Abstract
Background
Hemangioblastoma of the central nervous system is an uncommon benign neoplasm, with about 25% of cases in patients with von Hippel–Lindau disease. The incidence of metastasis is rare, particularly in patients without von Hippel–Lindau disease. We report a case of hemangioblastoma with leptomeningeal dissemination as a late recurrence.
Case presentation
A 65-year-old Caucasian man with a history of World Health Organization grade I hemangioblastoma of the cerebellar vermis underwent gross total resection in 1997. In early 2018, he developed intracranial recurrences with diffuse leptomeningeal disease of the entire spine. The patient underwent resection of intracranial recurrence, followed by palliative craniospinal irradiation. The disease progressed quickly, and he died 8 months after recurrence.
Conclusions
Despite a benign pathology, hemangioblastoma has a low risk of metastasis. The outcome for hemangioblastoma patients with metastasis is poor. Multidisciplinary care for patients with metastatic hemangioblastoma warrants further investigation, and an effective systemic option is urgently needed. Regular lifelong follow-up of at-risk patients is recommended.
Similar content being viewed by others
Background
Hemangioblastoma (HB) of the central nervous system is an uncommon benign neoplasm accounting for approximately 2% of intracranial neoplasms and 2–10% of primary spinal cord neoplasms [1]. Primary lesions most commonly arise in the cerebellum, with HBs comprising 7–12% of posterior fossa lesions in adults. While the majority of hemangioblastomas arise sporadically, about 25% of cases are in patients with von Hippel–Lindau (VHL) disease [2]. The presence of multiple lesions should raise suspicions of VHL. Due to the low metastatic potential of HB, disseminated hemangioblastomatosis in patients without VHL is a rare occurrence [2, 3]. We report a case of hemangioblastoma with leptomeningeal dissemination as a late recurrence. This is a very unusual presentation for this benign tumor, and it raised the need for long-term follow-up for patients with hemangioblastoma, particularly in high-risk patients.
Case presentation
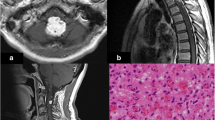
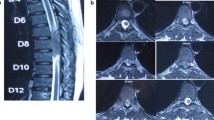
A 65-year-old Caucasian man with no significant past medical history presented with a World Health Organization (WHO) grade I hemangioblastoma of the cerebellar vermis post suboccipital craniotomy and resection in 1997. A VHL gene mutation test was not performed then. He had a gross total resection and was observed, and was later lost to follow-up. He presented again in early 2018 with 3 months of forgetfulness, worsening gait imbalance, and ataxia. Physical examination was significant for bilateral upper and lower extremity ataxia, worse in the left lower extremity. Magnetic resonance imaging (MRI) of the brain revealed a 2.8 cm brightly enhancing complex extra-axial solid/cystic mass arising from inferior surface of left tentorium, producing a local mass effect on the superior surface of the left cerebellar hemisphere and a second well-defined, 1 cm brightly enhancing extra-axial mass at the cerebellar vermis. There was also arachnoidal spreading of the tumor, most prominently in the suprasellar cistern and along the sylvian fissures, consistent with diffuse leptomeningeal disease (Fig. 1). Subsequently, a cervical, thoracic, and lumbar spine MRI was ordered, which revealed extensive extramedullary intradural enhancement consistent with extensive leptomeningeal involvement along the entire spinal cord, T8 to T11 cord enhancement, and thick plaque-like enhancing soft tissue within the thecal sac reflecting drop metastases (Fig. 2). A computed tomography (CT) scan of the chest, abdomen and pelvis was negative for metastatic disease. A lumbar puncture was performed, which was nondiagnostic but showed an atypical cerebrospinal fluid (CSF) cytology.
