Abstract
Background
Nicotinamide adenine dinucleotide (NAD+) is a critical molecule involved in various biological functions. Poly (ADP-ribose) polymerase 1 (PARP1) and sirtuin 1 (SIRT1) affect cellular NAD+ levels and play essential roles in regulating metabolism. However, there has been little research on the effects of PARP1 and SIRT1 crosstalk during senescence.
Methods
We isolated endothelial progenitor cells (EPCs) from human umbilical cord blood and treated them with a PARP1 inhibitor (PJ34).
Results
Using a stress-induced premature aging model built by H2O2, transfection with adenoviral vectors, and Western blot analysis, we observed that PJ34 treatment preserved intracellular NAD+ levels, increased SIRT1 activity, decreased p53 acetylation, and improved the function of stress-induced premature aging EPCs.
Conclusions
Our results suggest that PJ34 improves the function of aging-induced EPCs and may contribute to cellular therapies for atherosclerosis.
Similar content being viewed by others
Background
Atherosclerosis (AS) is a major cause of cardiovascular disease, and endothelial injury and dysfunction caused by aging are classical risk factors for AS [1,2,3]. In recent years, several studies have revealed that endothelial progenitor cells (EPCs) play a crucial role in the replacement of injured vascular endothelial cells. Patients with AS display a significant decrease in the number of EPCs in their peripheral blood [4]. Animal experiments have demonstrated that EPCs can repair injured endothelial cells and improve angiogenesis during ischemia [5]. Further research showed that the ability of EPCs to replace impaired endothelial cells depends on their number and functionality [6]. However, EPC function gradually degenerated with senescence, showing decreased cellular viability, slower migration, and degressive angiogenic ability [7,8,9,10]. Therefore, improving the function of aging EPCs may aid in the prevention of atherosclerosis and other vascular diseases caused by endothelial damage.
Nicotinamide adenine dinucleotide (NAD+) is a critical molecule involved in various biological functions such as energy generation, metabolism, and DNA repair. NAD+ also plays an important role during aging because it participates in oxidation-reduction (redox) reactions in the tricarboxylic acid (TCA) cycle [11,12,13,14].
Poly (ADP-ribose) polymerase 1 (PARP1) is a genome-stabilizing enzyme that catalyzes the covalent transfer of mono- or poly-adenosine diphosphate (ADP) units from NAD+ to glutamate or aspartate residues within target proteins, resulting in protein-conjugated chains of poly ADP-ribose (PAR) polymers [15]. PARP1 is involved in DNA repair and, in the presence of pathological DNA damage, its activity can result in excessive NAD+ consumption [16,17,18].
Sirtuin 1 (SIRT1) is a redox-sensitive protein involved in a wide range of cellular processes, including aging, oxidative stress responses, metabolism, circadian rhythm regulation, and proliferation [19,20,21,22]. It is a NAD+-dependent deacetylase that targets several transcription factors, including forkhead box O3, tumor protein p53, nuclear factor κB, and peroxisome proliferator-activated receptor γ coactivator 1 alpha. Therefore, its activity plays an important role in health maintenance [23, 24].
PARP1 and SIRT1 affect NAD+ levels and play crucial roles in regulating cellular metabolism [25]. However, there is little research on the effects of PARP1 and SIRT1 crosstalk on senescence. To study its effects on aging EPCs, we treated EPCs obtained from human umbilical cord blood with a PARP1 inhibitor (PJ34) and assayed the effects of PARP1 and SIRT1 activity on EPC senescence.
PJ34, a PARP inhibitor, can inhibit the activation from PARP to PAR and thereby preserve intracellular NAD+ levels, which improves mitochondrial function [26].
