Abstract
Background
Mycophenolate mofetil (MMF) is an immunosuppressive drug that is frequently prescribed to patients with rheumatological diseases. MMF’s side effects include abdominal discomfort, nausea, vomiting, and other gastro-intestinal side effects, which typically appear in the first few months of treatment. However, late-onset diarrhea does not rule out the presence of MMF-induced colitis, which can be misdiagnosed since it is linked to a broad range of histopathological characteristics, including alterations that resemble inflammatory bowel disease, graft-versus-host disease, and ischemia. The differences in treatment response may be explained by the complexity of the histopathologic characteristics.
Case presentation
Here we present a case of a 29-year-old Arabian female with lupus nephritis who started on MMF as induction therapy. In two months, the patient was presented to the Emergency Department with diarrhea and manifestations of severe dehydration. Infectious diseases and adverse drug events were suspected, so the patient was admitted for further workup, and MMF was stopped. The patient was diagnosed with MMF-induced colitis based on colonoscopy and histological findings. Fourteen days after stopping MMF, she was within her baseline.
Conclusion
The purpose of this paper is to report a case of early-onset MMF-induced colitis in a patient with lupus nephritis who had started MMF as induction therapy. A review of the available literature on this uncommon immunosuppressive effect is also presented.
Similar content being viewed by others
Introduction
Mycophenolate mofetil (MMF) is widely used as an immunosuppressive agent for various inflammatory and/rheumatic conditions, including lupus nephritis and organ transplantation. Mycophenolic acid is an active metabolite of MMF that reversibly inhibits inosine monophosphate (IMP) dehydrogenase, preventing purine synthesis in T and B cells [1, 2]. Dose modification or even discontinuation of MMF is quite common due to adverse effects, especially gastro-intestinal side effects, which occur in nearly 45% of cases [2]. Enterocytes are particularly susceptible to the antimetabolic effects of MMF due to their reliance on the de novo process of purine synthesis. This prevents the growth and reproduction of small bowel epithelial cells, which disrupts fluid absorption and causes diarrhea [3, 4]. However, MMF is a well-tolerated therapy in general.
One of the major adverse effects of MMF is colitis, which can lead to serious complications that include perforation, bleeding, and hospitalization. In recent years, several studies have investigated the factors associated with MMF-induced colitis. One study evaluated the incidence of gastro-intestinal complications following kidney transplant and showed that MMF-induced colitis was the most common type of colitis, occurring in 6–9% of patients, and the most common symptom was diarrhea [5, 6]. MMF is one of the common immunosuppression medications for rheumatological disease and has been used for the last two decades with very good outcomes in terms of different aspects and system involvement.
There are only a few reports of patients who developed MMF-related colitis (Table 1). A small retrospective study evaluated 11 patients with rheumatologic disease who had been treated with MMF and found that only one patient had medication-related colitis [7]. We report a case of a young female who had systemic lupus erythematosus and lupus nephritis and presented with abdominal pain and diarrhea. We also discuss the challenges in the diagnosis of MMF-induced colitis.
Written consent was obtained from the patient, and ethical approval was provided by the Institutional Review Board (IRB) of the Study and Research Department of King Fahad Hospital, Jeddah.
Case presentation
The patient was a 29-year-old Arabian woman with a known case of systemic lupus erythematosus, which had been diagnosed 5 years prior based on the criteria of the European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR). She was started on hydroxychloroquine at 200 mg orally once per day, and she had no comorbidities except for hypothyroidism.
The patient had not been followed up due to the COVID-19 pandemic, but in January 2023, she presented to the clinic with an incidental lab result showing a creatinine level of 4.3 mg/dL. Thus, a renal biopsy was planned, and she was diagnosed with class IV lupus nephritis. She received pulse methylprednisolone therapy at 500 mg intravenously for 3 days, which was then switched to a tapering dose of prednisolone. Induction therapy using MMF was initiated at 500 mg orally twice daily then titrated up weekly until she was discharged to home on 1.5 g orally twice per day, which is the maximum recommended dose of induction phase for lupus nephritis.
After 2 months, the patient presented to the emergency department with complaints of nausea, vomiting, and left-sided abdominal pain associated with diarrhea 5–6 times per day, which was watery but contained no blood or mucus. Her symptoms started at just 2 weeks after starting MMF therapy and had progressed over the last month. She denied having fever, weight loss, or night sweats. Upon physical examination, she was alert and oriented but in pain. The abdomen was tender, but there were no signs of peritonitis. The rest of the physical examination was unremarkable apart from Cushingoid face. She was afebrile, and her blood pressure, heart rate, respiratory rate, and oxygen saturation were within normal ranges. Her weight was 90 kg.
