Abstract
Background
The cause of systemic lupus erythematosus is not completely clear so far, but the prevalence of systemic lupus erythematosus is significantly increased in people with additional X chromosomes.
Case presentation
We report a 17-year-old Chinese female patient with systemic lupus erythematosus complicated with trisomy X, accompanied by lupus nephritis, pancytopenia, hemolytic anemia, and multiserous effusion. The patient recovered well after treatment and returned regularly. We review the previously reported cases to summarize the clinical characteristics of these patients.
Conclusion
The additional X chromosome is related to the development of systemic lupus erythematosus. Whether it is a subtype of systemic lupus erythematosus remains to be further confirmed.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease with circulating immune complexes deposition and multiorgan involvement. It predominantly affects females, especially women of childbearing age, with a female-to-male ratio of 9:1 [1]. Although the gender bias has often been attributed to sex hormones, the risk of developing SLE in men with an extra X chromosome is similar to that in 46,XX normal women and a 14-fold higher risk of developing SLE than 46,XY males, while female patients with Turner’s syndrome (45,X0) abstained from contracting SLE [2, 3]. In addition, the prevalence of SLE in 47,XXX women is approximately 2.5 times higher than that in 46,XX women [4]. It suggests X chromosomes may contribute to the susceptibility of human SLE. SLE in females with extra X chromosomes have been described in several cases.
Here, we present a case of a 17-year-old girl who developed SLE with 47,XXX, and review similar literature to discuss the relationship between X chromosomes and SLE.
Case presentation
A 17-year-old Chinese girl with no previous medical history or particular family history was admitted to our hospital with fever, headache, and weakness for 4 days. The patient developed paroxysmal headache and dizziness at school, followed by low-grade fever and weakness, without cough and expectoration. She then went to the emergency room of another hospital for treatment. During her hospitalization, she developed abdominal pain and computed tomography scan of the chest and abdomen revealed multiple lymphadenopathy and serous cavity effusion. After treatment with ceftriaxone and oseltamivir, the symptoms were not relieved, and her temperature peaked at 38.9 °C. Subsequently, the patient was admitted to our hospital for further diagnosis and treatment.
Physical examination at admission revealed a fever of 37.7 ℃, anemic, abdominal tenderness, and anterior tibial edema. Several enlarged lymph nodes with slight tenderness were palpable in her bilateral neck and armpit. The patient’s height and weight were 168 cm and 48 kg, respectively, and her expression was indifferent.
Laboratory findings revealed elevated acute phase reactants with erythrocyte sedimentation rate (ESR) at 112 mm, and C-reactive protein (CRP) at 213.5 mg/L. Blood tests revealed pancytopenia and anemia (total white blood cell count: 2.27 × 109/L; hemoglobin level: 65 g/L; red blood cells 1.81 × 109/L, platelets 89 × 109/L; reticulocyte 1.9%). Urine tests showed proteinuria (1.66 g/days) and serum albumin was 26.5/L. Coombs test was positive and platelet antibody test was negative. The immunoglobulin G (IgG) was 24.5 g/dL and complement (C3 and C4) levels were decreased. Anticardiolipin antibody IgG was positive. The patient tested positive for the following autoantibodies: high titer of antinuclear antibody of 1:1000, positivity for anti-double-stranded DNA (dsDNA) antibody, anti-nRNP/Sm antibody, anti-SS-A/Ro and anti-SS-B/La antibodies, anti-histone antibody, anti-ribosomal P protein antibody, and anti-nucleosome antibody. Tumor series and blood culture were normal.
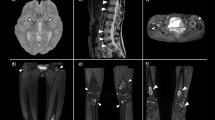
Ultrasonography showed bilateral pleural effusion. Magnetic resonance of the brain revealed a few small cavities in the left semi-soft circle area and bilateral frontal cortex. No organic lesions were found on enhanced computed tomography scan of small intestine. Low bone mineral density detected by the dual-energy X-ray absorptiometry (lumbar spine: Z-score, 1.7).
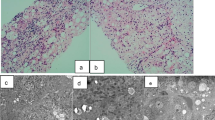
To rule out other hematologic diseases, bone marrow examination was performed, which showed no malignancy, but the Giemsa banding revealed a karyotype of 47,XXX in all 20 metaphase cells analyzed (Figure 1).