Methods
Study design
The overall objective of this study was to determine whether PJ34 is able to inhibit EPC senescence and, if so, the mechanism. Firstly, we used H2O2 to build a stress-induced premature aging model [27]. Endogenous DNA damage induced by sublethal oxidative stress is responsible for the initiation and progression of the senescent phenotype [28]. According to the changes of H2O2-activated PAR and the cellular states observed under a microscope, we selected an optimum concentration of H2O2. Furthermore, we used several concentrations of PJ34 to inhibit the activation of PARP and selected an optimum concentration on the basis of changes in PAR. Next, we established four treatments to study the effects of PJ34 on aging EPCs. Cells treated with PJ34 were used to measure the effects of PJ34 on young EPCs, cells treated with H2O2 were used to induce premature cellular senescence, and cells treated with H2O2 + PJ34 were compared with the cells treated with H2O2 to verify the effects of PJ34 on aging EPCs. Untreated cells were used as a negative control. Finally, we used SIRT1 short-hairpin RNA (Ad-sh-SIRT1) to silence SIRT1 expression in EPCs and measured the effects of PJ34 again. We measured and evaluated PARP1 activity by assaying the production of PAR [15, 26]. SIRT1 activity was evaluated by analyzing p53 acetylation [29, 30]. EPC functionality changes with senescence were evaluated by a series of functional experiments. For example, senescence-associated beta-galactosidase (SA-β-gal) staining was used to identify cellular senescence directly [31, 32], cell counting kit (CCK)-8 was used to analyze cell viability [7], transwell trials were used to observe cell migration [8, 9], and Matrigel angiogenesis assays were used to determine the angiogenic ability [10].
EPC isolation and culture
The Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine approved this study. EPCs were isolated from human umbilical cord blood by density gradient centrifugation with Histopaque-1077 (Sigma). We gently added human blood to the separation solution and centrifuged at 2000 rpm for 20 min at 4 °C. After density gradient centrifugation, the uppermost layer contains the serum, while the lowermost layer is composed of red blood cells, and the middle layer of white blood cells is suspended in the separation liquid. We withdrew the white blood cells, resuspended these in complete endothelial cell growth medium (EGM)-2 (Lonza), and then seeded them in six-well plates. Cells from approximately 10 mL of cord blood were plated per well. The medium was changed every 3 days, and cells were subcultured at a 1:3 ratio. EPC endothelial markers were detected by immunofluorescence and flow cytometry. For immunofluorescence, antibodies against CD31, CD34, von Willebrand factor (vWF), vascular endothelial growth factor receptor 2 (VEGFR2), and CD133 were purchased from Cell Signaling Technology. For flow cytometry, antibodies against CD34, CD133, and VEGFR2 were purchased from Invitrogen.
Western blot analysis
Cellular proteins were extracted using radioimmunoprecipitation assay buffer, and protein concentrations were measured using the bicinchoninic acid method. Approximately 30 μg of protein per well was loaded on 8 or 10% gels for sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore), and sequentially detected by primary antibodies, secondary antibodies, and enhanced chemiluminescence (Millipore). Antibodies against PARP1, SIRT1, acetylated (ac)-p53, p53, and cyclin-dependent kinase inhibitor 1A (p21), as well as anti-mouse and anti-rabbit secondary antibodies, were purchased from Cell Signaling Technology. The anti-PAR antibody was purchased from Invitrogen. An anti-β-actin antibody (Cell Signaling Technology) was used as an internal control.
Cell viability assays
CCK-8 (Dojindo) was used to analyze cell viability after various treatments. Cells (1 × 104/well) were suspended in 100 μL fresh medium containing the various treatments and then seeded. After treatment, CCK-8 (10 μL/well) was added for 3 h of additional incubation, and the absorbance was measured at 450 nm.
Migration assays
Transwell plates (Corning) were used to observe cell migration. Complete fresh EGM-2 containing the various treatments (600 μL) was added to the bottom chambers, and 5 × 104 cells suspended in 200 μL serum-free medium containing the various treatments were added to the top chamber. After incubation at 37 °C for 12 h, transmigrated cells were fixed in 4% paraformaldehyde and stained with crystal violet. Three random microscopic fields were selected, and the stained cells were counted.
Matrigel angiogenesis assays
Matrigel™ (50 μL; BD Biosciences) was added to the wells of 96-well plates and incubated at 37 °C for 30 min. Then, 2 × 104 cells/well were seeded on the Matrigel and incubated at 37 °C. Images were acquired after 8 h.
SA-β-Gal assays
An SA-β-gal staining kit (Cell Signaling Technology) was used to identify cellular senescence. Cells were fixed for 20 min at room temperature and then incubated in staining solution overnight at 37 °C. Three random microscopic fields were selected, and the stained cells were counted.