Upon admission, MMF was promptly discontinued due to the possibility that it might have led to an infection. The immediate care involved the delivery of intravenous fluids, a low-residue diet, analgesic medications like intravenous acetaminophen, and antispastic treatments. Routine blood tests performed at admission indicated leukopenia 3.800 × 106/L normal range (4–11 × 106/L), elevated creatinine 6.3 mg/dL normal range (07–1.3 mg/dL) while her baseline of creatinine was 2 mg/dL and GFR 60 ml/min, and noticeably increased inflammatory markers (C-reactive protein 32 mg/dL normal range < 5 mg/dL, erythrocyte sedimentation rate 40 mm/hour normal range < 20 mm/hour). The C3 and C4 complement levels were 0.6 g/L (0.8–1.6 g/L) and C4 0.18 g/L (0.20–0.65 g/L), respectively. Stool analysis indicated + 2 pus cells and a negative culture. Urine analysis indicated a protein level of + 1 with no red blood cell crystals or casts. An abdominal ultrasound was performed, but the result was unremarkable.
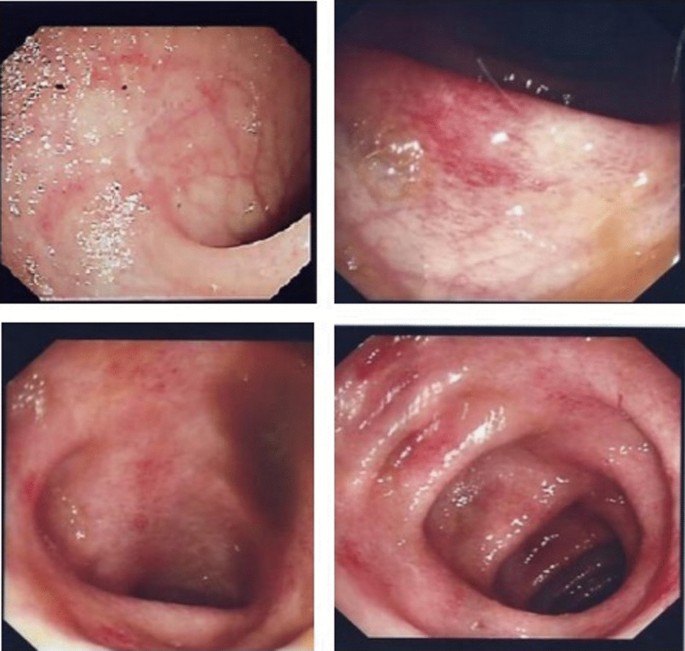
Later, computed tomography scan was performed, which did not reveal any other abnormalities and confirmed the ultrasound results. A colonoscopy showed erythematous patches with few erosions and rectal-sparing colitis. Multiple biopsies been taken (Fig. 1). Infectious colitis, drug-induced colitis, newly diagnosed inflammatory bowel disease (IBD), gastro-intestinal involvement associated with systemic lupus erythematosus, and mesenteric ischemia were all considered in the differential diagnosis. Cytomegalovirus (CMV) infection is the main concern among infectious causes of colitis in patients with impaired immune systems, and its possible endoscopic findings include diffuse erythema, ischemia, erosions, and ulcers.
