Abstract
Background
Cerebral cardiac embolism accounts for an increasing proportion of ischemic strokes and transient ischemic attacks. Calcified cerebral emboli are rare and mostly iatrogenic secondary to heart or aorta catheterization. However, spontaneous cerebral calcified embolism in the case of calcified aortic valve is very rare and there are less than 10 case reports in the literature. And a more interesting fact is that such an event, in the context of calcified mitral valve disease, has never been reported, at least to our knowledge. We are reporting a case of spontaneous calcified cerebral embolism revealing a calcified rheumatic mitral valve stenosis.
Case presentation
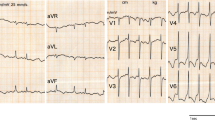
We report a case of a 59 year-old Moroccan patient, with a history of rheumatic fever at the age of 14 and no history of recent cardiac intervention or aortic/carotid manipulation, who was admitted to the emergency department after a transient ischemic attack. Physical examination at admission found normal blood pressure of 124/79 mmHg and heart rate of 90 bpm. A 12-lead electrocardiogram showed an atrial fibrillation, no other anomalies. Unenhanced cerebral computed tomography imaging was performed, revealing calcified material inside both middle cerebral arteries. Transthoracic echocardiography was performed, showing severe mitral leaflets calcification with a severe mitral stenosis, probably due to rheumatic heart disease. Cervical arteries Duplex was normal. A vitamin K antagonist (acenocoumarol) was prescribed, targeting an international normalized ratio of 2–3 and mitral valve replacement surgery was performed using mechanical prosthesis. Short- and long-term health, with a 1-year follow-up, were good and the patient did not experience any stroke.
Conclusion
Spontaneous calcified cerebral emboli secondary to mitral valve leaflet calcifications is an extremely rare condition. Replacement of the valve is the only option to prevent recurrent emboli and outcomes are still to be determined.
Similar content being viewed by others
Background
Cerebral cardiac embolism accounts for an increasing proportion of ischemic strokes and transient ischemic attacks [1]. Calcified cerebral emboli are rarely reported, but potentially cause of strokes and transient ischemic attacks and may be the first manifestation of vascular or cardiac disease. Identification of the source of embolization is crucial to prevent future emboli, neurological damage, and death. Non-contrast computed tomography (CT) scan of the head is the most common imaging procedure used as the initial assessment of suspected stroke or transient ischemic attack [2].
Cerebral calcified embolus can occur after percutaneous and surgical intervention in the context of calcified aortic or mitral valve disease [3]. These emboli are presumed to occur because of valve trauma. However, spontaneous cerebral calcified embolism in the case of calcified aortic valve is very rare and there are less than ten case reports in the literature [4]. And a more interesting fact is that such an event in the context of calcified mitral valve disease has never been reported, at least to our knowledge.
We are reporting a case of spontaneous calcified cerebral embolism revealing a calcified rheumatic mitral valve stenosis.
Case presentation
We are reporting a case of a 59-year-old Moroccan man presenting to the emergency department after a transient ischemic attack (right hemiparesia and left central facial paralysis resolving briefly and spontaneously). There was a history of rheumatic fever at age 14 and stage II New York Heart Association (NYHA) dyspnea on moderate exertion for 2 years, with no history of recent cardiac intervention or aortic/carotid manipulation and no other symptoms. Physical examination on admission found an irregular heart rhythm of 90 bpm, blood pressure (BP) of 124/79 mmHg, mid-diastolic rumble at apex, no signs of heart congestion, and no signs of neurological impairment. A 12-lead-electrocardiogram showed an atrial fibrillation without other anomalies. Two-dimensional (2D) transthoracic echocardiograms revealed important mitral valve leaflets calcifications, probably related to rheumatic heart disease; planimetry of the valve was not possible. Continuous wave Doppler interrogation of the mitral valve found a severe mitral stenosis with a mean gradient of 15 mmHg and a continuity equation surface of 1 cm2. The aortic valve was thickened, but not calcified, a moderate aortic regurgitation was noticed. Left ventricular (LV) function was normal and the LV ejection fraction (LVEF) was at 55%. There was a right ventricular (RV) longitudinal systolic dysfunction: TAPSE 11 mm and S’VD 6 cm/second. Tricuspid valve was thin with a mild tricuspid regurgitation. Continuous wave Doppler interrogation of the tricuspid valve allowed to estimate systolic pulmonary artery pressure at 69 mmHg. Mitral valve calcification was best shown on a cardiac CT (Fig. 1).