Cellular reactive oxygen species (ROS) measurements
The reactive oxygen species assay kit (Sigma) was used to measure cellular ROS. Cells were seeded in a 96-well plate and incubated with 10 μM dichloro-dihydro-fluorescein diacetate (DCFH-DA) at 37 °C for 30 min. Cells were washed with phosphate-buffered saline three times, and then incubated in fresh medium containing the various treatments. After treatment, the absorbance was measured using a fluorescence enzyme-labeling device at excitation and emission wavelengths of 485 and 535 nm, respectively.
NAD+ measurements
The NAD/NADH assay kit (Abcam) was used to measure NAD/NADH levels. The standard solution was prepared according to the manufacturer’s protocol, and the NAD/NADH concentration was calculated using the standard curve. The NAD+ concentration was calculated according to the formula NAD+ = total NADH − NAD.
Adenovirus transfection
Adenoviral vectors containing green fluorescent protein (Ad-GFP) and Ad-sh-SIRT1 were purchased from Hanheng Biotechnology. Ad-GFP was used as a control. Cells were transfected for 6 h and then incubated with fresh medium for 48 h, after which protein expression levels were analyzed by Western blot, or additional treatments were performed.
Statistical analysis
The results are expressed as the mean ± standard error. Comparisons between two groups were performed using the independent samples t test. P values < 0.05 were considered statistically significant. All experiments were performed independently in triplicate at a minimum.
Results
Identification of EPCs and analysis of their protein expression
The isolated cells exhibited monolayer growth and cobblestone morphology, like typical EPCs (Fig. 1a). Most of the cells expressed CD31, CD34, vWF, VEGFR2, and CD133, which are considered typical markers of EPCs (Fig. 1b) [33]. Flow cytometry analysis revealed that the positive expression rates of CD34, VEGFR2, and CD133 were 90.8%, 93.8%, and 95.0%, respectively (Additional file 1: Figure S1). VEGFR2 was considered as a classical endothelial cell marker. CD34 and CD133 were considered as classical progenitor cell markers [34].
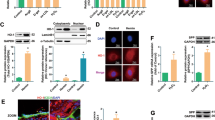
Identification of EPCs from human umbilical cord blood and protein expression during replicative aging. a EPCs (passage (P)1) after 2 weeks (100×). b Cells were characterized by immunofluorescence detection of CD31, CD34, von Willebrand factor (VWF), vascular endothelial growth factor receptor 2 (VEGFR2), and CD133. c Morphology of aging EPCs (100×). d, e Senescence-associated beta-galactosidase (SA-β-gal) staining at P3, P12, and P21. f–h Protein levels of poly (ADP-ribose) polymerase 1 (PARP1), poly ADP-ribose (PAR), sirtuin 1 (SIRT1), acetylated (ac)-p53, p53, and p21 during replicative senescence. *P < 0.05, **P < 0.01, ***P < 0.001, versus the control
To investigate the differences between young and senescent EPCs, we repeatedly subcultured EPCs for up to 21 passages (to P21). Senescence was confirmed through morphology and SA-β-gal assays. Cells at P1 were small and displayed typical cobblestone-like morphology. With repeated subculture, P21 cells became larger and more irregular in shape, and some were branch-like and polygonal or long and spindle-shaped (Fig. 1c). SA-β-gal positivity increased with repeated subculture (Fig. 1d, e).
We next examined the levels of SIRT1, PARP1, PAR, ac-p53, p53, and p21. Our results showed that with the repeated subculture SIRT1 decreased, while PAR, ac-p53, p53, and p21 increased. There was no significant difference in PARP1 levels between young and replicative aging EPCs (Fig. 1f–h).
Effects of H2O2 and PJ34 on protein expression
To verify the effects of crosstalk between PARP1 and SIRT1 on senescence, we first established a stress-induced premature aging model by treating young EPCs (P3–P5) with H2O2, and then used various concentrations of PJ34 to inhibit PARP1 activation. By Western blot, we found that H2O2 decreased SIRT1 levels and increased PAR synthesis by activating PARP1 (Fig. 2a, b). PJ34 inhibited PARP1 activation, resulting in decreased PAR synthesis, which may preserve intracellular NAD+ levels during cellular senescence (Fig. 2c, d).
