Abstract
Background
Hump-nosed pit viper is a medically important deadly venomous snake in Sri Lanka and is the commonest cause of venomous snakebites in the country. It frequently causes local effects and systemic manifestations such as acute kidney injury and coagulopathy that occur in less than 10% of all bites. This also includes some atypical presentations such as thrombotic microangiopathy and myocardial infarction. Currently, no antivenom is available for hump-nosed pit viper bites in Sri Lanka, and patients are managed with supportive treatment. This case illustrates an acute ischemic stroke following a hump-nosed viper bite, which is the second case in the literature.
Case presentation
A 71-year-old a Sinhalese male patient presented with left-sided hemiparesis with mouth deviation on day 2 of hump-nosed viper (Hypnale spp.) bite on the right foot. Non-contrast computed tomography of brain showed right ischemic stroke in internal capsule. He was given antiplatelets and statins and continued supportive treatment including limb physiotherapy and speech therapy. He recovered completely and was discharged on day 4 with clinic follow-up.
Conclusions
Physicians should be aware that ischemic cerebral infarcts may occur following hump-nosed pit viper bites.
Similar content being viewed by others
Background
Hump-nosed pit viper is a medically important deadly venomous snake in Sri Lanka (Fig. 1) and is the commonest cause of venomous snakebites in the country (22–77%) [1]. It frequently causes local envenoming and less commonly cause systemic effects such as acute kidney injury and venom-induced consumption coagulopathy (VICC) [2, 3]. Myocardial infarction (MI) [4], Kounis syndrome [5], microangiopathic hemolysis [6], thrombotic microangiopathy (TMA), hemolytic uremic syndrome (HUS), and thrombotic thrombocytopenic purpura (TTP) [2, 7] are some atypical presentations of hump-nosed pit viper bites. Currently, no antivenom is available for Hypnale bites in Sri Lanka or India, and supportive treatments are carried out for these bites. We describe such a presentation of acute ischemic stroke following a bite by hump-nosed viper as the second reported case of Sri Lanka.
Case presentation
A 71-year old previously well Sinhalese male was transferred from a local hospital for further management of weakness of left upper and lower limbs following a hump-nosed pit viper bite. At a local hospital, his 20 min whole blood clotting test (WBCT20) was normal. On the previous day, while the patient was working in the home garden at about 11:00, his right foot was bitten by a snake identified by the patient and the relatives as a hump-nosed pit viper. However, the snake was not caught or killed for religious reasons. When he was shown a formalin-preserved specimen of hump-nosed viper at the ward, he identified and confirmed the offending snake. He was given native treatment (local application and decoction) once and was admitted to a local hospital. The next day, he developed weakness of left lower and upper limbs at about 11:30. His urine output was normal, and there was moderate pain at site of bite. On admission to the tertiary care center, his Glasgow coma scale (GCS) was 15/15, his mouth deviated to the right, and he had reduced muscle power in both left upper and lower limbs (3/5) with exaggerated tendon reflexes. Ptosis or external ophthalmoplegia was not observed. Also, there was slurring of speech. However, there was no sensory impairment.
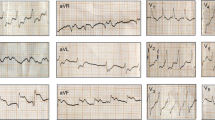
There was moderate swelling over the bitten foot, and no necrosis was observed. Fang punctures were difficult to identify owing to local application of herbal medicine over the bitten limb (Fig. 2). His blood pressure was 160/80 mmHg, pulse rate was 52 beats per minute, and O2 saturation was 97% on room air. Twenty-minute whole blood clotting test done three times 6 hours apart was normal, and the other laboratory findings are presented in Table 1. Electrocardiogram (ECG) showed bradycardia (Fig. 3). Blood picture and chest X-ray were normal. The non-contrast computed tomography scan of brain on admission (day 2 of snakebite) was normal, but on day 3 it showed an ischemic infarct in right internal capsule (Fig. 4).
The patient was started on aspirin 300 mg, clopidogrel 75 mg, and atorvastatin 20 mg nocte after giving loading doses of those medications. Intravenous clindamycin 600 mg 6-hourly and cefotaxime 1 g 8-hourly were administered. He continued limb physiotherapy and speech therapy. He was discharged on day 4 of snakebite by arranging clinic visits with a plan for stroke rehabilitation (physiotherapy) with aspirin and atorvastatin. At first clinic visit 2 weeks after the discharge, his limb weakness and pulse rate were improved. His lipid profile was normal.
