Abstract
Background
Chromosomal aberrations are as common as 13.8% in the infertile population. The incidence of pericentric inversion of chromosome 9 is approximately 1–3%. However, although these inversions do not alternate phenotype, there have been conflicting data about their effect as they were correlated with infertility, recurrent pregnancy loss, and deceased children, with no clear evidence of the inversions being the causative factor for these events.
Case presentation
We report a case report of an Arab family with many members with inv(9)(p22q13). Our proband male aged 35 years at time of presentation with primary infertility. Some members, such as a brother aged 34 years, who had this inversion suffered from recurrent pregnancy loss while other members of similar reproductive age did not.
Conclusions
inv(9)(p22q13) might be a hereditary anomaly that might be a risk factor for recurrent pregnancy loss in its members.
Similar content being viewed by others
Introduction
As much as 15% of infertility problems are caused by chromosomal anomalies [1], with the 9th chromosome having the highest degree of variability in structure [2]. Different breakpoints of chromosome 9 inversions can cause diverse disruptions in the carriers. Pericentric inversions occur when a double breakage occurs in each arm of an affected chromosome, which includes the centromere [3]. Although they are dominant in characters that affect both sexes equally, they rarely lead to phenotypic abnormality, and they are of an occurrence of 50% to be inherited if the parent was heterozygous. Having the inverted regions enclose nothing but the chromosomal centromere and its heterochromatin, it rarely causes any genetic aberrancy after crossing over. It was also reported that structural rearrangements of chromosomes could be inherited like dominant characteristics, leading to hypothesized phenotypic abnormality [4, 5]. Moreover, many cytogeneticists consider pericentric chromosome 9 inversions in the heterochromatin as a normal variance, especially when there is no corresponding phenotype. Such is the case of the relatively common pericentric inversion inv(9) (p11q12) and inv(9)(p11q13) [6]. In the general population, it is said that the incidence of which is about 1–3% [7]. However, regardless of being a minor chromosomal rearrangement not related to any alterations in the phenotype, it was reported in the literature with some conflicting data. It was associated with some medical conditions such as infertility, recurrent pregnancy loss, and even deceased infants. Concluding an unclarity on whether inv(9)(p11q12) should be considered normal or abnormal [8]. Heterochromatin variance was also reported to probably cause instability in the chromosome, cancer, and even congenital abnormalities. In addition, carriers of such genetic abnormality are at risk of developing abnormal gametes during meiosis resulting in unbalanced offspring [6, 9, 10]. The phenotype of heterozygosity of this inversion ranges from being normal to a wide range of abnormalities [11]. Furthermore, the chromosomal abnormality inv(9)(p11q13) mentioned as normal earlier was also noted to cause skull and facial abnormalities, mental and growth retardation, congenital skeletal and cardiac malformation, and habitual aborts [12,13,14]. Infertility is defined as the inability of a couple to conceive after 12 months of regular unprotected sexual intercourse [15]. The World Health Organization (WHO) defines the term primary infertility as when a couple has never achieved conception, and the term secondary is the inability to conceive for a couple who had at least one conception [16]. Recurrent pregnancy loss is defined as two or more pregnancy losses according to the American Society for Reproductive Medicine (ASRM) [17]. Our study presents cases of multiple familial obstetric medical outcomes affecting a whole family and suspected to be resulting from a hereditary 9th chromosomal abnormality.
Case
A nonconsanguineous proband couple with Syrian ethnicity presented to the infertility clinic with the complaint of primary infertility lasting for 7 years. The husband who was the proband for his family did not complain of any symptoms affecting ejaculation or sexual intercourse. He had a normal clinical examination, with both testes appearing normal in size and shape. The patient's wife had a history of mononeuritis multiplex, and the proband husband had no medical history but had a history of surgery for a bilateral varicocele of the spermatic cord. The patient was referred to conduct semen analysis which was within the normal ranges for each feature tested. They had no history of drug intake, or exposure to radiation or chemicals. Transvaginal ultrasonography showed a normal uterus with a normal antral follicle count; around 11 in each ovary (hyporesponder < 5 follicles, suboptimal responder 6–10, optimal 11–15, hyperresponder > 15), and the size of the follicles ranged from 5 to 10 mm (follicles were measured on day 3 of the cycles and only the follicles ≤ 10 are counted). Basal hormones on day 3 of cycle were normal [FSH 6.7 mIU/ml (normal range 3.4–10 mIU/ml) LH 4.6 mIU/ml (normal range 1.6–8.3 mIU/ml) Prolactin 13 ng/ml (normal range 3.6–20 ng/ml) Testosterone 0.6 nmol/l (normal range 0.5–2.5 nmol/l) Oestradiol 34 pg/ml (normal range < 50 pg/ml) Progesterone 0.4 ng/ml (normal range 0.32–2 ng/ml). hysterosalpingogram showed normal fallopian tubes. The wife also had some anovulatory cycles, so ovulation induction was tried with clomiphene citrate as simple treatment. Despite the successful ovulatory cycles and successful intrauterine inseminations with the addition of gonadotropins, she failed to conceive. The proband couple was later referred for in-vitro fertilisation (IVF) due to the unexplained infertility.