Effects of H2O2 and PJ34 treatment on EPC protein expression. a, b Expression of sirtuin 1 (SIRT1), poly (ADP-ribose) polymerase 1 (PARP1), and poly ADP-ribose (PAR) as analyzed by Western blot. c, d Expression of PARP1, PAR, and acetylated (ac)-p53 as analyzed by Western blot. *P < 0.05, **P < 0.01, ***P < 0.001, versus the control
Effects of PJ34 on aging EPCs
To further verify the effects of crosstalk between PARP1 and SIRT1 on senescence, we first determined the proper concentrations of H2O2 and PJ34. EPCs (P3–P5) were incubated with normal fresh culture medium, 2 μM PJ34, 100 μM H2O2, or 2 μM PJ34 + 100 μM H2O2 for 6 h. After incubation, the culture medium was removed. Cells treated with PJ34 were incubated in fresh culture medium containing 2 μM PJ34 for 18 h, and cells subjected to other treatments were incubated in normal fresh culture medium for 18 h.
ROS production increased 6 h after H2O2 treatment (Fig. 3a). In addition, intracellular NAD+ levels decreased markedly in cells treated with H2O2 alone. However, the intracellular NAD+ levels of cells treated with H2O2 + PJ34 were relatively maintained, although they did decrease compared with the control (Fig. 3b). To investigate the effects of increased SIRT1 activity on aging EPCs, we measured several indicators related to senescence, including cell viability, SA-β-gal activity, tube formation ability, and migration ability. The cell viability, tube formation ability, and migration ability of cells treated with H2O2 decreased significantly but were partially restored in cells treated with H2O2 + PJ34. The rate of SA-β-gal positivity markedly increased in cells treated with H2O2, and this increase was attenuated in cells treated with H2O2 + PJ34 (Fig. 3c–k).
Functional alterations in senescent EPCs after PJ34 treatment. a Reactive oxygen species (ROS) production was assessed by DCFH-DA staining after 6 h of treatment. b Intracellular nicotinamide adenine dinucleotide (NAD+) levels were measured after 6 h of treatment. c Cell viability was evaluated by CCK-8 after 24 h of treatment. d, e Senescence was analyzed by senescence-associated beta-galactosidase (SA-β-gal) staining after 24 h of treatment. f–i Tube formation ability was evaluated on Matrigel after 24 h of treatment. j, k Migration ability was analyzed by Transwell assay after 24 h of treatment. *P < 0.05, **P < 0.01, ***P < 0.001, versus the control; #P < 0.05, ##P < 0.01, ###P < 0.001, versus H2O2 treatment
To further examine the effects of PJ34, we used Western blot analysis to confirm the alteration in SIRT1 activity. In the two groups of cells treated with PJ34, PARP1 levels were barely changed but PAR levels decreased markedly. SIRT1 expression levels were no different between the two groups of cells treated with H2O2, but ac-p53 expression levels and the ac-p53/p53 ratio declined significantly after PJ34 treatment. Increased p21 levels were consistent with increased ac-p53 activity (Fig. 4). Therefore, we suggest that PJ34 activates SIRT1 by inhibiting PARP1 activation and preserving intracellular NAD+ levels in EPCs, and possibly reverses the effects of aging.
Protein expression changes in senescent EPCs with PJ34 treatment. a–c Poly (ADP-ribose) polymerase 1 (PARP1), poly ADP-ribose (PAR), sirtuin 1 (SIRT1), acetylated (ac)-p53, p53, and p21 were detected by Western blot after 24 h of treatment. *P < 0.05, **P < 0.01, ***P < 0.001, versus the control; #P < 0.05, ##P < 0.01, ###P < 0.001, versus H2O2 treatment
Mechanism of PJ34 action in aging EPCs
To further verify the mechanism of PJ34 in aging EPCs, we used Ad-sh-SIRT1 to attenuate SIRT1 expression (Fig. 5a–c). After 48 h, we treated cells with H2O2 and PJ34 as above. ROS production change and the effects of PJ34 on the preservation of intracellular NAD+ levels were again observed (Fig. 5d, e). However, the effects of PJ34 on reversing aging EPC functionality almost completely disappeared (Fig. 5f–n). By Western blot, we found that the effects of PJ34 on PARP1 inhibition remained but the previously observed decreases in ac-p53 and p21 levels were not evident (Fig. 6). Therefore, PJ34 may improve the function of aging EPCs through PARP1 inhibition, preservation of intracellular NAD+ levels, and increased SIRT1 activity without increased SIRT1 expression.