Discussion
Ischemic stroke following hump-nosed pit viper bite has previously been reported in a Sri Lankan patient who developed left-sided hemiparesis associated with acute kidney injury [8]. In vitro studies confirmed that Hypnale venom has potent cytotoxic, mild procoagulant, weak neurotoxic, and myotoxic activity [9]. However, procoagulant toxins of Hypnale venom have thus far not been isolated and characterized, and it is hypothesized that the procoagulant activity is due to thrombin-like enzymes (TLEs) of the venom [10], which may have caused the cerebral infarction in our patient. Thrombin-like enzymes activate clotting pathways, leading to the formation of thrombi, and hence deposit them as fibrin, causing vascular occlusion, which results in reduction of blood supply to the organ. This commonly occurs in vessels of kidneys, brain, heart, or pituitary gland. When red blood cells flow through these blocked vessels, they get distorted, which can be seen on peripheral blood microscopy as schistocytes (fragmented red blood cells). This condition is called microangiopathic hemolysis and can be observed in Hypnale bites [6]. When this organ ischemia occurs together with thrombocytopenia and microangiopathic hemolysis, the process is called TMA, which can also be observed in hump-nosed pit viper bites [7]. It was evidenced that, in our patient, cerebral infarction was due to the venom because he also had other venom effects, including bradycardia, mild reduction of hemoglobin (Hb), and mild elevation of creatinine. While thrombi are formed, circulating platelets can get trapped in fibrin mesh, resulting in thrombocytopenia as our patient’s platelet count on day 2 was near to the lower marginal limit (Table 1). As a drug therapy, these patients can be administered aspirin after the clotting abnormality is resolved, and physiotherapy plays a vital part in the management. To determine whether the type of snake venom is hemotoxic, neurotoxic, nephrotoxic, or myotoxic, there are various investigations that can be done according to the severity of the patient’s condition. Coagulopathy is detected from the clotting profile, including prothrombin time (PT)/international normalized ratio (INR), activated partial thromboplastin time (aPTT), WBCT20, fibrinogen, and D-dimers. Nephrotoxicity is detected from serum creatinine, blood urea, and renal ultrasound scan. Myotoxicity is assessed using laboratory findings related to rhabdomyolysis such as myoglobinuria, serum K+, and creatine phosphokinase.
Interestingly, the clotting profile of our patient was normal throughout (Table 1), and it could be hypothesized that he has depletion of the downstream clotting factors due to consumption and maintained factor levels marginally sufficient for normal clotting. At this stage, fibrinogen levels could be reduced. However, we were unable to measure fibrinogen levels owing to lack of that facility at the hospital. In Sri Lanka, both hemorrhagic and ischemic cerebral infarction is common with Russell’s viper (Daboia russelii) envenoming, and sometimes these may be fatal [11]. Cardiac effects such as MI, atrial fibrillation, acute ischemic changes, bradycardia, and cardiac arrest have previously been reported following hump-nosed pit viper bites [4, 12]. Our patient had bradycardia throughout. However, we expect heart rate to be high because of the local pain. Therefore, reduction of heart rate may be the effect of venom on heart, as previously suggested [12]. Initially, it was thought that bradycardia might also be due to the patient’s old age. However, his pulse rate improved to around 70 beats per minute 4 days after the snakebite. Our patient had pure motor involvement of arms, face, and legs, which was suggestive of ischemia mainly to the posterior limb of internal capsule and involvement of middle cerebral artery. Even though strokes are very rare in Hypnale bites, physicians should be aware of them, because as there is no antivenom, these patients should be managed with available supportive care. Otherwise, they may be administered available antivenom unnecessarily.
Conclusions
Physicians should be aware that ischemic cerebral infarcts may occur following hump-nosed pit viper bites.
Availability of data and materials
All the data generated and analyzed for this case report have been included in this article.
