Abstract
Background
The current guidelines have discouraged the routine use of intra-aortic balloon pump (IABP) in cardiogenic shock complicating acute coronary syndrome (ACS). Since then, the trend of IABP utilization in ACS has been declining. Nevertheless, the guidelines still preserve the recommendation of IABP use in hemodynamic instability or cardiogenic shock caused by post myocardial infarction (MI) ventricular septal rupture (VSR).
Case presentation
A 46-years-old diabetic Southeast Asian female was referred from a peripheral facility with intractable heart failure despite treatment with vasoactive agents and diuretics for five days. The ECG suggested a recent anteroseptal myocardial infarction with normal high-sensitivity troponin-I value. The echocardiography detected a regional wall motion abnormality and a 10 mm wide ventricular septal defect. Invasive coronary angiography revealed a severe two-vessel coronary artery disease. We planned a delayed surgical strategy with preoperative optimization using IABP as a bridge to surgery. IABP implantation followed by significant hemodynamic improvement and rapid resolution of heart failure without any inotrope support. Afterwards, coronary artery bypass grafting (CABG) and VSR surgical repair were performed. We safely removed IABP on the third postoperative day with proper weaning and minimal vasoactive support.
Conclusion
We report a case where IABP still provided benefits for a patient with intractable heart failure caused by undetermined onset MI complicated by VSR. The use of IABP in such a case is in accordance with the recommendation of the current guidelines. Several studies showed that IABP use during preoperative optimization in the case of post-MI VSR was associated with survival benefits.
Similar content being viewed by others
Introduction
Intra-aortic balloon pump (IABP) has been the most employed mechanical circulatory support (MCS) device for the past half-century [1, 2]. It provides hemodynamic support for various clinical conditions, ranging from prophylactic measures for high-risk invasive coronary artery procedures to managing cardiogenic shock or advanced heart failure [2]. However, its utilization in recent years has been shown to be progressively reduced, particularly in acute myocardial infarction (AMI) [3].
IABP use in cardiogenic shock complicating AMI was formerly assigned to a Class I recommendation [4,5,6]. This recommendation mainly was based on observational studies. However, after the publication SHOCK-II trial, a multi-centre randomized clinical trial (RCT) with 600 subjects [7], routine use of IABP in cardiogenic shock complicating AMI had been downgraded to a Class III recommendation [8]. The trial showed that IABP did not significantly reduce 30-day mortality [7]. Nonetheless, IABP use in hemodynamic instability or cardiogenic shock due to mechanical complications of AMI is still preserved as a Class IIa recommendation (level of evidence C) [8].
Ventricular septal rupture (VSR) is a lethal mechanical complication of AMI [9,10,11]. The current guidelines suggested early surgical repair as an urgent treatment [8, 12]. However, delayed surgical strategy may be considered in patients who respond well to heart failure therapy, allowing infarcted tissue maturation for better surgical outcomes [8, 13]. Preoperative optimization of the already poor cardiac function and hemodynamic status during the deferred period is a serious challenge [14]. Various types of MCS have been shown to be feasible and effective during the preoperative period [15,16,17]. However, according to the current guidelines, the widely used IABP is still the first-line MCS in this clinical setting [8, 18]. It is also the simplest, safest, cheapest, and most studied MCS [2, 16].
We report a case that demonstrated IABP preoperative roles in the treatment of a patient with an intractable heart failure caused by VSR complicating a late-onset AMI who was planned to undergo coronary artery bypass grafting (CABG) and surgical repair of VSR. We will discuss whether IABP use in this clinical setting is still relevant.
Case presentation
A 46-year-old Southeast Asian female was referred to our institution with persistent hypotension and intractable heart failure after five days of care in a peripheral hospital. In the peripheral hospital, the patient was initially treated for decompensated heart failure. Her condition worsened despite optimum available pharmacological treatment had been administered. The local physicians decided to refer the patient as soon as they suspected a concurrent ventricular septal defect.
The patient’s consciousness was still intact on admission. She complained of shortness of breath—especially when assuming a supinated position—excessive fatigue, and progressive legs swelling within the last month. There was no complaint of angina prior to or during hospitalization in the peripheral hospital. There was a history of poorly treated Type 2 Diabetes Mellitus for more than ten years. The presence of the other risk factors was denied.