Effects of PJ34 on senescent EPC functionality after silencing sirtuin 1 (SIRT1). a–c SIRT1 expression was detected by Western blot after 48 h of SIRT1 short-hairpin RNA (Ad-sh-SIRT1) transfection. d Reactive oxygen species (ROS) production was assessed by DCFH-DA staining. e Intracellular nicotinamide adenine dinucleotide (NAD+) levels were measured. f Cell viability was evaluated via the CCK-8 assay. g, h Senescence was analyzed by senescence-associated beta-galactosidase (SA-β-gal) staining. i–l Tube formation ability was evaluated by Matrigel assay. m, n Migration ability was analyzed by Transwell assay. *P < 0.05, **P < 0.01, ***P < 0.001, versus the control; #P < 0.05, ##P < 0.01, ###P < 0.001, versus H2O2 treatment. NS not significant
Effects of PJ34 on the protein levels of senescent EPCs after silencing sirtuin 1 (SIRT1). a–c Poly (ADP-ribose) polymerase 1 (PARP1), poly ADP-ribose (PAR), SIRT1, acetylated (ac)-p53, p53, and p21 were detected by Western blot. *P < 0.05, **P < 0.01, ***P < 0.001, versus the control; #P < 0.05, ##P < 0.01, ###P < 0.001, versus H2O2 treatment. NS not significant
Discussion
Our results suggested that SIRT1 levels decreased and P53 levels increased with replicative senescence, which is consistent with the results of previous studies [35, 36]. H2O2 promoted PARP1 to PAR activation. At the same time, H2O2 decreased SIRT1 expression and increased P53 expression; these results were similar to those of previous studies [28, 30]. Moreover, our results suggested that a certain concentration of PJ34 may revert the decreased functionality of aging-induced EPCs through SIRT1. In this process, PJ34 inhibits the PARP1 activation and thereby preserves intracellular NAD+ levels. Interestingly, SIRT1 expression levels were not changed, but its activity was enhanced. Therefore, with respect to the NAD+-dependent deacetylase (SIRT1), we speculated that increased NAD+ levels, as a cofactor of SIRT1, were unable to promote SIRT1 expression but activated the biological activity of SIRT1, which could be observed by the improvement of deacetylation functionality.
There are some advantages to our study. First, we used a novel approach to understand the effects and mechanism by treating EPCs with PJ34, which will aid further research on senescent EPCs (Fig. 7). Improvement of the functionality of aging EPCs may contribute to the development of cellular therapies for AS. Furthermore, we found that EPC functionality changed with senescence and treatment in various aspects. PAR, whose molecular weight was from 2 kD to 300 kD as a protein polymer, was very special. We demonstrated changes in its expression by Western blot.
Schematic illustration of poly (ADP-ribose) polymerase 1 (PARP1) and sirtuin 1 (SIRT1) crosstalk. In EPCs, H2O2 results in DNA damage, PARP1 activation, and consequent nicotinamide adenine dinucleotide (NAD+) consumption. PJ34 preserves intracellular NAD+ levels and increases SIRT1 deacetylation. ROS reactive oxygen species
There are several limitations to our study. Firstly, EPCs cultured from human umbilical cord blood entered a senescent stage after a limited number of cell divisions, which was identified as replicative senescence in our study [37, 38]. In previous experiments, we found that EPCs entered this stage at approximately P20. EPCs incubated with 100 μM H2O2 for 6 h and then with fresh culture medium for 18 h exhibited the same state as P20 EPCs, which were identified as being in stress-induced premature cellular senescence [32]. Therefore, we used H2O2 to establish a senescent model for further and easier research. However, there are many differences between induced senescence and natural senescence. Whether our results can be applied to naturally senescent cells in vitro, and even in vivo, remains to be discussed. Secondly, compared with knockdown, shRNA attenuated protein expression enormously. Hence, our results were not perfect. In addition, NAD+ is widespread in cells and is associated with various functions, and senescence is a slow and complex process involving many regulators and signaling pathways. Therefore, other signaling pathways may play roles in preventing senescence after the elevation of intracellular NAD+ levels. Finally, we found that excessive PJ34 was harmful to EPCs, but the most appropriate concentration remains to be determined.
Conclusions
PJ34 can improve the function of aging EPCs through PARP1 inhibition, preservation of intracellular NAD+ levels, and increased SIRT1 activity.