The patient was diagnosed with SLE on the basis of fever, hemolytic anemia, pancytopenia, low C3 and C4, proteinuria greater than 0.5 g/day, serous effusion, antinuclear antibody positive, and anti-dsDNA positive, fulfilling the 2019 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) criteria [5], complicated with lupus nephritis, pancytopenia, hemolytic anemia, serous effusion, lacunar cerebral infarction, and trisomy X syndrome. Although she had positive anti-SS-A/Ro and anti-SS-B/La antibodies, and anticardiolipin antibody, she did not fulfill the classification criteria of Sjögren’s syndrome (SS) or antiphospholipid syndrome. The SLE disease activity index (SLEDAI) score was 17, indicating the patient had high disease activity. The patient was treated successfully with intravenous gamma globulin at 17.5 g/day for 5 days to block antibodies, methylprednisolone 240 mg/day for 3 days (then the dose of methylprednisolone was gradually reduced), oral hydroxychloroquine 0.2 g/day, along with transfusion of washed red blood cells, albumin infusion, therapy of calcium supplement, stomach protection, diuresis, and anticoagulation. The symptoms were relieved after 2 weeks of treatment, edema disappeared, and ultrasonography did not show bilateral pleural effusion. Because of the lupus nephritis, the patient was treated with intravenous cyclophosphamide 0.6 g per 2 weeks. When the cumulative dose of cyclophosphamide reached 3 g, the blood cell count, serum albumin, C-reactive protein, and erythrocyte sedimentation rate were normal, anticardiolipin antibody IgG was negative, and urinary protein decreased to 0.44 g/day. Considering the patient has not given birth and the inhibitory effect of cyclophosphamide on gonads, we decided to discontinue the treatment of cyclophosphamide and start with belimumab as a remission-maintenance drug. After altering to belimumab, the patient's condition remained stable.
Discussion
Trisomy X syndrome (47,XXX) is characterized by the presence of an additional X chromosome, which presents in 1 of every 1000 live-born females [6]. Its incidence is related to increased age of the mother. Physical findings described in women with 47,XXX are the presence of epicanthal folds, hypertelorism, upslanted palpebral fissures, clinodactyly, overlapping digits, pes planus, pectus excavatum, hypotonia, and joint hyperextensibility. The extra chromosome may cause premature ovarian failure, and proportion of infertility is slightly higher than that of normal people [7]. The patient in our case presented menarche at the age of 15 years and had normal menstrual cycles. Some patients with trisomy X syndrome may have delayed development and personality disorder, but most patients with trisomy X syndrome have mild or asymptomatic symptoms. In this case, the patient’s hypertelorism and indifference may be related to trisomy X syndrome. However, she had no difficulty in learning and communication.
We summarized the clinical characteristics of patients with SLE with trisomy X syndrome [8,9,10] (Table 1). All patients presented with anemia, of which five cases were hemolytic anemia. In addition, three cases had arthritis and four cases had lupus nephritis. The results suggest that lupus nephritis, arthritis, and hemolytic anemia may be common clinical features of SLE in 47,XXX women. Among them, four patients tested positive for anti-RNP and anti-SSA/SSB antibodies, but there were no related clinical manifestations. In addition, the age of onset of these patients was relatively young, including three cases with diagnosis for childhood-onset systemic lupus erythematosus (cSLE).
SLE and 47,XXX both occur in 1 in 1000 women [11]; if the conditions were independent, only 1 in 1 million would have both SLE and 47,XXX. However, an increased incidence of 47,XXX in cSLE was observed. In another study involving 2826 patients with SLE, 7 were women with 47,XXX, with the prevalence of SLE with 47,XXX being about 2.5 times higher than that of 46,XX women [4]. Slae M et al. performed a systemic literature review for cases of polysomy X and SLE and summarized previously published cases, which showed that X chromosome polysomy may confer increased susceptibility to SLE [12]. Sharma et al. examined cohorts of patients with SS and SLE by constructing intensity plots of X chromosome single-nucleotide polymorphism alleles, along with determining the karyotype of selected patients. The results showed that 3 patients with a triple mosaic, consisting of 45,X/46,XX/47,XXX among ~ 2500 women with SLE, and 1 patient had 45,X/46,XX/47,XXX among ~ 2100 women with Sjögren’s syndrome(SS), and neither the triple mosaic nor the partial triplication were found among the controls. Thus, it indicated X-chromosome abnormalities were present among patients with either SS or SLE and may inform the location of genes that mediate an X dose effect [13]. The previous data suggested the number of X chromosomes was responsible for SLE in men [14].