The patient’s vital signs on arrival at the emergency department were as follows: blood pressure (BP) of 107/69 mmHg supported with continuous infusion of dobutamine 3 mcg/kg/min and norepinephrine 0.05 mcg/kg/min; heart rate (HR) was ranged between 95 to 115 beats/min; respiratory rate (RR) of 24 breaths/min; afebrile (36.9 °C); and peripheral oxygen saturation level (SpO2) of 96% aided by 4 L/min nasal cannula oxygen supplementation. Head and neck assessment showed anaemic conjunctiva and distended jugular vein. Chest auscultation revealed rales at the base of both lungs and a holosystolic murmur at the apex of the heart. There were ascites and lower limb oedema.
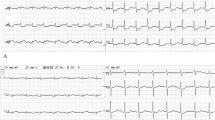
The ECG was sinus rhythm with poor R wave progression at precordial leads as well as biphasic and inverted T waves at leads V1 to V3 that may suggest a late onset anteroseptal myocardial infarction (Fig. 1). There was no clear information regarding the exact onset of acute coronary syndrome. Chest radiography displayed pulmonary congestion and pleural effusion of both lungs (Fig. 2A). Initial laboratory examination showed mild anaemia (haemoglobin of 10.9 g/dL and haematocrit of 31%) and hypoalbuminemia (2.46 mg/dL). High-sensitivity cardiac troponin I was normal. Other laboratory indices included liver function tests, renal function tests, arterial blood gas parameters, and electrolyte levels were unremarkable.
Chest radiograph evaluation on: A admition (first day) it showed congestive lungs with bilateral pleural effusion; B a week of hospitalization (seventh day) the sign of lungs congestion was still remain despite adequate diuresis; C second day of IABP implantation (eleventh day) the congestion remarkably reduced and heart border became more apparent; D second post-operative day (14th day). The yellow arrow indicates the tip of IABP
Echocardiography revealed hypokinetic anteroseptal and anterior segments of the left ventricular (LV) wall, while other segments’ motion remained normal. The LV ejection fraction (LVEF) was 50%. A left-to-right shunt was detected from a ventricular septal defect of 10 mm in size at the apical portion of the interventricular septum (Fig. 3A). The non-invasive hemodynamic assessment showed reduced cardiac output (CO), cardiac index (CI), LV outflow tract velocity time integral (LVOT VTI), respectively 2.87 L/min, 1.81 L/min m2 and 10 cm (the normal values are respectively 4–8 L/min, 2.8–4.2 L/min m2, and 20 ± 3 cm). The systemic vascular resistance (SVR) was relatively high (1997.7 dynes s/cm5). There was an increase in estimated right atrial pressure (RAP) (> 15 mmHg), LV filling pressure and pulmonary capillary wedge pressure (PCWP) (E/e’ ratio of > 14). The pulmonic to systemic blood flow (Qp/Qs) ratio was 2.1 (Fig. 3B). Lung ultrasonography (LUS) displayed pulmonary congestion and bilateral pleural effusion in the right and left costophrenic sinuses.
The patient was transferred to the intensive cardiac care unit (ICCU). The objectives were to improve the clinical status and preparation for coronary angiography. Dobutamine (5 mcg/kg/min) was administered to maintain adequate perfusion. Norepinephrine was safely tapped off, followed by the introduction of a low-dose ACE inhibitor, Ramipril 2.5 mg. Furosemide was administered through continuous infusion to achieve adequate decongestion. The dose was started by 10 mg/hour and gradually lowered following the improvement in patient’s volume status. Diabetes mellitus was treated with insulin, and comorbidities were treated accordingly.
During the first week in the ICCU, the urine output was adequate with diuretic and inotrope support (the total urine output was 17.7 L and the total water deficit was 11.3 L). However, the heart failure symptoms remained, and the echocardiography hemodynamic monitoring showed only slight improvement in CO, CI, and LVOT VTI. The LV filling pressure and PCWP were still high with an E/e’ ratio of > 14. Since inotrope can enhance left-to-right shunting through the septal defect, lowering the dose may reduce the shunt. However, several attempts to lower the inotrope dose resulted in hypotension, decreasing urine output, and worsening symptoms of congestion. The patient clinical profile may be described as “stable but inotrope dependent”. It took ten days for the patient to tolerate the supinated position without developing severe symptoms and to be considered eligible for a coronary angiography procedure. Within this duration, there were episodes of hypokalaemia and metabolic alkalosis secondary to loop diuretic therapy.