Change history
25 October 2018
The original article [1] contains an error regarding the erroneous inclusion of 3 μl as a parameter in the x-axis of Fig. 2c; the correct version of Fig. 2c can instead be seen below.
Abbreviations
- Ad-GFP:
-
Adenoviral vectors containing green fluorescent protein
- ADP:
-
Adenosine diphosphate
- Ad-sh-SIRT1:
-
SIRT1 short-hairpin RNA
- AS:
-
Atherosclerosis
- CCK:
-
Cell counting kit
- DCFH-DA:
-
Dichloro-dihydro-fluorescein diacetate
- EGM:
-
Endothelial cell growth medium
- EPC:
-
Endothelial progenitor cell
- NAD+ :
-
Nicotinamide adenine dinucleotide
- P:
-
Passage
- PAR:
-
Poly ADP-ribose
- PARP1:
-
Poly (ADP-ribose) polymerase 1
- ROS:
-
Reactive oxygen species
- SA-β-gal:
-
Senescence-associated beta-galactosidase
- SIRT1:
-
Sirtuin 1
- TCA:
-
Tricarboxylic acid
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
- vWF:
-
Von Willebrand factor
References
Wang F, et al. Treatment of atherosclerosis by transplantation of bone endothelial progenitor cells over-expressed Paraoxonase-1 gene by recombinant adeno-associated virus in rat. Biol Pharm Bull. 2010;33(11):1806–13.
Lu H, Daugherty A. Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(3):485–91.
Head T, Daunert S, Goldschmidt-Clermont PJ. The aging risk and atherosclerosis: a fresh look at arterial homeostasis. Front Genet. 2017;8:216.
Gong X, et al. Effects of olmesartan on endothelial progenitor cell mobilization and function in carotid atherosclerosis. Med Sci Monit. 2015;21:1189–93.
Werner N, et al. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93(2):e17–24.
Altabas V, Altabas K, Kirigin L. Endothelial progenitor cells (EPCs) in ageing and age-related diseases: how currently available treatment modalities affect EPC biology, atherosclerosis, and cardiovascular outcomes. Mech Ageing Dev. 2016;159:49–62.
Li L, et al. Exogenous H2S contributes to recovery of ischemic post-conditioning-induced cardioprotection by decrease of ROS level via down-regulation of NF-kappaB and JAK2-STAT3 pathways in the aging cardiomyocytes. Cell Biosci. 2016;6:26.
Chang HN, et al. The effect of aging on migration, proliferation, and collagen expression of tenocytes in response to ciprofloxacin. J Orthop Res. 2012;30(5):764–8.
Naaldijk Y, et al. Migrational changes of mesenchymal stem cells in response to cytokines, growth factors, hypoxia, and aging. Exp Cell Res. 2015;338(1):97–104.
Ahluwalia A, et al. Reduced ghrelin in endothelial cells plays important mechanistic role in aging-related impairment of angiogenesis. J Physiol Pharmacol. 2009;60(2):29–34.
Goody MF, Henry CA. A need for NAD+ in muscle development, homeostasis, and aging. Skelet Muscle. 2018;8(1):9.
Valerio D, et al. SA1/SA2 cohesion proteins and SIRT1-NAD+ deacetylase modulate telomere homeostasis in cumulus cells and are eligible biomarkers of ovarian aging. Hum Reprod. 2018;33(5):887–94. https://doi.org/10.1093/humrep/dey035.
Hou Y, et al. NAD(+) supplementation normalizes key Alzheimer's features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci U S A. 2018;115(8):E1876–85.
Zhang M, Ying W. NAD(+) deficiency is a common central pathological factor of a number of diseases and aging: mechanisms and therapeutic implications. Antioxid Redox Signal. 2018. https://doi.org/10.1089/ars.2017.7445.
Mohamed JS, et al. Dysregulation of SIRT-1 in aging mice increases skeletal muscle fatigue by a PARP-1-dependent mechanism. Aging (Albany NY). 2014;6(10):820–34.
Lu P, et al. Poly(ADP-ribose) polymerase-1 causes mitochondrial damage and neuron death mediated by Bnip3. J Neurosci. 2014;34(48):15975–87.