The mechanism between abnormal X chromosome and the risk of SLE remains unclear. At the stage of female embryo development, one X chromosome is inactivated randomly, but this inactivation is not complete. About 15% of the genes encoded by X chromosomes can escape inactivation [15]. Some microRNAs (miRNAs) encoded by X chromosome were already suggested as being involved in the pathogenesis of SLE. The expression of miR-106a is regulated by EGR1 and Sp1, and disordered expression of miR-106a leads to the pathogenesis of SLE [16]. Overexpression of miR-224 inhibits the protein expression of api5, which can promote cell death induced by T-cell activation in SLE [17]. In addition, downregulation of miR-98 may regulate the expression of Fas-mediated apoptosis, signaling pathway in CD4+T cells, which induces apoptosis death in patients with SLE [18]. Some genes related to SLE are located on the X chromosome; therefore, asymmetric X inactivation and chimerism can induce the pathogenesis of SLE. In addition, demethylation of CD40LG, a B cell costimulatory molecule encoded on the X chromosome, may lead to reactivation of inactive X chromosome, which contributes to SLE [19] and provides another possible explanation for the gene dose hypothesis.
Several genes on X chromosome have been confirmed to be associated with SLE in humans. IRAK1 and its adjacent gene MECP2, located at Xq28, are related genes for pathogenesis of SLE [20]. Three potential signals (rs2071128 in NAA10, rs5987175 in LCA10, and rs17422 in TMEM187) are related to the risk of SLE [21]. A meta-analysis of two genome-wide association studies (GWAS) regarding SLE in Chinese Han population identified a novel variant in PRPS2 on Xp22.3 as associated with SLE [22]. Other genes located on X chromosome, such as TLR7 (Xp22.2), TLR8 (Xp22.2), and OGT (Xq13.1) have been implicated in the development of SLE. Many immunity-related genes are located in X chromosome, which are overexpressed and cause diseases. As a result, humans with extra X chromosomes may suffer higher risk of developing SLE.
Most patients described in Table 1 are Asian, while other studies have proved that patients with SLE with 47,XXX are mainly European [4]. However, owing to selection bias and other factors, the results reported cannot reflect the real situation. Therefore, the relationship between ethnicity and the incidence of 47,XXX needs to be further studied. In other immune system diseases, such as SS, the incidence of 47,XXX was increased, while the prevalence of 47 XXX in primary biliary cirrhosis (PBC) and rheumatoid arthritis(RA) did not increase significantly [4]. Therefore, the difference in the incidence of 47,XXX in different immune diseases needs to be further studied.
It is reported that patients with SLE complicated with trisomy X may have a higher risk of ischemic necrosis and osteoporosis, which may be related to the long-term use of steroids, premature ovarian failure, and overexpression of FVII on X chromosome [10]. In addition, trisomy X syndrome has a high risk of infertility and SS. For this patient, we should pay attention to patient’s fertility and the risk of developing SS and bone complications in the follow-up.
Conclusion
This case report reveals that additional X chromosome is related to the development of SLE. However, SLE combined with 47,XXX is relatively rare in women, but whether it is a subtype of SLE remains to be further confirmed, and the exact mechanisms of X-chromosome dosage in SLE remain unknown.
Availability of data and materials
Not applicable.
References
Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929–39.
Scofield RH, Bruner GR, Namjou B, et al. Klinefelter’s syndrome (47, XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 2008;58(8):2511–7.
Balestrazzi P, Ferraccioli GF, Ambanelli U, Giovannelli G. Juvenile rheumatoid arthritis in Turner’s syndrome. Clin Exp Rheumatol. 1986;4(1):61–2.