Coronary angiography was performed on the tenth day of hospitalization. It revealed a chronic total occlusion of the left anterior descendent artery, up to 50% stenoses at distal portion of left circumflex artery, and severe stenoses of the right coronary artery (Fig. 4). The Heart Team unanimously decided that surgery would be performed after preoperative optimization. IABP was implanted as a bridge to surgical therapy. There were significant improvements in symptoms as well as hemodynamic parameters following the application of IABP (1:1 augmentation ratio). On the second day of IABP utilization, the inotrope was completely tapped off. Hemodynamic monitoring showed left-to-right shunting reduction (Qp/Qs ratio 1.55) (Fig. 3C) and significant augmentation in CO, CI, and LVOT VTI (respectively 4.14 L/min, 2.67 L/min m2, and 14.9 cm). There was also improvement in ventricular filling pressure and PCWP (E/e’ ratio < 14).
Invasive coronary angiography revealed: A and B a normal left main coronary artery, critical stenosis at mid left anterior descending artery, total occlusion at distal left artery descending, and 50% stenosis at mid left circumflex artery; C and D A significant 80% stenosis at mid right coronary artery
The surgery was performed four days after IABP implantation. Pre-procedural trans-esophageal echocardiography (TEE) showed a defect at the apical portion of the ventricular septum with a diameter of 10 mm (Fig. 5A). The surgery procedures included coronary artery bypass grafting (CABG) and VSR closure. During CABG, anastomoses were made from the Saphenous vein graft (SVG) to the right posterior descending artery (RPDA) and from Left Internal Mammary Artery (LIMA) to the middle segment of the Left Artery Descending (LAD) artery. A septal defect of 15 mm in diameter was revealed during left ventriculotomy (Fig. 5B). It was then closed with a polytetrafluoroethylene patch. Post-procedural TEE showed no residual flow through the septal patch.
Postoperative care in the intensive care unit was uneventful. The IABP was still maintained after the surgery and safely removed after proper weaning with minimal vasoactive drugs on the third postoperative day. The patient was transferred to the low care unit on the fifth postoperative day and was discharged after several days without relapsing symptoms of heart failure.
Discussion
VSR is a rare but devastating complication of AMI. Its prevalence has substantially declined in the recent era of early reperfusion therapy, ranging between 0.17 and 0.31% [9,10,11]. It is associated with extensive comorbidities, resulting in poor cardiac output, multiorgan failure and death. Despite improvement in early diagnosis and treatment, the current mortality rate remains high (41–80%) [9] and it is extremely high (> 90%) among patients whose VSR is left untreated. [11]
Hemodynamically, the occurrence of VSR gives rise to left-to-right shunt and reduces forward blood flow. Compensatory increase in systemic vascular resistance, thus the LV afterload, leads to an even more rigorous left-to-right shunt [18]. The management should be directed at reducing the vicious left-to-right shunt with afterload-reducing pharmacological agents and MCSs [9, 14]. Inotropes and vasopressors may worsen left-to-right shunting, whereas vasodilators decrease shunting at the expense of hypotension [19]. Our patient, in this case, was so prone to hypotension that a vasodilator agent such as nitroprusside or nitroglycerin was not a safe option.
Several studies have demonstrated IABP's beneficial hemodynamic effects in post-MI VSR [19,20,21,22]. Its application results in a significant reduction in left-to-right shunt volume and shunt flow ratio, with a concomitant increase in effective systemic CO [20]. These outcomes are mainly achieved from afterload reduction [14, 21]. IABP also increases coronary perfusion and decreases myocardial oxygen consumption. Both effects result in the amelioration of myocardial ischemia [23,24,25,26].