The husband's brother later on referred to the clinic complaining of recurrent pregnancy loss of four consecutive pregnancies over the course of 5 years. This couple did not have any medical history, and the surgical history was also unremarkable, but the husbands' family had a similar history of four spontaneous abortions for the husbands' parents. Physical examination of the reproductive system for the brother was normal. The brother’s seminogram was also within normal limits according to WHO 2010 criteria. His wife underwent a hysterosalpingogram which was also unremarkable. She also underwent immuno-testing and was lab tested for Anticardiolipin IgG and IgM, ANA, Lupus anticoagulant, Homocysteine, Antiphospholipid IgG and IgM, Thyroid peroxidase, Protein C resistance, TNF-alpha, and all were within normal ranges. Genetic causes were suspected and both couples were referred for karyotyping of peripheral blood cells. The karyotype was tested using the G-Banding technique [18], and all results were reported according to the standard of the 2016 protocols of the International System for Human Cytogenetic Nomenclature (ISCN) [19].
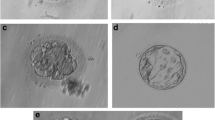
Both the husbands’ karyotype tests showed a common finding of 46,XY,inv(9)(p22q13), but the proband’s brother’s karyotype had a homozygous chromosomal variation, namely 46,XY,inv(9)(p22q13) × 2 (Fig. 1 and 2). Wives karyotyping results were normal for 46,XX.
However, the wives were normal (46,XX). The patients’ families were referred for genetic testing, and the results were that one parent of both affected husbands, that is, the mother, also was a carrier of the genetic abnormality (Fig. 3). Additionally, it was affecting a brother and a sister of both the husbands (Figs. 4 and 5), all sharing the same genetic abnormality of the ninth chromosome of inv(9)(p22q13) (Fig. 6).
Discussion
Familial pericentric inversion of chromosome 9 (p22q33) was described as early as 1980 to cause a malformed child, and it was found that even second-degree cousins had the same clinical features [12]. This emphasises the importance of the familial study when such an anomaly is found. It was believed that chromosomal inversions are rarely detected by using traditional staining. However, using band techniques, which allow a more precise method of detecting such anomalies, minor pericentric inversions were found to be more common than initially found [20, 21].
It was found that children born from a heterozygous mother are more than those born from a heterozygous father, which may suggest a relatively declined fertility in males compared to females, or a relatively improved fertility in females compared to males [22]. Although a study found that chromosome 9 inversions were more common in females, especially in those who suffer from infertility [23], Inv(9) was studied in the male sperm, and it was found that the DNA in the sperm had many more abnormalities such as fragmentations, alterations in the meiosis, aneuploidy, and changes in seminogram parameters [9]. This concurs with our study in that we found the proband couple’s infertility to be of male factor.
Furthermore, it was found in one study that all inverted chromosomes were identical within the family of the pedigree, having the same length in the normal homologous with the result that there is no loss of material. There was no evidence of new mutations in individuals coming from two such parents. Therefore, there were no de novo chromosomal inversions [24]. This could explain our finding of the same chromosomal aberration in all of our studied pedigree.
Chromosomal inversions might be even more common than anticipated because tests that detect them are only requested on clinical grounds, neglecting the subclinical presentations or cases that cause no phenotypic abnormality whatsoever [5, 25]. Such is the case in how our studied pedigree’s genetic abnormality was detected based on the symptomatic presentation in our clinic. Most patients appear normal and relatively can reproduce; hence, inversion carriers may propagate such chromosomal aberrations without even noticing any abnormalities [11].