Liu K, Kurien BT, Zimmerman SL, et al. X chromosome dose and sex bias in autoimmune diseases: increased prevalence of 47,XXX in systemic lupus erythematosus and Sjögren’s syndrome. Arthritis Rheumatol. 2016;68(5):1290–300.
Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78(9):1151–9.
Nielsen J, Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus. Denmark Hum Genet. 1991;87(1):81–3.
Jiao X, Qin C, Li J, et al. Cytogenetic analysis of 531 Chinese women with premature ovarian failure. Hum Reprod. 2012;27(7):2201–7.
Iwamoto T, Fujimoto M, Ikeda K, et al. Manifestations of systemic lupus erythematosus in female patients with polysomy X: possible roles of chromosome X. Mod Rheumatol. 2019;29(1):192–4.
Barbosa FB, Sinicato NA, Julio PR, et al. Trisomy X in a patient with childhood-onset systemic lupus erythematosus. J Transl Autoimmun. 2020;3: 100043.
Yamazaki S, Akutsu Y, Shimbo A, Shimizu M, Segawa Y, Mori M. Childhood-onset systemic lupus erythematosus with trisomy X and the increased risk for bone complications: a case report. Pediatr Rheumatol Online J. 2021;19(1):20.
Tartaglia NR, Howell S, Sutherland A, Wilson R, Wilson L. A review of trisomy X (47,XXX). Orphanet J Rare Dis. 2010;5:8.
Slae M, Heshin-Bekenstein M, Simckes A, Heimer G, Engelhard D, Eisenstein EM. Female polysomy-X and systemic lupus erythematosus. Semin Arthritis Rheum. 2014;43(4):508–12.
Sharma R, Harris VM, Cavett J, et al. Rare X chromosome abnormalities in systemic lupus erythematosus and Sjögren’s Syndrome. Arthritis Rheumatol. 2017;69(11):2187–92.
Dillon SP, Kurien BT, Li S, et al. Sex chromosome aneuploidies among men with systemic lupus erythematosus. J Autoimmun. 2012;38(2–3):J129–34.
Spolarics Z. The X-files of inflammation: cellular mosaicism of X-linked polymorphic genes and the female advantage in the host response to injury and infection. Shock. 2007;27(6):597–604.
Sharma A, Kumar M, Aich J, et al. Posttranscriptional regulation of interleukin-10 expression by hsa-miR-106a. Proc Natl Acad Sci U S A. 2009;106(14):5761–6.
Lu MC, Lai NS, Chen HC, et al. Decreased microRNA(miR)-145 and increased miR-224 expression in T cells from patients with systemic lupus erythematosus involved in lupus immunopathogenesis. Clin Exp Immunol. 2013;171(1):91–9.
Xie L, Xu J. Role of miR-98 and its underlying mechanisms in systemic lupus erythematosus. J Rheumatol. 2018;45(10):1397–405.
Lu Q, Wu A, Tesmer L, Ray D, Yousif N, Richardson B. Demethylation of CD40LG on the inactive X in T cells from women with lupus. J Immunol. 2007;179(9):6352–8.
Kaufman KM, Zhao J, Kelly JA, et al. Fine mapping of Xq28: both MECP2 and IRAK1 contribute to risk for systemic lupus erythematosus in multiple ancestral groups. Ann Rheum Dis. 2013;72(3):437–44.
Syrett CM, Paneru B, Sandoval-Heglund D, et al. Altered X-chromosome inactivation in T cells may promote sex-biased autoimmune diseases. JCI Insight. 2019;4:7.
Zhang Y, Zhang J, Yang J, et al. Meta-analysis of GWAS on two Chinese populations followed by replication identifies novel genetic variants on the X chromosome associated with systemic lupus erythematosus. Hum Mol Genet. 2015;24(1):274–84.
Acknowledgments
We thank the patient and all the staff who helped and took care of the patient in The Second Affiliate Hospital of Jiaxing University for support.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Fang Luo and Qiao Ye contributed equally to this work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been performed according to the Declaration of Helsinki.
Consent for publication
Written informed consent was obtained from the patient’s legal guardian for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Luo, F., Ye, Q. & Shen, J. Systemic lupus erythematosus with trisomy X: a case report and review of the literature. J Med Case Reports 16, 281 (2022). https://doi.org/10.1186/s13256-022-03478-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-022-03478-5