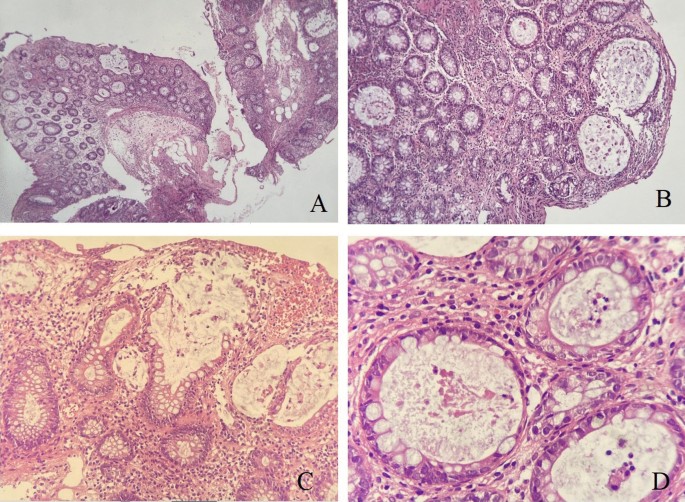
The endoscopic appearance of drug-induced colitis can resemble that of ulcerative colitis, infectious colitis, and ischemic colitis. Non-steroidal anti-inflammatory drugs (NSAIDs) are the most common cause of drug-induced colitis, but MMF was probably involved in the present case. Rectal sparing almost always occurs in MMF-induced colitis. Microscopically, the colonic mucosa displayed a mild architectural distortion, with ruptures of few dilated glands. Mild cryptitis is observed. However, no obvious apoptosis seen as been previously described in few cases of MMF induced colitis. There were no evidence of viral cytopathic changes, granuloma, dysplasia or malignancy (Fig. 2A–D),
Mycophenolate Mofetil induced colitis. A Colonic biopsy with mild architectural distortion, crypt hyperplasia and lamina propria inflammation (HEx 4 ×). B Higher magnification (HE 20 ×) show dilated colonic glands. C Destructed ruptured colonic glands with mucin spillage (HE × 20 ×). D Acute inflammation within the glands (cryptitis), at high power magnification (HE x 40 ×)
Following the cessation of MMF, the gastro-intestinal symptoms and the biomarkers for systemic inflammation gradually subsided and returned to base line levels after 14 days without further therapy, thus supporting the suspicion of drug-induced colitis. The patient was discharged 24 days after admission, MMF was discontinued, and the Euro-Lupus protocol was started with cyclophosphamide as induction therapy for lupus nephritis. The patient has been followed up closely and has shown improvement of active and chronic issues with 6 doses of cyclophosphamide completed. Azathioprine with a low dose of steroid was initiated. There have been no other gastro-intestinal manifestations.
Discussion
MMF is an immunosuppressive medication that was first used to decrease the risk of organ rejection after transplantation, but now, it is also being used to treat patients with autoimmune systemic disorders, including systemic lupus erythematosus. The target dose of MMF for the treatment of Lupus Nephritis is 2–3 g per day in combination with glucocorticoids especially for those high-risk patients for kidney failure including reduced GFR. Dosage may need to be adjusted according to adverse events, toxicity, efficacy and MPA blood level [according to 2019 European Leage Against Rheumatism and European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendation for the management of Lupus Nephritis]. General recommendation to not exceed 2 g in patients with chronic renal failure with GFR less than 25 mL/min.
As far as we are aware, there have been only a few documented cases of colitis caused by MMF in a patient with a rheumatological condition. The onset of MMF-induced colitis in a patient with lupus nephritis, sclerosis, mixed connective tissue disease (MCTD), and polymyositis has previously been documented by other authors (Table 1). In transplant recipients, however, MMF is a well-known trigger of drug-induced colitis [6].
Mycophenolate targets tissues with fast cell division and reliance on purine synthesis. Lymphocytes and gut cells are the two main organs in which regeneration is dependent on this system. Immunosuppression results from lymphocytes (B and T cells) being more dependent on this route (by 90%) [8]. The blood level of mycophenolic acid is directly inversely correlated with mycophenolate’s adverse effects [9]. Since 50% of enterocytes rely on the mycophenolate-targeting mechanisms, it is believed to explain why 45% of patients experience gastro-intestinal side effects, including simple diarrhea, esophagitis, gastroesophageal reflux disease, enteritis, and colitis, as in our patient [2]. The most typical gastro-intestinal mucosal pattern associated with MMF is mucosa that seems normal [10]. The histological changes in patients receiving MMF have mostly been classified in many studies as normal or near normal in around one-third of cases, followed by changes resembling IBD, graft-versus-host disease (GVHD), self-limited colitis, and ischemia [11,12,13]. Another study reported histological results that were in line with an acute colitis-like pattern in half of cases as being the most common, followed by IBD-like pathologic findings in 36% of cases, ischemia-like characteristics in 5.6% of cases, and GVHD-like abnormalities in 8.3% of cases [6]. Examples of specific histological characteristics of MMF-related colitis include crypt architectural disarray, increased lamina propria inflammation, dilated damaged crypts, increased crypt epithelial apoptosis, and GVHD-like alterations [14].
The wide morphological spectrum documented in MMF-induced colitis includes features that can lead to misdiagnosis and delayed intervention. Therefore, it is essential to discuss the clinical history of MMF therapy with pathologists and to take this diagnosis into consideration, regardless of the length of therapy, given the variations in the therapeutic management and prognosis of these disorders. The most frequent indication for a colonoscopy referral for patients on MMF medication is diarrhea. Nearly half of such patients have normal colonoscopy results. Other endoscopic findings include erythema (33%) and erosions/ulcers (19%), which indicate a need for routine biopsies to help with confirmation of the diagnosis [6].
