day 12 post-procedure while those in the SVF group had raised IL-10 levels noted since as early as day 7 (Fig. 3a). Besides, those in the SVF group also demonstrated raised TGF-β levels on postoperative day 7 and 12 (Fig. 3b). In contrast to the control group, lower TNF-α levels were noted in both the SVF and ADSC-treated groups at 7 days post-operatively while those in the SVF group had persistently low levels of this marker even on day 12 (Fig. 3c).
T cell subsets of three groups were measured on day 10 and day 14 post-procedure (Fig. 4). Both SVF and ADSC had lower levels of CD4+ T cells at both the aforementioned time points in contrast to the control group (Fig. 4b). The proportion of CD8+ T cells in SVF treated groups was also noted to be low on postoperative day 10 (Fig. 4c). Both SVF and ADSC groups had higher Treg levels at day 14 post-operatively in contrast to the control group (Fig. 4d).
Gating strategy and percentages of T cells. a Gating strategy for CD4+, CD8+, and Treg cells. b Percentage of CD4+ T cells of three groups at post-operative days 10 and 14. c Percentage of CD8+ T cells of three groups at post-operative days 10 and 14. d Percentage of Treg cells of three groups at post-operative days 10 and 14 (*P < 0.05, **P < 0.01, ***P < 0.001)
SVF treatment promotes vascularization
We observed CD31-expressing endothelial cells in immunohistochemical (IHC) stains done on allograft skin tissue at postoperative day 7 (Fig. 5). Stronger CD31 staining was noted in the SVF group in contrast to the control and ADSC group (Fig. 5a). Those in the SVF group also had much larger integrated optical density (IOD) of CD31-stained area and blood vessel numbers compared to the control and ADSC treated groups (Fig. 5b, c). We also tested blood VEGF-A levels at postoperative day 7 and found its expression to be significantly raised in the SVF group compared to the other two groups (Fig. 5d).
CD31 expression in allografts and VEGF-A expression in serum. a Images of allograft skin tissue subjected to immunohistochemical of three groups at post-operative day 7 (scale bar = 50 μm). b Quantitative analysis of integrated optical density (IOD) of CD31-stained area. c Quantification of blood vessel number in CD31-stained tissue sections. d Concentrations of VEGF-A in serum (*P < 0.05, **P < 0.01, ***P < 0.001)
Discussion
Lifelong administration of immunosuppressive drugs after VCA is a major impediment to the benefits of VCA reconstructive surgery. Therefore, the induction and maintenance of donor-specific tolerance towards allografts is an important field of research.
For decades, there have been several studies regarding the use of cellular therapy to promote peripheral tolerance or to modulate local inflammatory immune responses within the graft in transplantation research. Mesenchymal stem cells have been found to possess immunomodulatory effects in VCA. Kuo et al. [25] reported on the benefits of the systemic delivery of ADSC on prolonged survival of swine allografts. Moreover, both Jeong et al. [26] and Plock et al. [27] found out that graft survival time was significantly improved with multiple (as opposed to single dose) post-transplant injections of donor-derived ADSC, an effect that is attributed to increased circulating Treg numbers and reduced allograft infiltration of leukocytes. Our group previously demonstrated the importance of ADSC pre-treatment as an adjunct for improving allograft survival in VCA [18]. This article compares ADSC to SVF pre-treatment in improving allograft survival. Interestingly, we found that the latter was more efficient in enhancing allograft survival. We postulate that this effect is mediated by decreased proliferation and tissue infiltration of lymphocytes as well as modulation of immunomodulatory cytokines IL-10 and TGF-β and increased Treg expression levels. Furthermore, SVF treatment increases the expression of VEGF-A in serum and CD31 in cells of allografts which indicates that SVF could reduce the graft healing time by promoting neovascularization.