It is also difficult to conclude whether any of the inversions truly increases spontaneous abortions. One family whose mother had heterozygous inv(9) suffered from four spontaneous abortions with no children. The first and second metaphases appeared to be normal, which may suggest that the meiotic division was not grossly affected, though it does not rule out the effect of crossing over [24]. Accordingly, chromosomal aberrations could be responsible for as much as 50% of all spontaneous abortions and miscarriages, in addition to many congenital abnormalities [10, 26]. Many reports are emerging in the medical literature on the importance of chromosome 9 inversions in correlation with unfavourable obstetric history [27, 28]. Correspondingly, chromosome 9 inversion was reported in 2.3% of couples who suffered from recurrent spontaneous miscarriages and infertility [29]. One of our patients, namely the proband’s brother, and the family’s parents, also suffered from four spontaneous abortions. The brother’s abnormality was homozygous, and no other cause of spontaneous abortion was found. Also, we can only assume that the patient's brother with the homozygous variation might have suffered from heterodisomy in inheriting two copies from the heterozygous mother, similar to one case in a previously reported study [30]. Furthermore, the proband and his brother also had seminograms within normal limits, and despite the proband's surgical history, this did not affect the semen quality.
However, we cannot know for certain whether to count inv(9) as normal or abnormal [8] because it sometimes raises the instability of the chromosome, malignancies, and congenital malformations [12,13,14]. Chromosome 9 has the highest morphological variance, with inversions being the most common alteration, and it is believed that it is being inherited by Mendelian rules [8, 31]. Moreover, such a common abnormality was reported in a few studies to be related to infertility and congenital malformations [32]. Therefore, it is believed it should be considered harmful, especially with the aforementioned reports suggesting its negative effect on obstetric history, sterility, and frequent miscarriages [27, 28, 33, 34]. In contrast, other reports reported a correlation of such findings with abnormalities in chromosomes 1 and 10 [4, 9, 35]. However, our study was in line with the inherited chromosome 9 abnormality being the causative factor.
Phenotypes of Inv(9) depend on the breakpoint locations, for example, (p11q12), which is believed to have one type of breakpoint at (p11q12), with the result that it leads to no effect on the carrier nor causes any imbalances or miscarriages in the pedigree [36]. Moreover, during the reunion after the breakage, many alterations may occur in the euchromatic sequences ranging from suppressing to deleting, which can cause various abnormalities [37]. Such can explain the persistence of alterations in one pedigree. It was also reported that chromosome 9 [46,XX,inv(9)(p11-q13)] could phenotype as complete hydatidiform mole [38], and inv(9) can also predispose to schizophrenia [36]. Different breakpoint regions for inv(9) correlated with different clinical aspects. More studies using molecular cytogenetic probes on a genome-wide basis are needed in order to make causative relations between each type of chromosomal 9 inversion and their distinct clinical syndromes.
In Conclusion, inv(9)(p22q13) can be a hereditary chromosomal anomaly which might be a risk factor for recurrent pregnancy loss, though literature data might be conflicting on the matter. Cytogenetic investigation could be a mainstay diagnostic step in the standard of care for cases of unexplained recurrent pregnancy loss, in addition to unexplained infertility, especially in patient populations sharing a similar familial obstetric history.
Availability of data and materials
The data can be made available upon reasonable request.
Abbreviations
- ANA:
-
Antinuclear Antibody
- ASRM:
-
American Society for Reproductive Medicine
- FSH:
-
Follicle Stimulating Hormone
- Ig:
-
Immunoglobulin
- Inv:
-
Inversion
- ISCN:
-
International system for human cytogenetic nomenclature
- IVF:
-
In-vitro fertilisation
- LH:
-
Luteinizing Hormone
- TNF:
-
Tumour necrosis factor
- TNF-alpha:
-
Tumor Necrosis Factor alpha
- WHO:
-
World Health Organization
References
Beaumont M, Tucker EJ, Mary L, Launay E, Lurton Y, Pimentel C, et al. Pseudodicentric chromosome originating from autosomes 9 and 21 in a male patient with oligozoospermia. Cytogenet Genome Res. 2019;159(4):201–7.
Cheong K, Knight L, Tan M, Ng I. Variants of chromosome 9 in phenotypically normal individuals. Ann Acad Med Singapore. 1997;26(3):312–4.