Abbreviations
- MI:
-
Myocardial infarction
- VICC:
-
Venom-induced consumption coagulopathy
- HUS:
-
Hemolytic uremic syndrome
- TTP:
-
Thrombotic thrombocytopenic purpura
- TMA:
-
Thrombotic microangiopathy
- GCS:
-
Glasgow coma scale
- ECG:
-
Electrocardiogram
- TLEs:
-
Thrombin-like enzymes
- Hb:
-
Hemoglobin
References
Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, et al. The global burden of snakebite: a literature analysis and modeling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5(11): e218.
Namal Rathnayaka RMMK, Nishanthi Ranathunga PEA, Kularatne SAM. Kidney injury following envenoming by hump-nosed pit viper (genus: Hypnale) in Sri Lanka: proven and probable cases. Trans R Soc Trop Med Hyg. 2018;00:1–12.
Namal Rathnayaka RMMK, Ranathunga PEAN, Kularatne SAM. Venom induced consumption coagulopathy following hump-nosed pit viper (genus: Hypnale) envenoming in Sri Lanka: uncertain efficacy of fresh frozen plasma. Wilderness Environ Med. 2020;31(2):131–43.
Namal Rathnayaka RMMK, Nishanthi Ranathunga PEA, Janaka Ranaweera A, et al. Cardiac arrest and atrial fibrillation following hump-nosed pit viper (Hypnale hypnale) envenoming. Toxicon. 2018;148:33–9.
Namal Rathnayaka RMMK, Nishanthi Ranathunga PEA, Kularatne SAM. Kounis syndrome following Hypnale zara (hump-nosed pit viper) bite in Sri Lanka. Wilderness Environ Med. 2021;32(2):210–6.
Namal Rathnayaka RMMK, Nishanthi Ranathunga PEA, Kularatne SAM, Rajapakse J, Ranasinghe S, Jayathunga R. Microangiopathic haemolytic anaemia following three different species of hump-nosed pit viper (genus: Hypnale) envenoming in Sri Lanka. Wilderness Environ Med. 2018;29:94–101.
Namal Rathnayaka RMMK, Nishanthi Ranathunga PEA, Kularatne SAM. Thrombotic microangiopathy, haemolytic uremic syndrome and thrombotic thrombocytopenic purpura following hump-nosed pit viper (genus: Hypnale) envenoming in Sri Lanka. Wilderness Environ Med. 2018;00:1–13.
Jeevagan V, Chang T, Gnanathasan AC. Acute ischemic stroke following hump-nosed viper envenoming; first authenticated case. Thromb J. 2012;10:21.
Maduwagea K, Hodgson WC, Konstantakopoulos N, O’Leary MA, Gawarammana I, et al. The in-vitro toxicity of venoms from South Asian hump-nosed pit vipers (Viperidae: Hypnale). J Venom Res. 2011;2:17–23.
Maduwage K, Scorgie FE, Silva A, Shahmy S, Mohamed F, et al. Hump-nosed pit viper (Hypnale hypnale) envenoming causes mild coagulopathy with incomplete clotting factor consumption. Clin Toxicol. 2013;51(7):527–31.
Namal Rathnayaka RMMK, Kularatne SAM, Kumarasinghe KDM, Ranaweera J, Nishanthi Ranathunga PEA. Ischemic brain infarcts and intracranial haemorrhages following Russell’s viper (Daboia russelii) bite in Sri Lanka. Toxicon. 2017;125:70–3.
Namal Rathnayaka RMMK, Nishanthi Ranathunga PEA, Kularatne SAM. Clinico-epidemiology of Hypnale zara (Hump-nosed pit viper) envenoming in Sri Lanka. Trans R Soc Trop Med Hyg. 2021;115:603–12.
Acknowledgements
We are thankful to staff of medical unit Teaching Hospital, Ratnapura, Sri Lanka.
Funding
No.
Author information
Authors and Affiliations
Contributions
RMMKNR and SJ examined, assessed, and were involved in the management of the patient. RMMKNR, PEANR, and SAMK were involved in literature search. All authors collected and analyzed data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our institution does not require ethical approval for reporting individual cases.
Consent for publication
Written informed consent was obtained from the son of the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Namal Rathnayaka, R.M.M.K., Nishanthi Ranathunga, P.E.A., Kularatne, S.A.M. et al. Acute ischemic stroke: a rare complication of hump-nosed pit viper (Hypnale spp.) bite: a case report. J Med Case Reports 16, 218 (2022). https://doi.org/10.1186/s13256-022-03442-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-022-03442-3