Abstract
Background
The loss of limbal stem cells owing to either corneal burn or inflammation leads to the repopulation of opaque skin over the raw surface of the cornea. It has been proposed that reconstitution of oral mucosal stem cells over this raw surface will mimic the limbal stem cells and restore vision. The efficacy and safety of applying a sheet of cultivated oral mucosal cells as an autologous graft for corneal replacement were evaluated.
Case presentation
The study was conducted during 2014–2015 and involved a total of six patients, of whom three had suffered a chemical burn and three had Stevens-Johnson Syndrome (SJS). Oral mucosal tissue was dissected from each patient, seeded onto irradiated J2 fibroblast feeder cells for 14 days, and analyzed for quality and safety 1 day before being transplanted onto the cornea of the affected eyes. After transplantation, topical antibiotic and anti-inflammatory eye drops were instilled four times daily, and the patients wore contact lenses. Subjects were clinically followed for visual acuities and adverse effects at 2, 4, and 6 weeks, 3 and 6 months, and 1 year post-transplantation. Data were presented descriptively. Visual acuities in patients improved at 2 weeks post-surgery. However, two patients with SJS had corneal ulcer at 2 weeks postoperatively. At the 1-year postoperative examination, the eyes of two patients were in good condition with decreased vascularization and epithelial defect.
Conclusions
Cultivated oral mucosal epithelial sheet transplantation in limbal stem cell deficiency had a favorable efficacy. In this study, patients with chemical burn had more clinical benefit than those with SJS.
Trial registration ClinicalTrials.gov: NCT02415218. Registered retrospectively 4 Apr 2015 (https://clinicaltrials.gov/ct2/show/NCT02415218).
Similar content being viewed by others
Background
Management of limbal stem cell deficiency (LSCD) due to Stevens Johnson–Syndrome (SJS) or chemical burn to preserve vision is challenging. In Thailand, the treatment of penetrating keratoplasty is particularly cumbersome and difficult, with few eye donations. Patients who may receive allogeneic transplants often suffer eventual conjunctivalization, graft failure, and blindness. Success rates of 20% at 16 months and 27.3% at 36 months have been reported in the few studies performed [1, 2]. Complications, such as infection and liver and kidney injury, have been reported due to the use of long-term immunosuppressive medications [3,4,5,6,7]. Cultivated autologous oral mucosal epithelial sheet is a cell sheet that can be grafted onto the corneal stroma, replacing the corneal epithelium [8,9,10,11,12,13]. The presence of transparent mucosal stem cell in the mucosal sheet may restore the corneal surface with intact visual functions and circumvent allogeneic complications [14,15,16]. The purpose of the present study was to evaluate the efficacy and safety of cultivated oral mucosal epithelial sheet transplantation in patients with total LSCD.
Case presentation
Subjects
Six patients are reported here, of whom three suffered chemical burn and three had SJS. Of these six patients, three were men, with four right eyes affected. The mean age was 46.2 (range 34–66) years. All patients were prospectively enrolled as a single group and gave informed consent to the investigators at the Faculty of Medicine Siriraj Hospital (Institute Review Board [IRB] Ethical Approval No. SI 227/2013). The protocol could be terminated at any time due to loss to follow-up, inability to obtain mucosal tissue, or patient’s request. The inclusion criteria included patients aged > 20 years with total LSCD or total conjunctivalization from any cause. The exclusion criteria included pregnancy, severe infection, keratitis, and eye or buccal mucosal complications. All standard preparations, including surgery, work flow, laboratory tests, environmental controls, materials, chemical materials, records, pharmacological storage, quality/safety checks, and transportation, were managaged by Siriraj Hospital and complied with GMP guidelines for medicinal products. All procedures strictly followed the regulations in the U.S. Food and Drug Administration Code of Federal Regulations (CFR) Title 21 Part 1271 (Human cells, tissue and cellular and tissue-based products; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1271) and Title 21 CFR Part 600 (Biological product, current good tissue practice [CGTP]; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=600), as well as with additional requirements for manufacturers of human cells, tissues and cellular and tissue-based products. Any adverse event, deviation, or intervention would be spontaneously reported to the IRB for review and further actions.