The current guidelines suggest urgent surgical repair as the definitive treatment for post-MI VSR, but there is no consensus on the optimal timing of the surgery [8]. In all patients with severe heart failure that do not respond rapidly to aggressive heart failure therapy, early surgery should be performed [8]. However, delayed surgical repair may be considered in those who respond well to aggressive therapy. Delayed surgery is beneficial as it allows infarcted tissue maturation and firm scar formation for better anchoring of suture and patch material [12]. Nonetheless, this strategy also carries the risk of rupture extension and death while waiting for surgery [8]. Preoperative optimization of the already poor cardiac function and hemodynamic status during the waiting periods is challenging [27]. MCSs utilization, either as a sole device or in combination, is often required. [15]
Kettner et al. discovered that IABP support in shock caused by post-MI mechanical complications—primarily due to VSR—significantly reduces preoperative mortality [22]. Furui et al. reported that surgery could be delayed for an average of 9 days from acute VSR onset using IABP or respiratory management without deterioration of organ function [28]. The strategy was also associated with favorable 30-day mortality and long-term outcome. However, IABP hemodynamic support is often insufficient in more critical patients, and combination with other MCS may be beneficial [29]. Morimura et al. reported that a combination of IABP and venoarterial extracorporeal membrane oxygenation (VA ECMO) during preoperative optimization might improve outcomes of patients with post-MI VSR complicated by cardiogenic shock [30]. 33 It is worth mentioning that transcutaneous VSR closure has been proposed as a preoperative strategy prior to definitive surgical repair to reduce shunt and thereby stabilize the patient’s clinical condition [15, 31]. However, it adds up the potential risk of complications such as device dislodgement and hemolysis [32, 33].
Our patient presented with late AMI and was revealed to have a severe two-vessel coronary artery disease that required CABG revascularization. A meta-analysis that included 9212 patients undergoing CABG showed that preoperative implanted IABP was associated with a mortality relative risk reduction of more than 4% [34]. There were also reductions in the risk of MI and renal failure. Additionally, intensive care and total hospital stays were significantly reduced, indicating possible health and economic benefits [34]. Yang et al. reported a study of 416 patients with LV dysfunction undergoing off-pump CABG that showed preoperative IABP was linked to lower 30-day mortality. [35]
Conclusion
Despite the declining trend of IABP use in cardiogenic shock complicating AMI, IABP is still the first-line MCS in the case of post-MI VSR. Its use during the preoperative optimization period is associated with survival benefits in patients who were planned for delayed surgical repair of VSR and CABG. However, its hemodynamic support is insufficient in more critical patients. This situation may call for more advanced measures such as IABP combination with the other type of MCS or preoperative transcutaneous closure of VSR.
Availability of data and materials
Not applicable.
Abbreviations
- IABP:
-
Intra-aortic balloon pump
- MCS:
-
Mechanical circulatory support
- ACS:
-
Acute coronary syndrome
- AMI:
-
Acute myocardial infarction
- RCT:
-
Randomized clinical trial
- VSR:
-
Ventricular septal rupture
- CABG:
-
Coronary artery bypass grafting
- HR:
-
Heart rate
- BP:
-
Blood pressure
- RR:
-
Respiratory rate
- CO:
-
Cardiac output
- CI:
-
Cardiac index
- LVOT VTI:
-
Left ventricle outflow tract velocity time integral
- SVR:
-
Systemic vascular resistance
- RAP:
-
Right atrial pressure
- PCWP:
-
Pulmonary capillary wedge pressure
- Qp/Qs:
-
Pulmonic to systemic blood flow
References
Kantrowitz A, Tjonneland S, Freed PS, Phillips SJ, Butner AN, Sherman JLJ. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA. 1968;203(2):113–8.
Rossini R, Valente R, Furio C, et al. ANMCO POSITION PAPER : Role of intra-aortic balloon pump in patients with acute advanced heart failure and cardiogenic shock. Eur Heart J Suppl. 2021;23:204–20. https://doi.org/10.1093/eurheartj/suab074.
Shah M, Patnaik S, Patel B, et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;107(4):287–303. https://doi.org/10.1007/s00392-017-1182-2.
Wijns W, Kolh P, Danchin N, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31(20):2501–55. https://doi.org/10.1093/eurheartj/ehq277.
Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29(23):2909–45. https://doi.org/10.1093/eurheartj/ehn416.
Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 199. Circulation. 2004;110(5):588–636. https://doi.org/10.1161/01.CIR.0000134791.68010.FA.
Thiele H, Zeymer U, Neumann F-J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–96. https://doi.org/10.1056/nejmoa1208410.
Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39(2):119–77. https://doi.org/10.1093/eurheartj/ehx393.
Jones BM, Kapadia SR, Smedira NG, et al. Ventricular septal rupture complicating acute myocardial infarction: a contemporary review. Eur Heart J. 2014;35(31):2060–8. https://doi.org/10.1093/eurheartj/ehu248.
French JK, Hellkamp AS, Armstrong PW, et al. Mechanical complications after percutaneous coronary intervention in ST-elevation myocardial infarction (from APEX-AMI). Am J Cardiol. 2010;105(1):59–63. https://doi.org/10.1016/j.amjcard.2009.08.653.
Crenshaw BS, Granger CB, Birnbaum Y, et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. Circulation. 2000;101(1):27–32. https://doi.org/10.1161/01.CIR.101.1.27.
Thiele H, Kaulfersch C, Daehnert I, et al. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J. 2009;30(1):81–8. https://doi.org/10.1093/eurheartj/ehn524.
Yang C, Sun Y, Zou D, et al. Transcatheter closure of ventricular septal rupture with prolonged support of intra - aortic balloon pump after primary PCI : a case report. BMC Cardiovasc Disord. 2021;21(1):1–8. https://doi.org/10.1186/s12872-021-02392-w.
Żbikowska K, Wróbel K. Mechanical circulatory support in delayed surgery of post-infarction ventricular septal rupture in patients in cardiogenic shock—a review. J Clin Med. 2022. https://doi.org/10.3390/jcm11164728.
Ronco D, Matteucci M, Ravaux JM, et al. Mechanical circulatory support as a bridge to definitive treatment in post-infarction ventricular septal rupture. JACC Cardiovasc Interv. 2021;14(10):1053–66. https://doi.org/10.1016/j.jcin.2021.02.046.
Gambaro A, Rosenberg A, Galiatsou E, Stock UA. Pros and cons of different types of mechanical circulatory support device in case of postinfarction ventricular septal defect. ASAIO J. 2021;67(6):E110–3. https://doi.org/10.1097/MAT.0000000000001290.
O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362-425. https://doi.org/10.1161/CIR.0b013e3182742cf6.
Birnbaum Y, Fishbein MC, Blanche C, Siegel RJ. Ventricular septal rupture after acute myocardial infarction. N Engl J Med. 2002;347(18):1426–32. https://doi.org/10.1056/NEJMra020228.
Pahuja M, Schrage B, Westermann D, Basir MB, Garan AR, Burkhoff D. Hemodynamic effects of mechanical circulatory support devices in ventricular septal defect: results from a computer simulation model. Circ Hear Fail. 2019;12(7):1–12. https://doi.org/10.1161/CIRCHEARTFAILURE.119.005981.
Thiele H, Lauer B, Hambrecht R, et al. Short- and long-term hemodynamic effects of intra-aortic balloon support in ventricular septal defect complicating acute myocardial infarction. Am J Cardiol. 2003;92(4):450–4. https://doi.org/10.1016/s0002-9149(03)00665-9.
Testuz A, Roffi M, Bonvini RF. Left-to-right shunt reduction with intra-aortic balloon pump in postmyocardial infarction ventricular septal defect. Catheter Cardiovasc Interv. 2013;81(4):727–31. https://doi.org/10.1002/ccd.24290.
Kettner J, Sramko M, Holek M, Pirk J, Kautzner J. Utility of intra-aortic balloon pump support for ventricular septal rupture and acute mitral regurgitation complicating acute myocardial infarction. Am J Cardiol. 2013;112(11):1709–13. https://doi.org/10.1016/j.amjcard.2013.07.035.
Kimman JR, Van Mieghem NM, Endeman H, et al. Mechanical support in early cardiogenic shock: what is the role of intra-aortic balloon counterpulsation? Curr Heart Fail Rep. 2020;17(5):247–60. https://doi.org/10.1007/s11897-020-00480-0.