A majority of studies focus on immunomodulatory properties regarding ADSC, with a paucity of literature surrounding SVF. ADSC have the advantage of a uniform preparation. However, the ADSC phenotype is easily influenced by their microenvironment [28, 29]. The immunomodulatory properties of ADSC may be lost or seriously diminished during long-term storage [30]. In contrast, freshly isolated SVF do not require in vitro culture and expansion, reducing the risks of contamination and phenotype variation [31]. Moreover, SVF contains a multitude of regulatory immune cells that are involved in the maintenance of immune homeostasis in the adipose tissue microenvironment [32]. These cells improve anti-inflammatory capabilities of other resident cells in adipose tissue, collectively [33]. This impact is likely to continue after isolation, suggesting that it may be essential in maintaining the immunomodulatory functions of the post-transplant environment. Furthermore, SVF treatment could shorten the graft healing time by promoting neovascularization [34, 35]. In sum, we believe that SVF is more appropriate in modulating acute rejection-associated inflammation, thereby inducing peripheral allograft tolerance and vastly improving the overall outcome of VCA recipients.
As a preliminary exploration, this study only contrasts the effectiveness of ADSC and SVF. Future studies regarding the optimal number of injected cells and the time interval of injected cells as well as the relationship between systemic and local treatments are necessary.
Conclusion
Allograft pre-treatment with SVF enhances allograft survival through suppression of T lymphocyte proliferation and infiltration and augmented immunomodulatory cytokine secretion as well as induction of Treg expression and CD31 and VEGF-A expressions. This treatment modality may be a key adjunct in the introduction and maintenance of immunotolerance in VCA.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Abbreviations
- VCA:
-
Vascularized composite allotransplantation
- SVF:
-
Stromal vascular fraction
- BMSC:
-
Bone marrow-derived stromal cells
- ADSC:
-
Adipose-derived stem cells
- IHC:
-
Immunohistochemical staining
- TNF:
-
Tumor necrosis factor
- TGF:
-
Transforming growth factor
- IL:
-
Interleukin
- VEGF:
-
Vascular endothelial growth factor
- IOD:
-
Integrated optical density
References
Shores JT, Brandacher G, Lee WP. Hand and upper extremity transplantation: an update of outcomes in the worldwide experience. Plast Reconstr Surg. 2015;135(2):351e–60e.
Cetrulo CL Jr, et al. The advent of vascularized composite allotransplantation. Clin Plast Surg. 2017;44(2):425–9.
Bamoulid J, et al. The need for minimization strategies: current problems of immunosuppression. Transpl Int. 2015;28(8):891–900.
Soares MA, et al. Ex vivo allotransplantation engineering: delivery of mesenchymal stem cells prolongs rejection-free allograft survival. Am J Transplant. 2018;18(7):1657–67.
Carretero-Iglesia L, et al. Comparative study of the immunoregulatory capacity of in vitro generated tolerogenic dendritic cells, suppressor macrophages, and myeloid-derived suppressor cells. Transplantation. 2016;100(10):2079–89.
Schweizer R, Gorantla VS, Plock JA. Premise and promise of mesenchymal stem cell-based therapies in clinical vascularized composite allotransplantation. Curr Opin Organ Transplant. 2015;20(6):608–14.
Gonzalez-Rey E, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69(1):241–8.
Almasbak H, Aarvak T, Vemuri MC. CAR T cell therapy: a game changer in cancer treatment. J Immunol Res. 2016;2016:5474602.
Rasmusson I, et al. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76(8):1208–13.
Ding Y, et al. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes. 2009;58(8):1797–806.
Peng Y, et al. Donor-derived mesenchymal stem cells combined with low-dose tacrolimus prevent acute rejection after renal transplantation: a clinical pilot study. Transplantation. 2013;95(1):161–8.
Pileggi A, et al. Mesenchymal stromal (stem) cells to improve solid organ transplant outcome: lessons from the initial clinical trials. Curr Opin Organ Transplant. 2013;18(6):672–81.
Shi M, et al. A pilot study of mesenchymal stem cell therapy for acute liver allograft rejection. Stem Cells Transl Med. 2017;6(12):2053–61.
Tan J, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307(11):1169–77.
Ikeguchi R, et al. Recipient bone marrow-derived stromal cells prolong graft survival in a rat hind limb allotransplantation model. Microsurgery. 2017;37(6):632–40.
Zuk PA, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28.
Valencia J, et al. Comparative analysis of the immunomodulatory capacities of human bone marrow- and adipose tissue-derived mesenchymal stromal cells from the same donor. Cytotherapy. 2016;18(10):1297–311.