Mangoni M, et al. Soft tissue sarcomas: new opportunity of treatment with PARP inhibitors? Radiol Med. 2018. https://doi.org/10.1007/s11547-018-0877-4.
Sizemore ST, et al. Synthetic lethality of PARP inhibition and ionizing radiation is p53-dependent. Mol Cancer Res. 2018;16(7):1092–1102. https://doi.org/10.1158/1541-7786.MCR-18-0106.
Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464–71.
Chang HC, Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell. 2013;153(7):1448–60.
Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25(3):138–45.
Li Z, et al. SIRT1 inhibits TGF-beta-induced endothelial-mesenchymal transition in human endothelial cells with Smad4 deacetylation. J Cell Physiol. 2018. https://doi.org/10.1002/jcp.26846.
Kiga K, et al. Comprehensive silencing of target-sharing microRNAs is a mechanism for SIRT1 overexpression in cancer. RNA Biol. 2014;11(11):1347–54.
Ong ALC, Ramasamy TS. Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Res Rev. 2018;43:64–80.
Rappou E, et al. Weight loss is associated with increased NAD(+)/SIRT1 expression but reduced PARP activity in white adipose tissue. J Clin Endocrinol Metab. 2016;101(3):1263–73.
Huang S, et al. Poly(ADP-ribose) polymerase inhibitor PJ34 attenuated hepatic triglyceride accumulation in alcoholic fatty liver disease in mice. J Pharmacol Exp Ther. 2018;364(3):452–61.
Nopparat C, Sinjanakhom P, Govitrapong P. Melatonin reverses H2O2-induced senescence in SH-SY5Y cells by enhancing autophagy via sirtuin 1 deacetylation of the RelA/p65 subunit of NF-kappaB. J Pineal Res. 2017;63(1). https://doi.org/10.1111/jpi.12407.
Venkatachalam G, Surana U, Clement MV. Replication stress-induced endogenous DNA damage drives cellular senescence induced by a sub-lethal oxidative stress. Nucleic Acids Res. 2017;45(18):10564–82.
Yang H, et al. Acetylation of HDAC1 and degradation of SIRT1 form a positive feedback loop to regulate p53 acetylation during heat-shock stress. Cell Death Dis. 2015;6:e1747.
Furukawa A, et al. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem. 2007;20:45–54.
Feng L, et al. Roles of progesterone receptor membrane component 1 in oxidative stress-induced aging in chorion cells. Reprod Sci. 2018:1933719118776790. https://doi.org/10.1177/1933719118776790.
Bielak-Zmijewska A, et al. A comparison of replicative senescence and doxorubicin-induced premature senescence of vascular smooth muscle cells isolated from human aorta. Biogerontology. 2014;15:47–64.
Chen I-C, et al. Chronic hyperuricemia impairs blood flow recovery in the ischemic hindlimb through suppression of endothelial progenitor cells. Oncotarget. 2018;9(10):9285–98.
Wang C, et al. MeCP2-mediated epigenetic regulation in senescent endothelial progenitor cells. Stem Cell Res Ther. 2018;9(1):87.
Wang C, et al. MeCP2 mediated dysfunction in senescent EPCs. Oncotarget. 2017;8(45):78289–99.
Rossman MJ, et al. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol. 2017;313(5):H890–5.
Hanzelmann S, et al. Replicative senescence is associated with nuclear reorganization and with DNA methylation at specific transcription factor binding sites. Clin Epigenetics. 2015;7:19.
Ballew BJ, Lundblad V. Multiple genetic pathways regulate replicative senescence in telomerase-deficient yeast. Aging Cell. 2013;12(4):719–27.
Funding
This study was supported by the National Natural Science Foundation of China (grant number 81471399).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
SZ and ZL conceived the idea and designed the experiments. QC collected human umbilical cord blood. SZ performed all experiments. FL and FW analyzed the results. SZ wrote the manuscript and edited it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Figure S1. Identification of EPCs from human umbilical cord blood. Cells were characterized by flow cytometry detection of CD34, VEGFR2, and CD133. (TIF 138 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zha, S., Li, Z., Cao, Q. et al. PARP1 inhibitor (PJ34) improves the function of aging-induced endothelial progenitor cells by preserving intracellular NAD+ levels and increasing SIRT1 activity. Stem Cell Res Ther 9, 224 (2018). https://doi.org/10.1186/s13287-018-0961-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-018-0961-7