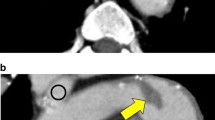
Unenhanced cerebral CT was performed, revealing calcified emboli in both middle cerebral arteries (M3 and M4 segments) (Fig. 2). Susceptibility weighted magnetic resonance cerebral sequences result is reported in Fig. 3.
Cervical arteries Duplex showed normal carotid and vertebral arteries.
A vitamin K antagonist (acenocoumarol) was prescribed, targeting an international normalized ratio (INR) of 2–3 and replacement of mitral valve using a mechanical prosthesis was performed with a good short-term and long-term outcome. The patient did not experience ischemic stroke during a 1-year follow-up.
Discussion
In our patient, transient ischemic attack (TIA) was certainly due to atrial fibrillation (AF), and cerebral imaging lead to the discovery of calcified emboli.
Approximately 6%–31% of TIA are caused by a cardiogenic cerebral embolism (cardioembolic TIA) [5, 6]. Determining TIA etiology is important before administering therapy. Permanent or paroxysmal, valvular and non-valvular, AF is associated with a three- to five-fold increased risk of TIA and stroke [7, 8]. Cardiogenic cerebral embolization is common among patients with any cause of AF, but particularly in AF resulting from rheumatic and arteriosclerotic heart disease [9]. It is recommended to prescribe these patients oral anticoagulant therapy in case of valvular AF and, according to CHA2DS2VASc score, in case of non-valvular AF.
Calcified cerebral emboli are an infrequent, but increasingly recognized cause of TIA and ischemic stroke, although recognition among general radiologists and clinicians can be limited. Unenhanced CT and computed tomography angiography (CTA) are the imaging techniques of choice for the diagnosis [10, 11]. First described on CT by Yock in 1981, calcified cerebral emboli were previously thought to be unusual, and to most commonly arise following instrumentation of calcified cardiac valves or direct aortic/carotid artery manipulation [12, 13]. However, there is growing evidence that spontaneous calcified cerebral embolism is more common, with a recent study and review of published cases reporting a 2.7% prevalence among a group of patients presenting with suspected stroke over a 1-year period. In this report, the middle cerebral artery was the site of 83% calcified emboli. Cardiac valvular disease was more common than carotid atheromatous disease, with calcified aortic stenosis three times more common than mitral valve disease as the embolic source [3].
Iatrogenic calcified embolus following cardiac surgery or catheterization is common [4, 14, 15]. These emboli are presumed to occur because of valve trauma. According to a most recent postmortem analysis of iatrogenic embolization cases, the source of calcified cerebral emboli was attributed to dislodgement and displacement of calcified material from calcified aortic valves and ulcerated aortic atherosclerotic plaques during therapeutic and investigative procedures [16].
There are some reported cases of cerebral calcific emboli following open heart mitral valvotomy and percutaneous mitral valvuloplasty. Mitral calcification accounts for fewer than 1% of cerebral cardiac embolism, and in all described cases, it was secondary to mitral valve intervention [16]. However, there is not any case report published describing spontaneous calcified cerebral emboli in the context of calcified rheumatic mitral stenosis.
In case of stroke secondary to calcified emboli, the role of thrombolysis remains uncertain, as there are conflicting reports regarding its efficacy in this setting [17,18,19]. There is debate and very limited experience regarding the place of mechanical thrombectomy [18, 20]. Subsequent imaging evaluation of this subgroup of patients who have suffered from ischemic strokes requires caution because the calcified nature of the embolus may be obscured on CT angiography or magnetic resonance imaging (MRI). Clinical evaluation should include consideration of potential proximal source of calcified material and recent cardiac intervention or aortic/carotid manipulation. Although there is no data showing benefit of valve replacement, most authors advocate valve replacement to remove the source of emboli.