Encephalomalacia in bilateral medial cerebellum. Enhancing soft tissue nodule along anteromedial left cerebellum/vermis and enhancing mass along left cerebellopontine angle. Abnormal enhancement along pre-chiasmatic optic nerves and chiasm, without intraorbital involvement. Leptomeningeal enhancement involving bilateral frontal lobes and bilateral ambient and suprasellar cistern, with extension into interhemispheric fissure
Extensive nodular and linear enhancement in posterior fossa and along the surface of the spinal cord representing leptomeningeal metastasis. Diffuse abnormal plaque-like and nodular enhancement along the cord surface throughout the thoracic spine and clearly identifiable ventral intrathecal tumor below T9. Thick plaque-like enhancing soft tissue within the thecal sac at the L5–S1 level reflecting drop metastasis
The patient underwent a suboccipital craniotomy for resection of the recurring tumor in the vermis, as well as the new infratentorial tumor. Immunohistochemistry for inhibin was strongly positive within the tumor cells, which supported the diagnosis of hemangioblastoma (Fig. 3). The postoperative course was complicated by communicating hydrocephalus requiring a ventriculoperitoneal shunt (VPS) placement. Subsequently, the patient received palliative craniospinal irradiation. He received 30 Gy in ten fractions to the whole brain and whole spine. The patient tolerated radiation treatment well without significant side effects. MRI of the brain, cervical, thoracic and lumbar spine 2 months post-radiation demonstrated new bilateral complex subdural hygromas, stable diffuse pachymeningeal, and leptomeningeal disease. Approximately 4.5 months post-radiation treatment, the patient presented once again with altered mental state and MRI of the brain revealed increased subdural hygromas, which was worse on the left. He underwent a left frontal craniotomy for evacuation of the left hygroma but his condition deteriorated with admission complicated by aspiration pneumonia, urinary tract infection (UTI), multifactorial encephalitis, and respiratory distress requiring intubation. The patient was placed on comfort care and died from respiratory failure shortly after, 6 months post-radiation and 8 months following presentation with disseminated disease.
A–F Lateral cerebellar tumor, histologically consistent with hemangioblastoma (A, hematoxylin and eosin stain, 200× magnification). Extramedullary hematopoiesis is seen (A, circle). A high-power image shows hemangioblastoma stromal cells with vacuolated cytoplasm surrounded by thin-walled capillaries ( B, hematoxylin and eosin stain, 400× magnification). Immunohistochemical staining for inhibin is strongly positive in tumor cells (C, 200× magnification). Reticulin stain highlights the delicate capillary network that surrounds the nests of hemangioblastoma stromal cells (D, 200× magnification). Cytokeratin CAM 5.2 (E, 200× magnification) and renal cell carcinoma (RCC, F, 200× magnification) stains are negative in the hemangioblastoma cells, ruling out metastatic renal cell carcinoma
Discussion
Primary hemangioblastomas are rare tumors of the central nervous, often slow growing and most commonly occurring in the cerebellum, brainstem, or spinal cord. Hemangioblastomas can arise sporadically; however, they are classically associated with von Hippel–Lindau (VHL) disease, with approximately 25% of hemangioblastomas being attributed to the disease [1]. Multiple hemangioblastomas can be seen in at least 60% of VHL patients, and is one of the most common causes of demise in VHL patients, in addition to renal cell carcinoma. However, disseminated leptomeningeal involvement of hemangioblastomas is exceedingly rare in patients without a diagnosis of VHL disease, with only ~ 33 published cases [2, 3].
Radiation therapy, either stereotactic radiosurgery or external beam radiotherapy, can be used as primary, adjuvant or a salvage treatment strategy for localized disease with excellent local control [4,5,6] [16,18,19]. Local control is 98%, 88%, and 73% of intracranial hemangioblastomas at 1, 2, and 6 years, respectively, with marginal dose and fractions ranging from 10–32 Gy and 1–10 fractions, respectively [7]. However, in patients with diffuse leptomeningeal involvement treated with palliative radiation, outcomes are poor [8,9,10]. In reviewing all prior cases of diffuse leptomeningeal HB, a majority of patients had experienced disease progression or complications due to disease burden, with 79%, 47%, and 18% survival at 1, 2, and 5 years,respectively, in case reports with long-term follow-up. In addition to surgery and radiation, several other systemic therapies have been explored with disappointing results. Systemic therapies also have poor results in patients with metastatic disease [11, 12]. With all reported cases of diffuse leptomeningeal HB, there are similar rates of overall survival in patients with 76%, 45%, and 18% survival at 1, 2, and 5 years, respectively, in patients with long-term follow-up. Our patient died shortly after palliative craniospinal radiation, with progression of disease and subsequent respiratory failure 6 months post-treatment. The clinical course of our patient closely aligns with prior case reports, with a quick declining following palliative radiation in patients with diffuse dissemination of HB in the brain and spine [13,14,15]. In reported cases, dissemination occurred approximately 8.5 years after the primary hemangioblastoma. As a result, long-term follow-up of these tumors, particularly in multifocal and recurrent patients, may be necessary.
Systemic therapy has also been used for management of metastatic hemangioblastoma. Although anthracycline-based therapies are frequently used, limited data is available regarding its efficacy. Given the rich vascular characteristics of hemangioblastoma, antiangiogenic therapies have also been investigated, such as bevacizumab, imatinib, sorafenib, sunitinib, and pazopanib. However, further studies are needed to better define the optimal systemic therapeutic regimen.