Annamalai SK, Buiten L, Esposito ML, et al. Acute hemodynamic effects of intra-aortic balloon counterpulsation pumps in advanced heart failure. J Card Fail. 2017;23(8):606–14. https://doi.org/10.1016/j.cardfail.2017.05.015.
Thomaz PG, Moura LAJ, Muramoto G, Assad RS. Intra-aortic balloon pump in cardiogenic shock: state of the art. Rev Col Bras Cir. 2017;44(1):102–6. https://doi.org/10.1590/0100-69912017001006.
Parissis H, Graham V, Lampridis S, Lau M, Hooks G, Mhandu PC. IABP: history-evolution-pathophysiology-indications: what we need to know. J Cardiothorac Surg. 2016;11(1):1–13. https://doi.org/10.1186/s13019-016-0513-0.
Khazi FM, Al-Safadi F, Karaly Y, Siddiqui NR, Al-Zamkan B, Aljassim O. Management issues during postinfarction ventricular septal defect and role of perioperative optimization: a case series. Ann Card Anaesth. 2019;22(1):30–4. https://doi.org/10.4103/aca.ACA_189_17.
Furui M, Yoshida T, Kakii B, Uchino G, Nishioka H. Strategy of delayed surgery for ventricular septal perforation after acute myocardial infarction. J Cardiol. 2018;71(5):488–93. https://doi.org/10.1016/j.jjcc.2017.10.016.
Hobbs R, Korutla V, Suzuki Y, Acker M, Vallabhajosyula P. Mechanical circulatory support as a bridge to definitive surgical repair after post-myocardial infarct ventricular septal defect. J Card Surg. 2015;30(6):535–40. https://doi.org/10.1111/jocs.12561.
Morimura H, Tabata M. Delayed surgery after mechanical circulatory support for ventricular septal rupture with cardiogenic shock. Interact Cardiovasc Thorac Surg. 2020;31(6):868–73. https://doi.org/10.1093/icvts/ivaa185.
Jorge C, de Oliveira EI, Martins SR, Nobre Â, da Silva PC, Diogo AN. Hybrid closure of postinfarction ventricular septal rupture enlargement after transcathether closure with Amplatzer occluder. Eur Heart J Acute Cardiovasc Care. 2012;1(1):57–9. https://doi.org/10.1177/2048872612441578.
Giblett JP, Jenkins DP, Calvert PA. Transcatheter treatment of postinfarct ventricular septal defects. Heart. 2020. https://doi.org/10.1136/heartjnl-2019-315751.
Amoozgar H, Soltani R, Edraki M, et al. Hemolysis and its outcome following percutaneous closure of cardiac defects among children and adolescents: a prospective study. Ital J Pediatr. 2019;45(1):1–6. https://doi.org/10.1186/s13052-019-0728-5.
Deppe AC, Weber C, Liakopoulos OJ, et al. Preoperative intra-aortic balloon pump use in high-risk patients prior to coronary artery bypass graft surgery decreases the risk for morbidity and mortality—a meta-analysis of 9,212 patients. J Card Surg. 2017;32(3):177–85. https://doi.org/10.1111/jocs.13114.
Yang F, Wang J, Hou D, et al. Preoperative intra-aortic balloon pump improves the clinical outcomes of off-pump coronary artery bypass grafting in left ventricular dysfunction patients. Sci Rep. 2016;6:27645. https://doi.org/10.1038/srep27645.
Acknowledgements
We would like to thank the Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia for providing support for our study and to all those who have helped this study until the completion of the manuscript.
Funding
No funding was used to complete this case report and literature review.
Author information
Authors and Affiliations
Contributions
MYA: literature review, manuscript revision, data presentation, figure revisions, and final manuscript approval. OR: manuscript review and revision. YS: gathered data, and generated figures. MII: drafted manuscript, gathered data, and generated figures. REI: manuscript review and revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval or consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alsagaff, M.Y., Revianto, O., Sembiring, Y.E. et al. Intra-aortic balloon pump still has a role in late-onset myocardial infarction complicated by ventricular septal rupture with intractable heart failure: a case report. J Med Case Reports 18, 8 (2024). https://doi.org/10.1186/s13256-023-04284-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-023-04284-3