Young D, Klepacka D, McGarvey M, Schoolcraft W, Katz-Jaffe M. Infertility patients with chromosome inversions are not susceptible to an inter-chromosomal effect. J Assist Reprod Genet. 2019;36(3):509–16.
Zhang X, Shi Q, Liu Y, Jiang Y, Yang X, Liu R, et al. Fertility problems in males carrying an inversion of chromosome 10. Open Medicine. 2021;16(1):316–21.
Entesarian M, Carlsson B, Mansouri MR, Stattin EL, Holmberg E, Golovleva I, et al. A chromosome 10 variant with a 12 Mb inversion [inv(10)(q11.22q21.1)] identical by descent and frequent in the Swedish population. Am J Med Genet A. 2009;149(3):380–6.
Ait-Allah AS, Ming P-ML, Salem HT, Reece EA. The clinical importance of pericentric inversion of chromosome 9 in prenatal diagnosis. J Maternal Fetal Invest. 1997;7:126–8.
Amiel A, Sardos-Albertini F, Fejgin MD, Sharony R, Diukman R, Bartoov BJ. Interchromosomal effect leading to an increase in aneuploidy in sperm nuclei in a man heterozygous for pericentric inversion (inv 9) and C-heterochromatin. J Hum Genet. 2001;46(5):245–50.
Ceylan G, Ceylan C, Yuce H. A rare seen case with homozygosity for pericentric inversion of chromosome 9 and primary infertility. Am J Case Rep. 2008;9:385–8.
Balasar Ö, Zamani AG, Balasar M, Acar H. Male infertility associated with de novo pericentric inversion of chromosome 1. Turk J Urol. 2017;43(4):560–2.
Zhang H, Wang R, Yu Y, Zhu H, Li L, Yang X, et al. Non-Robertsonian translocations involving chromosomes 13, 14, or 15 in male infertility: 28 cases and a review of the literature. Medicine. 2019;98(9):e14730.
Sudha T, Jayam S. Pericentric inversion in homologues of chromosome 9. Jpn J Hum Genet. 1993;38(3):341–3.
Mattei J, Mattei M, Balestrazzi P, Giraud F. Familial pericentric inversion of chromosome 9, INV (9)(p22q32) with recurrent duplication-deletion. Clin Genet. 1983;24(3):220–2.
Lourenço GJ, Silva PM, Bognone RA, De Souza RA, Delamain MT, Lima CS. Inherited pericentric inversion of chromosome 9 in acquired hematological disorders. Ann Hematol. 2007;86(6):465–7.
Ramegowda S, Savitha MR, Krishnamurthy B, Doddaiah N, Prasanth SN, Ramachandra NB. Association between pericentric inversion in chromosome 9 and congenital heart defects. Int J Human Genet. 2007;7(3):241–8.
Gurunath S, Pandian Z, Anderson RA, Bhattacharya S. Defining infertility–a systematic review of prevalence studies. Hum Reprod Update. 2011;17(5):575–88.
Tabong PT, Adongo PB. Infertility and childlessness: a qualitative study of the experiences of infertile couples in Northern Ghana. BMC Pregnancy Childbirth. 2013;13:72.
Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–11.
Babu A, Verma RS. Characterization of human chromosomal constitutive heterochromatin. Can J Genet Cytol. 1986;28(5):631–44.
McGowan-Jordan J, Simons A, Schmid M. Iscn 20162016.
Hamerton JL, Ray M, Abbott J, Williamson C, Ducasse GC. Chromosome studies in a neonatal population. Can Med Assoc J. 1972;106(7):776.
Guttenbach M, Engel W, Schmid M. Analysis of structural and numerical chromosome abnormalities in sperm of normal men and carriers of constitutional chromosome aberrations. A review. Human Genet. 1997;100(1):1–21.
Kara M, Sen A, Cetin ES, Kargun K. Chromosomal translocation t (10; 19)(q11/ 2; q13/ 4) in an infertile male. Eurasian J Med. 2014;46(3):220.
Šípek A Jr, Panczak A, Mihalová R, Hrčková L, Suttrová E, Sobotka V, et al. Pericentric inversion of human chromosome 9 epidemiology study in czech males and females. Folia Biol. 2015;61:140–6.
de la Chapelle A, Schröder J, Stenstrand K, Fellman J, Herva R, Saarni M, et al. Pericentric inversions of human chromosomes 9 and 10. Am J Human Genet. 1974;26(6):746.
