Abstract
Background
Streptococcus cristatus is a member of the Mitis streptococcus group. Like other members of this group, it resides on mucosal surfaces of the oral cavity. However, little is known about its ability to cause disease as there are only a handful of cases in the literature. Two of these cases involved infective endocarditis with significant complications. However, these cases involved additional microbes, limiting the inferences about the pathogenicity of Streptococcus cristatus.
Case presentation
A 59-year-old African American male with end-stage cryptogenic cirrhosis and ascites presented with fatigue and confusion. A paracentesis was negative for spontaneous bacterial peritonitis, but two separate blood cultures grew Streptococcus cristatus. Our patient had a history of dental caries and poor oral hygiene, which were likely the source of the infection. Echocardiograms revealed new aortic regurgitation, indicating “possible endocarditis” per the Modified Duke Criteria. However, since his clinical picture and cardiac function were reassuring, we elected against treatment for infective endocarditis. He was treated for bacteremia with a 2-week course of cephalosporins consisting of 8 days of ceftriaxone, transitioning to cefpodoxime after discharge. Despite having end-stage liver disease, our patient did not experience any significant complications from the infection.
Conclusion
A patient with end-stage cirrhosis and poor oral hygiene developed bacteremia with an oral bacterium called Streptococcus cristatus. Unlike previous cases in literature, our patient did not meet criteria for a definitive diagnosis of infective endocarditis, and he experienced no other complications from the infection. This suggests coinfectants may have been primarily responsible for the severe cardiac sequelae in prior cases, whereas isolated Streptococcus cristatus infection may be relatively mild.
Similar content being viewed by others
Background
Streptococcus cristatus (S. cristatus) belongs to the Mitis group of the Streptococcus genus. Like many Streptococcus species, S. cristatus is a commensal bacterium of the human oral cavity. It was first characterized in 1991 [1], as phenetic tests became more sophisticated. One species of oral streptococcus resembled Streptococcus sanguis, except with tufts of lateral fibrils. With new phenetic tests and DNA–DNA hybridization experiments, this species was reliably distinguished from other closely related streptococci. It was named Streptococcus crista and first described as “Gram-positive, catalase-negative cocci that are approximately 1 μm in diameter and grow in chains,” along with various biochemical descriptors, e.g., α-hemolytic and arginine metabolizer [1].
More than 30 years later, the pathogenicity of S. cristatus remains somewhat of a mystery. It appears to be a rare cause of disease, as there are only five cases in the literature [2,3,4]. They are summarized in Table 1. One other report referred to a patient’s history of “recurrent Streptococcus cristatus bacteremia originating from poor dentition” [5] but did not elaborate further.
The reports suggest S. cristatus can cause infective endocarditis (IE) with major complications, even in healthy adults [2, 3]. There are three cases in adults, two of which involved IE with valvular dysfunction of the aortic and mitral valves, requiring valvular surgery in one patient and progressing to heart failure in the other [2]. The third adult case [3] involved postoperative endophthalmitis after glaucoma surgery, suggesting S. cristatus may also inhabit the periocular flora and become pathogenic after breach of the globe exterior.
Case presentation
A 59-year-old African American male with end-stage cryptogenic cirrhosis presented to the emergency room (ER) endorsing progressive fatigue and confusion for 4 days. At baseline, the patient could complete all activities of daily living but lived with three family members who helped him with instrumental activities of daily living such as cooking and shopping. Per his niece who had accompanied him to the ER, he was now rarely leaving his bed due to fatigue, and his confusion had progressed such that he was now answering basic questions incorrectly.
His cirrhosis had been characterized as cryptogenic after a transplant evaluation failed to reveal an etiology. It had been classified as “end-stage” due to a Model for End-stage Liver Disease (MELD-Na) score of 27 (estimated 90-day mortality of 20%) [6] and a Class C Child–Pugh Score (life expectancy 1–3 years) [7]. He required monthly paracenteses and, in the past, required treatment for spontaneous bacterial peritonitis (SBP), hepatic encephalopathy, and gastrointestinal bleeds (GIBs) secondary to esophageal varices and portal hypertensive gastropathy.
In the ER, he was noted to be somnolent and oriented only to self. A physical exam revealed a distended abdomen with shifting dullness, and a lower extremity exam revealed +2 pitting edema. Labs were remarkable for ammonia of 106 µmol/L and a hemoglobin of 7.0 g/dL, down from a baseline of ~ 9 g/dL. He denied melena or any outright bleeding. Because of his ongoing ascites and altered mental status, he underwent a combined diagnostic/therapeutic paracentesis in the ER. Additionally, two blood cultures were drawn from separate venipuncture sites. He was initiated on treatment for suspected GIB (two large bore intravenous drips, intravenous pantoprazole, intravenous octreotide, intravenous ceftriaxone) and hepatic encephalopathy (lactulose and rifaximin titrated to 3–4 bowel movements per day) and admitted to the general practice unit.
