Abstract
Background
Lumbosacral radiculoplexus neuropathy, also known as amyotrophy, is an uncommon monophasic disorder characterized by inflammation of the lumbosacral nerve roots and plexuses. Lumbosacral radiculoplexus neuropathy is usually associated with diabetes mellitus, is typically painful at presentation, and often associated with long-term residual neurologic deficits. We report a case of painless, nondiabetic lumbosacral radiculoplexus neuropathy in a young Chinese woman, who made a full recovery after treatment with intravenous immunoglobulin, adding an atypical case to the scarce literature on lumbosacral radiculoplexus neuropathy.
Case presentation
A 35-year-old Chinese woman presented to our emergency department with 1-week history of painless left lower limb weakness and numbness. Examination revealed weakness confined to the left lower limb but spanning various nerves and myotomes, with abnormal sensation. Clinical localization to the lumbosacral plexus was supported by neurodiagnostic tests, and magnetic resonance imaging of the lumbosacral plexus showed that the nerve roots were also involved. After treatment with intravenous immunoglobulin for nondiabetic lumbosacral radiculoplexus neuropathy, the patient had a full recovery.
Conclusion
Our patient’s case highlights that lumbosacral radiculoplexus neuropathy, an already rare disorder, can occur in the absence of diabetes mellitus and pain, making it even harder to recognize. A systematic and meticulous clinical approach, supported by intelligent selection of adjunctive tests, is required for localization and diagnosis. With an accurate diagnosis, our case also demonstrates that appropriate and prompt treatment can lead to complete recovery, despite previous reports suggesting a high prevalence of long-term residual deficits after lumbosacral radiculoplexus neuropathy.
Similar content being viewed by others
Introduction
Lumbosacral radiculoplexus neuropathy (LRPN), also known as amyotrophy, is an uncommon monophasic disorder characterized by inflammation of the lumbosacral nerve roots and/or plexuses, usually associated with diabetes mellitus [1,2,3]. The disorder is typically associated with pain and long-term residual deficits [7, 8]. Herein, we aim to raise awareness of this rare disorder and its heterogeneous presentation by reporting an unusual case of painless, nondiabetic LRPN in a young Chinese lady with full recovery after treatment with intravenous immunoglobulin (IVIg).
Case presentation
A 35-year-old Chinese woman with no significant past medical history, family history, or medication history was admitted via our emergency department with a 1-week history of gradually worsening left lower limb weakness and numbness. She was without pain, and had no significant past medical history or recent illnesses. On examination, her left-sided ankle deep-tendon reflex was absent, and there was diffuse weakness (modified medical research council scale grade 4) of hip extension, hip adduction, knee extension, knee flexion, ankle plantar flexion, and ankle dorsiflexion in the left lower limb. Sensory testing demonstrated diminished proprioception and pain perception in the left lower limb, distal to the mid-calf. The rest of the neurological examination was unremarkable. Her gait was slow and cautious, but otherwise normal despite her subjective complaints of gait difficulty.
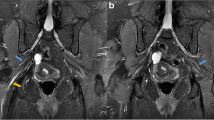
A nerve conduction study (NCS) and electromyography (EMG) performed eight days from symptom onset suggested a plexopathy. Specifically, there was an asymmetrically absent left-sided tibial H-reflex, diminished left saphenous sensory response of 1.93 μV compared with a right saphenous sensory response of 4.2 μV, and reduced recruitment on EMG of the left-sided vastus lateralis, gastrocnemius, and tibialis anterior muscles. Gadolinium-enhanced magnetic resonance imaging (MRI) of the lumbosacral plexuses and lumbar spine demonstrated enhancement of the cauda equina nerve roots bilaterally, sparing the lumbosacral plexuses (Fig. 1). A lumbar puncture was performed 12 days from symptom onset, showing a normal opening pressure of 12 cm H2O. Importantly, cerebrospinal fluid (CSF) examination revealed cytoalbuminologic dissociation (CSF protein 1.03 g/L, with a CSF white blood cell count of 1/μL) but was otherwise negative for oligoclonal bands. Microbiological and autoimmune assays performed on blood and CSF samples were unyielding (Table 1). Computed tomography (CT) of her neck, thorax, abdomen, and pelvis showed no suspicious masses.
MRI of the lumbar spine. A Axial projection, T1 with gadolinium contrast on the eighth day of symptoms, showing enhancement of the cauda equina nerve roots (arrow) at the L4 vertebral level. B Axial projection, T1 with gadolinium contrast on the 18th week from symptom onset, showing resolution of the nerve root enhancement
The patient was diagnosed with nondiabetic LRPN and was treated with IVIg at 2 g/kg divided over 5 days (13–17 days from symptom onset). The patient was reviewed by a physiotherapist once during her hospital stay, but did not require further rehabilitation. Other than a brief 3-day period of postdural puncture headache, which resolved with bed rest and hydration, she tolerated the evaluation and treatment well. She was discharged upon resolution of headache (18 days from symptom onset) with no change in clinical examination findings. On subsequent outpatient follow-up, she reported that her symptoms gradually resolved over the 2 weeks succeeding her discharge from hospital, and a subsequent MRI scan of her lumbar spine performed 18 weeks after symptom onset showed resolution of the cauda equina nerve root enhancement (Fig. 1). She remained asymptomatic when reviewed 20 weeks after symptom onset, and examination was normal, demonstrating a return of her left ankle tendon reflex and full power in all limbs. One year after the episode, the patient reported by phone interview that she had no recurrence of symptoms.