Conclusion
We report a case of cerebral hemangioblastoma with leptomeningeal metastasis as a late recurrence. Treatment outcomes of hemangioblastoma patients with metastasis are poor. A multidisciplinary care for patients with metastatic hemangioblastoma warrants further investigation and an effective systemic option is urgently need. Regular lifelong follow-up in at-risk (multifocal and recurrent) patients is recommended.
Data availability statement
Data available on request from the authors.
References
Brundl E, Schodel P, Ullrich OW, Brawanski A, Schebesch KM. Surgical resection of sporadic and hereditary hemangioblastoma: our 10-year experience and a literature review. Surg Neurol Int. 2014;5:138.
Conway JE, Chou D, Clatterbuck RE, Brem H, Long DM, Rigamonti D. Hemangioblastomas of the central nervous system in von Hippel-Lindau syndrome and sporadic disease. Neurosurgery. 2001;48(1):55–62; discussion 62-53.
Kim H, Park IS, Jo KW. Meningeal supratentorial hemangioblastoma in a patient with von hippel-lindau disease mimicking angioblastic menigioma. J Korean Neurosurg Soc. 2013;54(5):415–9.
Kano H, Shuto T, Iwai Y, et al. Stereotactic radiosurgery for intracranial hemangioblastomas: a retrospective international outcome study. J Neurosurg. 2015;122(6):1469–78.
Liao CC, Huang YH. Clinical features and surgical outcomes of sporadic cerebellar hemangioblastomas. Clin Neurol Neurosurg. 2014;125:160–5.
Koh ES, Millar BA, Menard C, et al. Fractionated stereotactic radiotherapy for acoustic neuroma: single-institution experience at The Princess Margaret Hospital. Cancer. 2007;109(6):1203–10.
Puataweepong P, Dhanachai M, Hansasuta A, et al. The clinical outcome of intracranial hemangioblastomas treated with linac-based stereotactic radiosurgery and radiotherapy. J Radiat Res. 2014;55(4):761–8.
Bains SJ, Niehusmann PF, Meling TR, Saxhaug C, Zuchner M, Brandal P. Disseminated central nervous system hemangioblastoma in a patient with no clinical or genetic evidence of von Hippel-Lindau disease—a case report and literature review. Acta Neurochir (Wien). 2019;161(2):343–9.
Chung SY, Jeun SS, Park JH. Disseminated hemangioblastoma of the central nervous system without Von Hippel-Lindau disease. Brain Tumor Res Treat. 2014;2(2):96–101.
Courcoutsakis NA, Prassopoulos PK, Patronas NJ. Aggressive leptomeningeal hemangioblastomatosis of the central nervous system in a patient with von Hippel-Lindau disease. AJNR Am J Neuroradiol. 2009;30(4):758–60.
Reyes-Botero G, Gallego Perez-Larraya J, Sanson M. Sporadic CNS hemangioblastomatosis, response to sunitinib and secondary polycythemia. J Neurooncol. 2012;107(2):439–40.
Hanse MC, Vincent A, van den Bent MJ. Hemangioblastomatosis in a patient with von Hippel-Lindau disease. J Neurooncol. 2007;82(2):163–4.
Mohanty CB, Rao KN, Sampath S. Brainstem hemangioblastoma and sellar mass. J Neurosci Rural Pract. 2012;3(3):429–30.
Koo HW, Park JE, Cha J, et al. Hemangioblastomas with leptomeningeal dissemination: case series and review of the literature. Acta Neurochir (Wien). 2016;158(6):1169–78.
Piribauer M, Czech T, Dieckmann K, et al. Stabilization of a progressive hemangioblastoma under treatment with thalidomide. J Neurooncol. 2004;66(3):295–9.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
SP and AR: manuscript development. SP, AR, and WS: development of the case report. CT, LK, KJ, and WS: provided data. All authors reviewed revised, read, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approved by Thomas Jefferson University IRB.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
Wenyin Shi has received research grants from Regeneron, Novocure, Brainlab, and consulting fee from Brainlab, Varian, Zai lab. Kevin Judy has received research grant from Imvax. Spencer Poiset, Aneesh Reddy, Catherine Tucker, Lawrence Kenyon declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Poiset, S.J., Reddy, A., Tucker, C.M. et al. Hemangioblastoma with late leptomeningeal metastasis: a case report. J Med Case Reports 17, 102 (2023). https://doi.org/10.1186/s13256-023-03812-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-023-03812-5