Wang Y, et al. Ex-vivo treatment of allografts using adipose-derived stem cells induced prolonged rejection-free survival in an allogenic hind-limb transplantation model. Ann Transl Med. 2020;8(14):867.
Han S, et al. Adipose-derived stromal vascular fraction cells: update on clinical utility and efficacy. Crit Rev Eukaryot Gene Expr. 2015;25(2):145–52.
Dong Z, et al. The survival condition and immunoregulatory function of adipose stromal vascular fraction (SVF) in the early stage of nonvascularized adipose transplantation. PLoS One. 2013;8(11):e80364.
Yoshimura K, et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34(9):1178–85.
Astori G, et al. “In vitro” and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J Transl Med. 2007;5:55.
Oda H, et al. Relative antigenicity of components in vascularized composite allotransplants: an experimental study of microRNAs expression in rat hind limb transplantation model. Microsurgery. 2019;39(4):340–8.
Bunnell BA, et al. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45(2):115–20.
Kuo YR, et al. Recipient adipose-derived stem cells enhance recipient cell engraftment and prolong allotransplant survival in a miniature swine hind-limb model. Cell Transplant. 2017;26(8):1418–27.
Jeong SH, Ji YH, Yoon ES. Immunosuppressive activity of adipose tissue-derived mesenchymal stem cells in a rat model of hind limb allotransplantation. Transplant Proc. 2014;46(5):1606–14.
Plock JA, et al. The influence of timing and frequency of adipose-derived mesenchymal stem cell therapy on immunomodulation outcomes after vascularized composite allotransplantation. Transplantation. 2017;101(1):e1–e11.
Yoshimura K, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208(1):64–76.
Mitchell JB, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24(2):376–85.
Kim N, Cho SG. Overcoming immunoregulatory plasticity of mesenchymal stem cells for accelerated clinical applications. Int J Hematol. 2016;103(2):129–37.
Patrikoski M, et al. Different culture conditions modulate the immunological properties of adipose stem cells. Stem Cells Transl Med. 2014;3(10):1220–30.
Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12(1):15–28.
Cautivo KM, Molofsky AB. Regulation of metabolic health and adipose tissue function by group 2 innate lymphoid cells. Eur J Immunol. 2016;46(6):1315–25.
Carstens MH, et al. Non-reconstructable peripheral vascular disease of the lower extremity in ten patients treated with adipose-derived stromal vascular fraction cells. Stem Cell Res. 2017;18:14–21.
Ataman MG, et al. The effect of adipose stromal vascular fraction on transverse rectus abdominis musculocutaneous flap: an experimental study. J Plast Surg Hand Surg. 2016;50(5):272–80.
Acknowledgements
Not applicable.
Funding
This study was supported by the Laboratory of tissue engineering at Shanghai 9th People’s Hospital, Shanghai Jiao Tong University, and Fundamental Research Program Funding of National Natural Science Foundation of China (No. 81871576 and 82002065).
Author information
Authors and Affiliations
Contributions
JTC and YMW designed the research and performed the experiments. HYH prepared the figures and analyzed the data. YX and SBW designed the research, performed the experiments, analyzed the data, and drafted the manuscript. JY designed the study, discussed the data, and wrote and reviewed the manuscript. All authors have read and approved the final version of the manuscript for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by the ethics committee of Shanghai Ninth People’s hospital Affiliated to Shanghai Jiao Tong University, School of Medicine (Shanghai, China), following the guidelines of the Laboratory Animal Manual of the NIH Guide to the Care and Use of Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Characterization of ADSC. A. Flow cytometric analysis of ADSC, demonstrating proportions of CD90+ (99.5%), CD44+ (99.7%) and CD45+ (1.45%) cells. B. ADSC images as visualized through microscopy (scale bar =100 μm), C. Oil red O-stained adipocytes 3 weeks after induction (scale bar = 40 μm), D. Alizarin red-stained osteocytes 2 weeks after induction (scale bar = 100 μm). E. Alcian blue-stained chondrocytes 4 weeks after induction (scale bar = 50 μm).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, J., Wang, Y., Hu, H. et al. Adipose-derived cellular therapies prolong graft survival in an allogenic hind limb transplantation model. Stem Cell Res Ther 12, 94 (2021). https://doi.org/10.1186/s13287-021-02162-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-021-02162-7