Soukup SW. Human cytogenetics. Vol. 1. General Cytogenetics. Vol. 2. Clinical Cytogenetics. By J. L. Hamerton. Academic Press, New York. 412 and 545 pp. 1971. Teratology. 1972;6(2):248–50.
Rimoin DL, Connor JM, Pyeritz RE, Korf BR. Emery and Rimoin’s principles and practice of medical genetics. Churchill Livingstone Elsevier; 2007.
Mohsen-Pour N, Talebi T, Naderi N, Moghadam MH, Maleki M, Kalayinia S. Chromosome 9 Inversion: pathogenic or Benign? A comprehensive systematic review of all clinical reports. Curr Mol Med. 2022;22:385.
Khaleghian M, Azimi C. Homozygosity for pericentric inversions of chromosome 9 in a patient’s parents with stillbirth-report of a new case and review of literature. Iran J Public Health. 2006;35(3):22–7.
Sasiadek M, Haus O, Lukasik-Majchrowska M, Slezak R, Paprocka-Borowicz M, Busza H, et al. Cytogenetic analysis in couples with spontaneous abortions. Ginekol Pol. 1997;68(5A):248–52.
Fritz B, Aslan M, Kalscheuer V, Ramsing M, Saar K, Fuchs B, et al. Low incidence of UPD in spontaneous abortions beyond the 5th gestational week. Eur J Hum Genet. 2001;9(12):910–6.
Luke S, Verma R, Conte RA, Mathews T. Molecular characterization of the secondary constriction region (qh) of human chromosome 9 with pericentric inversion. J Cell Sci. 1992;103(4):919–23.
Malan V, Pipiras E, Sifer C, Kanafani S, Cedrin-Durnerin I, Martin-Pont B, et al. Chromosome segregation in an infertile man carrying a unique pericentric inversion, inv (21)(p12q22. 3), analysed using fluorescence in situ hybridization on sperm nuclei: significance for clinical genetics. A case report. Human Reprod. 2006;21(8):2052–6.
Srebniak MG, Wawrzkiewicz A, Wiczkowski A, Kazmierczak W, Olejek A. Subfertile couple with inv (2), inv (9) and 16qh+. J Appl Genet. 2004;45(4):477–9.
Mozdarani H, Meybodi AM, Karimi H. Impact of pericentric inversion of Chromosome 9 [inv (9)(p11q12)] on infertility. Indian J Human Genet. 2007;13(1):26.
Li R, Fan H, Zhang Q, Yang X, Zhan P, Feng S. Pericentric inversion in chromosome 1 and male infertility. Open Med (Wars). 2020;15(1):343–8.
Lee KB, Kunugi H, Nanko S. Familial schizophrenia with pericentric inversion of chromosome 9: a case report. Schizophr Res. 1998;32(2):123–6.
Rao BV, Kerketta L, Korgaonkar S, Ghosh K. Pericentric inversion of chromosome 9 [inv (9)(p12q13)]: its association with genetic diseases. Indian J Hum Genet. 2006;12(3):129–32.
Abdalla EM, El-Kharadly RN. Pericentric inversion of chromosome 9 in a consanguineous couple with molar pregnancies and spontaneous abortions. Laboratory Medicine. 2012;43(5):212–6.
Acknowledgements
Not applicable.
Funding
We received no funding in any form.
Author information
Authors and Affiliations
Contributions
MMA: Validation; Supervision; Original draft; Investigation; Resources. AK:; Original draft; Writing—review & editing. MA: Conceptualization; Methodology; Project administration; Resources. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research article's ethical standards were reviewed and approved by the Damascus University Faculty of Medicine's ethical committee.
Consent for publication
Written informed consent was obtained from the patients for publication of this case report and any accompanying images. A copy of the written consents is available for review by the Editor-in-Chief of this journal.
Competing interests
We have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alhalabi, M.M., Kakaje, A. & Alhalabi, M. Hereditary chromosomal 9 inversion (p22q13) 9 as a cause for recurrent pregnancy loss: a case report. J Med Case Reports 17, 427 (2023). https://doi.org/10.1186/s13256-023-04137-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-023-04137-z