Discussion
LRPN is an uncommon, monophasic disease characterized by inflammation of multiple lumbosacral nerve roots and the lumbosacral plexuses, usually in association with diabetes mellitus [1]. While diabetic LRPN was first described in 1890, nondiabetic LRPN was more recently reported in 1981 by Evans et al. and appears rarer, with a postulated annual incidence rate of 1.27 per 100,000 [2, 4, 7]. The clinical features of nondiabetic LRPN are thought to be similar to diabetic LRPN [3]. In a 2019 study of 62 diabetic and nondiabetic LRPNs, Ng P.S. et al. reported that nearly two-thirds of cases (62.9%, 39/62) had unilateral symptoms at presentation, and unilateral deficits appeared more often among nondiabetic LRPN cases (80%, 16/20) than in diabetic LRPNs (54.8%, 23/42) [7]. In a follow-up analysis of these 62 patients, Pinto et al. reported that the temporal profile was hyperacute (< 24 hours to nadir) in 3.2%, rapidly progressive (1 day to 1 week) in 24.2%, and subacute to chronic (> 1 month) in 66.1% [8]. Our patient’s symptoms represented a case of rapidly progressive LRPN, reaching a nadir after 1 week from onset.
Our patient’s case of nondiabetic LRPN highlights important learning points.
First, we wish to raise awareness among clinicians that LRPN, known to some as amyotrophy, can occur in nondiabetic patients. Furthermore, LRPN can be painless at presentation. In the 2019 study, Ng et al. reported that five patients (8.1%) were without pain, amongst whom three were nondiabetic, representing the first descriptions of painless nondiabetic LRPN [4, 7]. Our patient therefore adds to this rare and recently described phenomenon, and clinicians should give due consideration to a diagnosis of nondiabetic LRPN even when there is no pain.
Secondly, a meticulous neurological examination is vital in localizing and diagnosing LRPN. Such keen clinical attention to detail is even more important when dealing with an uncommon presentation of an already rare disorder, such as a painless, nondiabetic variant of LRPN in our patient. Clinically, our patient’s weakness of left hip extension indicated involvement of the gluteus maximus and hamstring muscles, or the nerves that supply them, which are the inferior gluteal nerve (S1 myotome) and sciatic nerve (L5 and S1 myotomes) respectively [9]. Weakness of left ankle plantar flexion and dorsiflexion confirmed that both tibial and peroneal divisions of the sciatic nerve were involved, and expanded the myotomes involved to include L4 [9]. Weakness of left knee extension suggested involvement of the quadriceps femoris muscle, supplied by the femoral nerve (L2, L3, and L4 myotomes) [9]. Finally, weakness of left hip adduction further implicated the adductors of the lower limb, supplied by the obturator nerve (mainly L3 and L4 myotomes) [9]. Given the multiple nerves and myotomes involved, one should consider a plexopathy or multilevel radiculopathy to be the most likely localization. The fact that there were also sensory deficits in the left lower limb ruled out a primary neuromuscular junction disorder or focal myopathy. In addition, pin-prick sensation was diminished in the ipsilateral (left) lower limb but normal in the contralateral (right) lower limb, therefore excluding a hemispinal cord localization (that is, Brown Sequard syndrome). With the above considerations, neurodiagnostic tests were selectively performed. The finding of reduced recruitment on EMG of several left lower limb muscles confirmed that the pathology was a neuropathic rather than myopathic disorder. Importantly, as radiculopathy should not cause abnormal sensory responses, the asymmetrically diminished left saphenous sensory response indicated a plexopathy, with or without nerve root involvement [9]. Building on this localization, MRI of the lumbosacral plexus was arranged, showing enhancing nerve roots and thus suggesting that the disorder involved both nerve roots and plexus. The focal nature of presenting symptoms, mostly preserved reflexes, absence of cranial neuropathies, absence of demyelination on NCS, and negative ganglioside antibodies further made a diagnosis of Guillain Barré syndrome (GBS) unlikely. Due to the absence of specific distinguishing clinical features of LRPN, the astute clinician needs to maintain an appropriate index of suspicion even in the absence of diabetes and pain.
Our third learning point is that patients with LRPN may have bilateral enhancement on MRI scans, even when the symptoms and signs are unilateral. One possible explanation is that clinical presentation depends on the severity of inflammation, whereas the MRI findings indicate the extent of involvement without necessarily reflecting severity. This explanation is further supported by previous observations that electrophysiological tests can also demonstrate more widespread involvement than the clinical deficits suggest [3].