The next day, he underwent esophagogastroduodenoscopy (EGD), which revealed grade one varices and diffuse portal hypertensive gastropathy, but no active bleeding. Octreotide was discontinued at this point. Peritoneal cultures obtained in the ER revealed no evidence of SBP, but both blood cultures grew Streptococcus cristatus. Minimal inhibitory concentration using the ETEST method reveal antibiotic susceptibility to ceftriaxone, clindamycin, erythromycin, tetracycline, and vancomycin. The patient had an ongoing history of poor oral hygiene and dental caries, which were likely the source of this bacteremia.
Because of his positive blood cultures, we assessed for the presence of IE. We did not appreciate any cardiac murmurs on physical exam or other findings suggestive of IE such as Roth’s spots, Osler’s nodes, or Janeway lesions. However, transthoracic echocardiogram revealed new mild-to-moderate aortic regurgitation compared with prior echocardiograms, without other evidence of IE, in other words no vegetations, abscesses, or perforations. This new-onset aortic regurgitation with positive blood cultures indicated “possible endocarditis” per the modified Duke Criteria [8]. As such, a transesophageal echocardiogram was obtained to further assess for IE. This echocardiogram (Fig. 1) similarly showed mild-to-moderate aortic regurgitation without other findings of IE. Per Duke’s Criteria guidelines, we used clinical judgement at this point and elected not to treat him for IE.
A Mid-esophageal transesophageal echocardiogram image displaying two regurgitant jets (arrows) flowing from the aorta (Ao) to the left ventricle (LV), indicative of new onset mild-to-moderate aortic regurgitation; RV, right ventricle; LA, left atrium. B A structurally normal aortic valve (arrow) observed in the same view using standard ultrasound mode
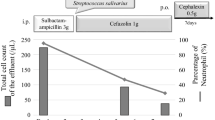
He was treated for bacteremia with a two-week course of cephalosporins. He received 8 days of 1 g/day intravenous ceftriaxone, which was started on day one for prophylactic treatment of suspected upper GIB. Upon discharge, he was transitioned to 400 mg/day of oral cefpodoxime for 6 days. Despite his advanced comorbidities and ongoing GIB, the infection was mild, and he recovered well. He did not exhibit any constitutional symptoms or any Systemic Inflammatory Response Syndrome (SIRS) criteria throughout his entire hospital stay. His mentation improved after lactulose, and subsequent blood cultures remained negative. The infection did not delay his discharge, which was determined primarily by the stability of his hemoglobin. His clinical course is summarized in Table 2.
Microbiology
The identification process started with the preparation of blood, MacConkey (Mac), and chocolate agar plates. Each plate was cultured and incubated overnight at 35 ± 2 °C. The following day, alpha-hemolytic growth was seen in the blood and chocolate agar, but not in the Mac agar. The colonies were also found to be catalase-negative, and Gram staining revealed Gram-positive cocci in pairs.
To determine the presence of S. cristatus, MALDI-TOF and Vitek 2 biochemical panels were performed. In addition, the Vogus-Proskauer (VP) test was negative, which was used to rule out Streptococcus sanginosus. Since S. cristatus is a member of the Mitis/Oralis group of the streptococcal species, the biochemical profile was negative for mannitol, urea hydrolysis, and VP, but variable for arginine hydrolysis, esculin, and sorbitol. No other organism was identified at any point in the culturing process.
Discussion
To summarize, this was a case of Streptococcus cristatus bacteremia and “possible endocarditis” in a 59-year-old male with a history of dental disease and end-stage cryptogenic cirrhosis. To our knowledge, this is the sixth clinical case of S. cristatus. As outlined in Table 1 [2,3,4], the other reports involved cases of bacteremia, infective endocarditis, endophthalmitis, and septic arthritis, and they involved both pediatric and middle-aged patients.
Our case is the only case in an adult in which a likely source was identified for the infection. Our patient had a history of poor oral hygiene and dental caries, and S. cristatus is an oral bacterium. Like other members of the streptococcus genus, S. cristatus can live in harmony with the healthy oral mucosa but with breaks in this protective layer, it appears capable of entering the blood and causing secondary infections. This highlights the importance of the oral exam, especially in patients with higher comorbidities, as poor oral hygiene is a known risk factor for bacteremia and IE [9].
Our case involved an inconclusive diagnosis of “possible endocarditis” based on the presence of bacteremia with new valvular regurgitation. We elected not to treat him for IE on the basis of clinical judgement. If there was any endocardial involvement, it appeared to be minimal, as the patient was asymptomatic, with normal hemodynamic parameters (aside from chronic hypotension secondary to cirrhosis) and relatively normal cardiac parameters on echocardiograms. As such, we were comfortable with a two-week course for bacteremia. Conversely, there are two cases of IE in the literature [2], one of which required surgical valve replacement and the other progressing to heart failure. However, unlike our case, these cases involved a second microbe known to cause IE [10], namely Coagulase-negative Staphylococcus and Streptococcus mitis. Thus, the endocardial affinity and virulence of S. cristatus may be significantly lower than suggested on the basis of these other cases with coinfectants.