Feeder cells
3T3-J2 fibroblasts were used as feeder cells. They were seeded to tissue culture flasks and maintained in Dulbecco's Modified Eagle Medium and 10% fetal bovine serum for 7–14 days to reach confluence, following which the 3T3-J2 cells were irradiated twice with 34 cGy X-ray each time to inhibit cell division. The irradiated cells (11.9 × 104 cells/mL) were plated in 2-mL aliquots onto Nunc UpCell 3.5-cm dishes (Thermo Fisher Scientific, Waltham, MA, USA) or as 4.7-mL aliquots onto 60-mm tissue culture dishes and kept overnight prior to the seeding of mucosal epithelial cells.
Oral mucosal sample collection
About 12–14 days before the planned transplant, an oral mucosal tissue sample (0.5 × 1.5 × 0.3 cm; width × length × depth) was excised under local anesthesia using disposable blade. The wound was sutured with Dacron 5-0 fiber to stop bleeding. Antibiotic and pain-relief medication were provided for 3 days. The mucosal tissue was soaked in 10% betadine in distilled water (1:8 dilution) for 10 min, then rinsed with 0.5% levofloxacin before being transported in 30 mL FDM (DMEM, 10% FBS, 0.125 μg/mL amphotericin B, 100 IU/mL penicillin G, 100 μg/mL kanamycin) in a sterilized box to the tissue culture facility. The dissected tissue was exposed to 1000 PU/mL dispase to separate the mucosal epithelial layer from the subcutaneous layer. The epithelial layer was digested with 0.25% trypsin EDTA to segregate epithelial cells. The isolated epithelial cells were seeded onto the irradiated feeder cells at a density of 2–3 × 105 cells/35-mm dish and maintained in 2 mL KCM medium with epidermal growth factor at 37 °C, 5% CO2 for 12–14 days before the transplant. For the colony-forming assay (CFA), the epithelial cells were seeded at 3000–5000 cells/60-mm dish; the mucosal epithelial sheets were then examined for quality and safety (Table 1), including by immunohistochemistry, flow cytometry, and real-time PCR. Samples were submitted for sterility tests including bacterial culture, fungal culture and endotoxin assay at the Department of Microbiology, Siriraj Hospital.
Transplantation
The mucosal sheet was transferred in a close-system box kept at 20 °C to the operating room. The cell sheet was harvested on a ring of PVDF membrane (outer diameter: 25 mm; inner diameter: 15 mm). The transplantation of the cultivated oral mucosal epithelial sheet was performed at 20°C with the patient under general anesthesia. The first step was the excision of conjunctiva and fibrous tissue on the cornea, namely symblepharon lysis, prior to the actual transplant. Before and after surgery, the subjects received topical antibiotic every 2 hours, and topical corticosteroid 4 times daily. Intravenous ceftriaxone (1 g) was given every 12 hours for 3 days, then oral antibiotic for the next 5 days. Intravenous solumedrol (125 mg) was given every 12 hours for the first day and once daily for 2 days, and oral prednisolone (25 mg) was given twice daily for 2–4 weeks. The subjects wore protective contact lenses for 1 year following surgery. The symptoms, clinical findings of inflammation, and the accompanying images were recorded in a dedicated hard disk and graded for severity at the follow-up periods: 2 and 4 weeks and 3, 6, and 12 months. The main outcome included corneal epithelial defect (0–100%) and conjunctivalization on the cornea (25–100%) within 1 year (graded from 0–5, with 0 = bad and 5 = excellent). The secondary outcome included visual acuity, corneal opacity, corneal vascularization, and complications. Only the investigators could access the collected data.
Statistical analysis
The results are shown as the mean ± standard error of the mean of at least triplicate determinants. Student’s t-test was used for the analysis. A p value < 0.05 was considered to be significant.
Results
The freshly obtained oral mucosal cells together with the resulting cell sheets were evaluated physically and quantitatively (Table 2). The differentiation status of the cell sheets was determined using immunofluorescence staining with the respective antibodies (Fig 1; Table 3). The epithelial cell sheets from all patients expressed tumor protein p63 (p63), the marker of epithelial stem cells. The presence of cytokeratin 3 (AE5), the unique marker of corneal epithelium, was clearly observed in subjects 1, 2, and 3, but was faint in patients 4, 5, and 6. Likewise, the presence of ZO-1, the epithelial tight junction protein 1, was also clearly observed in patients 1, 2, and 3. The proliferative activity of all cell sheets was confirmed using the CFA (Fig. 2; Table 4).