Treatment options range from stopping MMF use to using specialized immunosuppressive medications to correct the histological pattern replicated by MMF-induced colitis. There are no recommendations available to help clinicians treat colitis induced by MMF. Case reports have frequently shown that after stopping MMF, diarrhea symptoms improve within a week. In another study, after unsuccessful attempts with MMF cessation, a patient was given 50 mg of intravenous steroids daily for two weeks and a single infusion of 5 mg/kg of infliximab, which led to decreased stool frequency within three days after infusion [28].
Conclusion
It is well known that MMF causes drug induced colitis with a variety of patterns and clinical manifestations. When caring for people with autoimmune systemic disorders, colitis should be recognized as a rare side effect of MMF therapy. It is necessary for physicians to be aware that discontinuing the medicine is typically effective without the need for extra treatments.
Availabilty of data and materials
Not applicable.
Abbreviations
- MMF:
-
Mycophenolate mofetil
- MPS:
-
Mycophenolate sodium
- IMP:
-
Inosine monophosphate
- GI:
-
Gastro-intestinal
- IBD:
-
Inflammatory bowel disease
- CMV:
-
Cytomegalovirus
- APECED:
-
Polyendocrinopathy-candidiasis-ectodermal dystrophy
References
Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2–3):85–118. https://doi.org/10.1016/s0162-3109(00)00188-0.
Allison AC, Eugui EM. Purine metabolism and immunosuppressive effects of mycophenolate mofetil (MMF). Clin Transplant. 1996;10(1 Pt 2):77–84.
Tierce JC, Porterfield-Baxa J, Petrilla AA, Kilburg A, Ferguson RM. Impact of mycophenolate mofetil (MMF)-related gastrointestinal complications and MMF dose alterations on transplant outcomes and healthcare costs in renal transplant recipients. Clin Transplant. 2005;19(6):779–84. https://doi.org/10.1111/j.1399-0012.2005.00421.x.
Isaacs PE, Sladen GE, Filipe I. Mefenamic acid enteropathy. J Clin Pathol. 1987;40(10):1221–7. https://doi.org/10.1136/jcp.40.10.1221.
Gioco R, Puzzo L, Patanè M, Corona D, Trama G, Veroux P, Veroux M. Post-transplant colitis after kidney transplantation: clinical, endoscopic and histological features. Aging (Albany NY). 2020;12(24):24709–20. https://doi.org/10.18632/aging.202345.
Calmet FH, Yarur AJ, Pukazhendhi G, Ahmad J, Bhamidimarri KR. Endoscopic and histological features of mycophenolate mofetil colitis in patients after solid organ transplantation. Ann Gastroenterol. 2015;28(3):366–73.
Bandelier C, Guerne PA, Genevay S, Finckh A, Gabay C. Clinical experience with mycophenolate mofetil in systemic autoimmune conditions refractory to common immunosuppressive therapies. Swiss Med Wkly. 2009;139(3–4):41–6. https://doi.org/10.4414/smw.2009.12441.
Lamba V, Sangkuhl K, Sanghavi K, Fish A, Altman RB, Klein TE. PharmGKB summary: mycophenolic acid pathway. Pharmacogenet Genomics. 2014;24(1):73–9.
Md Dom ZI, Coller JK, Carroll RP, Tuke J, McWhinney BC, Somogyi AA, et al. Mycophenolic acid concentrations in peripheral blood mononuclear cells are associated with the incidence of rejection in renal transplant recipients. Br J Clin Pharmacol. 2018;84(10):2433–42.
Lee S, de Boer WB, Subramaniam K, Kumarasinghe MP. Pointers and pitfalls of mycophenolate-associated colitis. J Clin Pathol. 2013;66(1):8–11. https://doi.org/10.1136/jclinpath-2012-200888.
Histological spectrum of mycophenolate mofetil-related colitis: association with apoptosis.
Selbst MK, Ahrens WA, Robert ME, Friedman A, Proctor DD, Jain D. Spectrum of histologic changes in colonic biopsies in patients treated with mycophenolate mofetil. Mod Pathol. 2009;22(6):737–43. https://doi.org/10.1038/modpathol.2009.44.
Mycophenolate mofetil-induced colitis with graft versus host disease-like features in a renal transplant recipient: case report and literature review.