The final learning point is the potential value of IVIg in the treatment of patients with nondiabetic LRPN. LRPNs often result in long-term residual neurological deficits, but our patient represents a rare case of complete recovery [8]. While it remains uncertain if our patient’s excellent outcome was entirely influenced by IVIg treatment, histological evidence by preceding studies demonstrating underlying immune-mediated, inflammatory pathogenic processes behind LRPN lends reason to the administration of IVIg [3, 5]. There have also been previous case reports and uncontrolled case series demonstrating recovery in LRPNs after treatment with immunotherapy (involving varying combinations of IVIg, intravenous methylprednisolone, and oral prednisolone), providing further support [6, 10,11,12].
Conclusion
While current clinical descriptions of painless, nondiabetic LRPN remain scarce, our patient’s clinical presentation, treatment, and recovery can add further to the characterization of this uncommon neurological disorder. The learning points illustrated: (1) LRPN can occur in the absence of diabetes mellitus and can be painless at presentation, (2) meticulous examination with targeted adjunctive tests is important for diagnosing LRPN, (3) imaging findings can be more extensive and bilateral even if clinical deficits are focal, and (4) immunotherapy may be useful in patients with nondiabetic LRPN, can help augment the current understanding of this uncommon disorder.
Availability of data and materials
Not applicable.
Abbreviations
- ANA:
-
Antinuclear antibody
- ANCA:
-
Antineutrophil cytoplasmic antibody
- CMV:
-
Cytomegalovirus
- CRMP5:
-
Collapsing response mediator protein 5
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- EBV:
-
Epstein–Barr virus
- EMG:
-
Electromyography
- GAD65:
-
Glutamic acid decarboxylase 65-kilodalton isoform
- GBS:
-
Guillain Barré syndrome
- HIV:
-
Human immunodeficiency virus
- HSV:
-
Herpes simplex virus
- IVIg:
-
Intravenous immunoglobulin
- LRPN:
-
Lumbosacral radiculoplexus neuropathy
- MAG:
-
Myelin-associated glycoprotein
- MRI:
-
Magnetic resonance imaging
- NCS:
-
Nerve conduction study
- PCR:
-
Polymerase chain reaction
- PNMA2:
-
Paraneoplastic antigen Ma2
- SOX1:
-
Sry-like high mobility group box 1
- VDRL:
-
Venereal disease research laboratory
- VZV:
-
Varicella zoster virus
References
Bhanushali MJ, Muley SA. Diabetic and non-diabetic lumbosacral radiculoplexus neuropathy. Neurol India. 2008;56(4):420–5.
Bruns L. Ueber neuritsche Lahmungen beim diabetes mellitus. Berlin Klin Wochenschr. 1890;27:50.
Dyck PJ, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve. 2002;25(4):477–91.
Evans BA, Stevens JC, Dyck PJ. Lumbosacral plexus neuropathy. Neurology. 1981;31(10):1327–30.
Garces-Sanchez M, Laughlin RS, Dyck PJ, Engelstad JK, Norell JE, Dyck PJ. Painless diabetic motor neuropathy: a variant of diabetic lumbosacral radiculoplexus Neuropathy? Ann Neurol. 2011;69(6):1043–54.
Imam Y, Deleu D, Salem K. Idiopathic lumbosacral plexitis. Qatar Med J. 2013;2012(2):85–7.
Ng PS, Dyck PJ, Laughlin RS, Thapa P, Pinto MV, Dyck PJB. Lumbosacral radiculoplexus neuropathy: incidence and the association with diabetes mellitus. Neurology. 2019;92(11):e1188–94.
Pinto MV, Ng PS, Howe BM, Laughlin RS, Thapa P, Dyck PJ, Dyck PJB. Lumbosacral radiculoplexus neuropathy: neurologic outcomes and survival in a population-based study. Neurology. 2021;96(16):e2098–108.
Preston DC, Shapiro BE. Radiculopathy. In: Electromyography and Neuromuscular Disorders. Elsevier; 2021. p. 557–575.
Lee S-E, Lee Y-W, Yoon I-H, Lee C-M. Nondiabetic lumbosacral radiculoplexus neuropathy with abnormal resonance neurography findings. J Neurosonol Neuroimag. 2019;11(1):100–3.
Triggs WJ, Young MS, Eskin T, Valenstein E. Treatment of idiopathic lumbosacral plexopathy with intravenous immunoglobulin. Muscle Nerve. 1997;20(2):244–6.
Dyck PJ, Norell JE, Dyck PJ. Methylprednisolone may improve lumbosacral radiculoplexus neuropathy. Can J Neurol Sci. 2001;28(3):224–7.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
TZB was the primary physician in the patient’s care and was the main author of the manuscript. FWD was involved in the patient’s care and provided key input in establishing the diagnosis. TYJ was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional review board approval was not applicable for this case report.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tan, Z., Foong, W.D. & Tan, YJ. Painless nondiabetic lumbosacral radiculoplexus neuropathy with complete recovery: a case report. J Med Case Reports 17, 485 (2023). https://doi.org/10.1186/s13256-023-04227-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-023-04227-y