Hematoxylin and eosin (H&E) staining (objective lens: 40×) and immunofluorescence staining (objective lens: 20×) for the markers of epithelial stem/progenitor cells (tumor protein p63 [p63]), corneal differentiation (cytokeratin 3 [AE5]), and barrier function (membrane-anchored mucin-16 [MUC16] and tight junction protein-1 [ZO-1]) in the epithelial cell sheets prepared from the oral mucosal cells from subject #1 (A) and #3 (B)
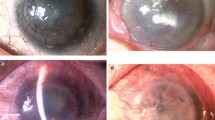
The scoring of clinical outcomes and severity (Table 5) was applied throughout the study (Tables 6, 7, 8, 9, 10, 11). The physical appearance of the affected eyes of successful responders (subjects #1 and #3; Fig. 3a) and less successful responders (subjects #2, #5, and #6; Fig. 3b) was assessed before and after the operation. The transplanted grafts were in good condition within a few days after the operation until at least 5 months post-surgery. At 2 weeks post-surgery, visual acuities had improved in 5 patients (Table 7). Two patients (subjects #5 and #6) had corneal ulcer requiring systemic and topical antibiotics. Subject #5 needed to stop wearing contact lenses in the first month post-surgery while subject #6 stopped wearing contact lenses at 4 months post-surgery. Subjects #4 lost her contact lenses with no corneal infection and stopped taking medication before the end of the study. At the 1-year postoperative check-up, the grading of tissue transplantation was excellent in two patients (subjects #1 and #3) who had chemical injuries (Table 11). The visual outcome of four patients (subjects #1, #2, #3, #5) along the post-operative course.
Discussion
The cultivated oral mucosal epithelial sheet requires expertise in cell culture, with temperature control and efficiency, including careful transplantation. Anti-inflammatory medicine, such as corticosteroid, is needed to maintain cell recovery, decrease fibrous adhesion (symblepharon), and promote healing with artificial tears. The result is critical at each evaluation time point to avoid the side effects of the corticosteroid treatment, especially infection. In cell culture preparation, the viability of cells from subjects with acid burn (92.1%) was higher than that from subjects with SJS and others. Chemical injuries that involve only the eyes, not the mouth, may result in better cell viability. Most patients in our study had severe dry eyes, neovascularization grade 3, and symblepharon.
At 1 year post-surgery, vision had improved compared to the pre-operation condition, there was more tear production, minimal symblepharon, and not corneal epithelial defect. The excellent grading result at 1 year was achieved in two patients with chemical burn. To the contrary, those with SJS may have had oral involvement that resulted in a lower number of viable mucosal epithelial cells, of which the viability may also have been lower. All of these patients had severe dry eyes and a higher risk of infection that ended up with a fair result. Tears of the severe chronic SJS eyes contained cytokines (interleukin-8 and granzyme B) [17] that reflected an ongoing immune reaction. The presence of both of these cytokines in the tears of patients with SJS could induce angiogenesis and cytotoxicity in the graft. Both the presence of these cytokines and the impaired treatment regimen could contribute towards the unsuccessful outcome in these patietns. Postoperative management required long-term usage of anti-inflammatory drug with different regimens and variations to prevent infection. Any recurring inflammation would result in increasing fibrosis.
Conclusions
Cultivated oral mucosal epithelial cell sheet transplantation was successful in the treatment of eyes with chemical injury at 1 month post-surgery. Long-term management and follow-up are required for all patients who need to strictly adhere to the instructions to achieve the optimal result of transplantation.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Abbreviations
- CFA:
-
Colony-forming assay
- LSCD:
-
Limbal stem cell deficiency
- SJS:
-
Stevens-Johnson Syndrome
References
Ilari L, Daya SM. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109(7):1278–84.