Our case is also the only case with a past medical history of severe chronic disease, as our patient was suffering from end-stage liver disease. Thus, there are now reports of S. cristatus infections in generally healthy patients, as well as severely ill patients.
Our patient was successfully treated with an antibiotic regimen consisting of 8 days of intravenous ceftriaxone, transitioning to 6 days of oral cefpodoxime. Ceftriaxone was used in one other case [2], and it did not lead to any clinical or biochemical improvement, prompting a switch to a different regimen. It is not clear from this case whether cephalosporins failed because of progression of S. cristatus or the coagulase-negative staphylococcus.
The main limitation of our case was the inconclusive diagnosis of “possible endocarditis.” Isolating S. cristatus from our patient’s endocardium, as was done in one previous case [2], could have established a more conclusive result, but our patient did not require valvular surgery. Further reports of isolated S. cristatus IE, especially those requiring valvular surgery, could help elucidate S. cristatus’s pathogenicity toward the endocardium. Similarly, it would also have been useful to isolate S. cristatus from our patient’s oral cavity to directly link his dental disease to the bacteremia.
Conclusion
A patient with dental disease developed bacteremia and “possible endocarditis” with an oral bacterium called Streptococcus cristatus. Whereas other case reports in literature involved severe infective endocarditis, our patient’s infection was mild and was adequately treated with cephalosporin antibiotics.
Availability of data and materials
Data sharing is not applicable to this article as no data sets were generated or analyzed during this study.
Abbreviations
- ER:
-
Emergency room
- GIB:
-
Gastrointestinal bleed
- IE:
-
Infective endocarditis
- MELD:
-
Model for End-Stage Liver Disease
- SBP:
-
Spontaneous bacterial peritonitis
- SIRS:
-
Systemic inflammatory response syndrome
- pRBCs:
-
Packed red blood cells
- VP:
-
Vogus–Proskauer
References
Handley P, Coykendall A, Beighton D, Hardie JM, Whiley RA. Streptococcus crista sp. nov., a viridans streptococcus with tufted fibrils, isolated from the human oral cavity and throat. Int J System Evol Microbiol. 1991;41(4):543–7. https://doi.org/10.1099/00207713-41-4-543.
Matthys C, Claeys G, Verschraegen G, Wauters G, Vogelaers D, De Baere T, Verhelst R, Van Nooten G, Van Coster R, Vaneechoutte M. Streptococcus cristatus isolated from a resected heart valve and blood cultures: case reports and application of phenotypic and genotypic techniques for identification. Acta Clin Belg. 2006;61(4):196–200. https://doi.org/10.1179/acb.2006.034.
Choe GS, Kim HD, Ahn BH, Jung JJ. Late-onset endophthalmitis caused by Streptococcus cristatus after trabeculectomy. J Korean Ophthalmol Soc. 2021;62(11):1570–4. https://doi.org/10.3341/jkos.2021.62.11.1570.
Gupta G, Chaudhary M, Khunt A, Shah MM. An unreported case of Streptococcus cristatus septic arthritis of wrist in a neonate. J Clin Orthopaedics Trauma. 2020;11(2):328–31. https://doi.org/10.1016/j.jcot.2019.02.002.
Hang D, Schmitt MC, Gonzalez LS, Zdanovec A, Pagel PS. Left atrial appendage confusion: a mobile echodensity in a patient with endocarditis. J Cardiothorac Vasc Anesth. 2022;36(8):2829–32. https://doi.org/10.1053/j.jvca.2022.01.008.
Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018–26. https://doi.org/10.1056/NEJMoa0801209.
Child CG, Turcotte JG. Surgery and portal hypertension. In: Child CG, editor. The liver and portal hypertension. Philadelphia: Saunders; 1964. p. 50–64.
Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of endocarditis. Clin Infect Dis. 2000;30(4):633–8. https://doi.org/10.1086/313753.
Lockhart PB, Brennan MT, Thornhill M, Michalowicz BS, Noll J, Bahrani-Mougeot FK, Sasser HC. Poor oral hygiene as a risk factor for endocarditis–related bacteremia. J Am Dental Assoc. 2009;140(10):1238–44. https://doi.org/10.14219/jada.archive.2009.0046.
Mitchell J. Streptococcus mitis: walking the line between commensalism and pathogenesis. Mol Oral Microbiol. 2011;26(2):89–98. https://doi.org/10.1111/j.2041-1014.2010.00601.x.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
CG: conceptualization, literature review, writing—original draft, writing—review and editing. AZ: writing—original draft, writing—review and editing. JM: writing—original draft, writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethical approval was required as only a single participant was involved in the study, and no HIPAA identifiers were included in the manuscript.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Video of the transesophageal echocardiogram from Figure 1, showing new-onset mild-tomoderate aortic regurgitation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guzman, C., Zaclli, A. & Molinari, J. Streptococcus cristatus bacteremia in a patient with poor oral hygiene: a case report. J Med Case Reports 17, 218 (2023). https://doi.org/10.1186/s13256-023-03818-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-023-03818-z