Parfitt JR, Jayakumar S, Driman DK. Mycophenolate mofetil-related gastrointestinal mucosal injury: variable injury patterns, including graft-versus-host disease-like changes. Am J Surg Pathol. 2008;32(9):1367–72. https://doi.org/10.1097/pas.0b013e31816bf3fe.
Al Saadi W, Al Salmi I, Mohammed E, Al Ajmi Z, Hannawi S. Mycophenolate induced colitis: one-year post-kidney transplantation. Oman Med J. 2023;38(2): e489. https://doi.org/10.5001/omj.2023.14.
Schmitt MM, Ferré EMN, De Melo MS, Cooper MA, Quezado MM, Heller T, Lionakis MS. Mycophenolate-induced colitis in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients. JPGN Rep. 2021;2(4): e131. https://doi.org/10.1097/pg9.0000000000000131.
Bani Fawwaz BA, Aldwairy A, Farooq A. Mycophenolate-induced colitis: a rare side effect. Cureus. 2021;13(9): e18250. https://doi.org/10.7759/cureus.18250.
Farooqi R, Kamal A, Burke C. Mycophenolate-induced colitis: a case report with focused review of literature. Cureus. 2020;12(1): e6774. https://doi.org/10.7759/cureus.6774.
Tayyem O, Saraireh H, Al Hanayneh M, Stevenson HL. Heart transplant recipient with mycophenolate mofetil-induced colitis: the great imitator. BMJ Case Rep. 2018;2018:bcr2017224035. https://doi.org/10.1136/bcr-2017-224035.
Moroncini G, Benfaremo D, Mandolesi A, Gabrielli A. Mycophenolate mofetil-induced colitis in a patient with systemic sclerosis. BMJ Case Rep. 2018;2018:bcr2018224829. https://doi.org/10.1136/bcr-2018-224829.
Sonoda A, Wada K, Mizukami K, Fukuda K, Shuto M, Okamoto K, Ogawa R, Okimoto T, Murakami K. Deep ulcers in the ileum associated with mycophenolate mofetil. Intern Med. 2017;56(21):2883–6. https://doi.org/10.2169/internalmedicine.8815-17.
Dhakal P, Gami R, Giri S, Bhatt VR. Mycophenolate mofetil (MMF)-induced colitis. Blood. 2016;128:4795.
Goyal A, Salahuddin M, Govil Y. A unique case of mycophenolate induced colitis after 10 years of use. Case Rep Gastrointest Med. 2016;2016:3058407. https://doi.org/10.1155/2016/3058407.
Johal K, Ratuapli SK, Lam-Himlin DM, Gurudu SR. Mycophenolate mofetil-induced segmental colitis mimicking ischemic colitis. Case Rep Gastroenterol. 2014;8(1):95–100. https://doi.org/10.1159/000360847.
Hamouda M, Mahmoudi H, Skhiri H, Elmay M. Mycophenolate mofetil-related pancolitis in a kidney transplant recipient [published correction appears in Exp Clin Transplant. 2012 Aug;10(4):418]. Exp Clin Transplant. 2012;10(5):501–5. https://doi.org/10.6002/ect.2011.0200.
Patra S, Vij M, Sukanya B, Kapoor D. Mycophenolate mofetil-induced colitis with graft versus host disease-like features in a liver transplant recipient. Indian J Pathol Microbiol. 2012;55(4):506–8. https://doi.org/10.4103/0377-4929.107792.
Gorospe EC. Chronic diarrhoea from mycophenolate mofetil-induced colitis. BMJ Case Rep. 2012;2012:bcr2220115344. https://doi.org/10.1136/bcr.12.2011.5344.
Bouhbouh S, Rookmaaker MB. Rapid resolution of persistent mycophenolate mofetil-induced diarrhoea with a single dose of infliximab. Nephrol Dial Transplant. 2010;25(10):3437–8. https://doi.org/10.1093/ndt/gfq379.
Acknowledgements
None.
Guarantor of submission
The corresponding author is the guarantor of submission.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Ziyad Alakkas: Literature review and writing manuscript. Abdulaziz M. Gari: case review and got ethical approval. Sara Makhdoum: review histology slides and review literature. Eman A. AlSindi: mentor and most responsible physician (MRP) of the patient.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study complies with the Declaration of Helsinki and was approved by the local ethical research committee. Patient provided a written informed consent. All authors declare their participation.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that there is no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alakkas, Z., Gari, A.M., Makhdoum, S. et al. Mycophenolate-induced colitis in a patient with lupus nephritis: a case report and review of the literature. J Med Case Reports 18, 229 (2024). https://doi.org/10.1186/s13256-024-04539-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-024-04539-7