Conclusion
Spontaneous calcified cerebral emboli, secondary to mitral valve leaflet calcification is an extremely rare condition. Replacement of the valve is the only option to prevent recurrent emboli and outcomes are still to be determined.
Availability of data and materials
The published information is available from the corresponding author on reasonable request.
References
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial TOAST Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41.
Walker BS. Calcified cerebral emboli, a “do not miss” imaging diagnosis: 22 new cases and review of the literature. AJNR Am J Neuroradiol. 2014;35:1515–9. https://doi.org/10.3174/ajnr.A3892.
Kapila A, Hart R. Calcific cerebral emboli and aortic stenosis: detection by computed tomography. Stroke. 1986;17:619–21.
Khetarpal V. Calcific aortic valve and spontaneous embolic stroke: a review of literature. J Neurol Sci. 2009;287:32–5.
Bogousslavsky J, Hachinski VC, Boughner DR. Cardiac and arterial lesions in carotid transient ischemic attacks. Arch Neurol. 1986;43:223–8.
Sempere AP, Duarte J, Cabezas C, et al. Etiopathogenesis of transient ischemic attacks and minor ischemic strokes: a community-based study in Segovia, Spain. Stroke. 1998;29:40–5.
Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8.
Wolf PA, Dawber TR, Thomas HE Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28:973–7.
Stirling J. Cerebral embolism as a cause of stroke and transient ischemic attack. Echocardiography. 1996;13(5):513–8.
Rancurel G, Marelle L, Vincent D, Catala M, Arzimanaglou A, Vacheron A. Spontaneous calcific cerebral embolus from a calcific aortic stenosis in a middle cerebral artery infarct. Stroke. 1989;20:691–3.
Oliveira-Filho J, Massaro AR, Yamamoto F, Bustamante L, Scaff M. Stroke as the first manifestation of calcific aortic stenosis. Cerebrovasc Dis. 2000;10:413–6.
Yock DH. CT demonstration of cerebral emboli. J Comput Assist Tomogr. 1981;5:190–6.
Khaw N, Gailloud P. CT of calcific cerebral emboli after carotidmanipulation. Am J Roentgenol. 2000;174:1467.
Salka S, Almassi H, Leitshuh M. Spontaneous coronary artery embolus associated with calcific artery stenosis. Chest. 1994;105:1289–90.
Wilson JH, Cranley JJ. Recurrent calcium emboli in a patient with aortic stenosis. Chest. 1989;96:1433–4.
Hickey TBM. Iatrogenic embolization following cardiac intervention: postmortem analysis of 110 cases. Cardiovasc Pathol. 2019;40:12–8.
O’Cearbhaill RM, Moriarty HK, Crosbie I, et al. Calcified cerebral emboli: a case series and review of the literature. J Syst Int Neurosci. 2016;2:180–3.
Halloran JI, Bekavac I. Unsuccessful tissue plasminogen activator treatment of acute stroke caused by a calcific embolus. J Neuroimaging. 2004;14:385–7.
Raghib MF, Mutzenback JS, Rosler C, et al. Acute treatment of stroke due to spontaneous calcified cerebral emboli causing large vessel occlusion. J Clin Neurosci. 2018;47:56–61.
Katsamakis G, Lukovits TG, Gorelick PB. Calcific cerebral embolism in systemic calciphylaxis. Neurology. 1998;51:295–7.
Acknowledgements
Not applicable.
Funding
The authors have no funding to declare.
Author information
Authors and Affiliations
Contributions
MH conceived the study, participated in its design, acquired the data, performed a literature review, and drafted the manuscript. SA participated in the design of the study and helped with the literature review. HM helped with the literature review. GM helped with the literature review. AM helped with the literature review. SA participated in the design of the study. MEGB helped with the literature review, and helped draft and edit the manuscript. AD helped draft the manuscript. LA helped draft and edit the manuscript. RH helped draft and edit the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The need for ethics approval was waived.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Haboub, M., Abouradi, S., Mechal, H. et al. Spontaneous calcific cerebral embolization revealing a calcified rheumatic mitral stenosis: a case report. J Med Case Reports 17, 254 (2023). https://doi.org/10.1186/s13256-023-03982-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-023-03982-2