Gomes JA, Santos MS, Ventura AS, Donato WB, Cunha MC, Hofling-Lima AL. Amniotic membrane with living related corneal limbal/conjunctival allograft for ocular surface reconstruction in Stevens-Johnson syndrome. Arch Ophthalmol. 2003;121(10):1369–74.
Samson CM, Nduaguba C, Baltatzis S, Foster CS. Limbal stem cell transplantation in chronic inflammatory eye disease. Ophthalmology. 2002;109(5):862–8.
Tsubota K, Satake Y, Kaido M, et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340(22):1697–703.
Williams KA, Brereton HM, Aggarwal R, et al. Use of DNA polymorphisms and the polymerase chain reaction to examine the survival of a human limbal stem cell allograft. Am J Ophthalmol. 1995;120(3):342–50.
Tsai RJ, Tseng SC. Effect of stromal inflammation on the outcome of limbal transplantation for corneal surface reconstruction. Cornea. 1995;14(5):439–49.
Tseng SC, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116(4):431–41.
Yamato M, Konno C, Utsumi M, Kikuchi A, Okano T. Thermally responsive polymer-grafted surfaces facilitate patterned cell seeding and co-culture. Biomaterials. 2002;23(2):561–7.
Shimizu T, Yamato M, Isoi Y, et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90(3): e40.
Hayashida Y, Nishida K, Yamato M, et al. Ocular surface reconstruction using autologous rabbit oral mucosal epithelial sheets fabricated ex vivo on a temperature-responsive culture surface. Invest Ophthalmol Vis Sci. 2005;46(5):1632–9.
Hayashi R, Yamato M, Takayanagi H, et al. Validation system of tissue-engineered epithelial cell sheets for corneal regenerative medicine. Tissue Eng Part C Methods. 2010;16(4):553–60.
Hori Y, Sugiyama H, Soma T, Nishida K. Expression of membrane-associated mucins in cultivated human oral mucosal epithelial cells. Cornea. 2007;26(9 Suppl 1):S65–9.
Sumide T, Nishida K, Yamato M, et al. Functional human corneal endothelial cell sheets harvested from temperature-responsive culture surfaces. FASEB J. 2006;20(2):392–4.
Nishida K, Yamato M, Hayashida Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351(12):1187–96.
Ikuno Y, Ikeda T, Sato Y, Tano Y. Tractional retinal detachment after branch retinal vein occlusion. Influence of disc neovascularization on the outcome of vitreous surgery. Ophthalmology. 1998;105(3):417–23.
Sotozono C, Ang LP, Koizumi N, et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology. 2007;114(7):1294–302.
Yoshikawa Y, Ueta M, Nishigaki H, Kinoshita S, Ikeda T, Sotozono C. Predictive biomarkers for the progression of ocular complications in chronic Stevens-Johnson syndrome and toxic Eeidermal necrolysis. Sci Rep. 2020;10(1):18922.
Acknowledgements
The authors would like to thank Dr. Kohji Nishida, Dr. Yoshinori Oie and Dr. Yuki Kobayashi from Osaka University for the technology transfer and instruments for mucosal epithelial sheet preparation.
Funding
This work was supported by a research grant from the Faculty of Medicine Siriraj Hospital, Mahidol University (to PK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. PK and AW are recipients of Chalermphrakiat Grant at the Faculty of Medicine Siriraj Hospital, Mahidol University.
Author information
Authors and Affiliations
Contributions
PK was responsible for the study concept and design. PK, WB, SD, KK, and KS acquired data. PK, SD, KK, and AW analyzed and interpreted data. AW and PK drafted the manuscript. All authors critically reviewed the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study report has received approval from the IRB of Siriraj Hospital (approval # SI 227/2013) and was conducted according to the Declaration of Helsinki and subsequent amendments. All participating subjects provided informed consent to investigators prior to the participation. A copy of the written consent is available for review by the Editor-in- Chief of this journal.
Consent for publication
Written informed consent was obtained from the patients for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declared that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Booranapong, W., Kosrirukvongs, P., Duangsa-ard, S. et al. Transplantation of autologous cultivated oral mucosal epithelial sheets for limbal stem cell deficiency at Siriraj Hospital: a case series. J Med Case Reports 16, 298 (2022). https://doi.org/10.1186/s13256-022-03502-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-022-03502-8