Abstract
Background
Neurologic impediments occur in only 0.1% of Mycoplasma pneumoniae infections. Although direct intracerebral infection can occur in these patients, autoimmune-mediated reactions secondary to molecular mimicry are the most common pathophysiology of such neurological complications. These complications include immune-mediated encephalitis, peripheral neuritis such as Guillain–Barré syndrome, and many others. Miller Fisher syndrome is a one of the variants of Guillain–Barré syndrome that has been rarely linked to Mycoplasma pneumoniae infection. It is a condition classically characterized by the triad of ophthalmoplegia, areflexia, and ataxia. Most patients with Miller Fisher syndrome will have positive anti-ganglioside GQ1b antibodies found in their serum, making this autoantibody a very useful serological confirmation parameter. We report a case of a Miller Fisher syndrome in a woman with Mycoplasma pneumoniae infection. To the best of the authors’ knowledge, such cases have been only rarely described in literature.
Case presentation
A 35-year-old Chinese woman presented with sudden onset of double vision and ataxia 5 days after fever and mild flu symptoms. Her Mycoplasma pneumoniae antigen was positive with 1 over 2560 titer of total mycoplasma antibody and presence of immunoglobulin M antibody, suggesting acute infection, and her nerve conduction study revealed mild sensory axonal polyneuropathy with segmental demyelination. the Miller Fischer syndrome variant of Guillain-Barré syndrome secondary to Mycoplasma pneumonia was suspected and later confirmed by presence of serum anti-GQ1b autoantibody. She was treated with intravenous immunoglobulin 0.4 g/kg once daily for 5 days.
Conclusions
The objective of this report is to share a case of an uncommon neurological complication of Mycoplasma pneumoniae infection, to increase the level of suspicion among clinicians that Miller Fischer syndrome can occur as an atypical presentation of an atypical pneumonia.
Similar content being viewed by others
Introduction
Mycoplasma pneumoniae (MP) is an atypical microorganism that commonly causes community-acquired pneumonia. This organism has peculiar properties that not only make it invisible on the usual Gram stain but also nonsusceptible to the broad-spectrum beta-lactam drugs usually applied as first-line antibiotics to treat community-acquired infection [1]. MP causes atypical pneumonia, associated with a list of extrapulmonary manifestations. These include hemolytic anemia, myringitis, Guillain–Barré syndrome and its variant, and many others. Miller Fisher syndrome is one of the rare extrapulmonary manifestations of MP infection [2]. This atypical presentation of an atypical pneumonia is the center of discussion in this case report.
Case report
A 35-year-old Chinese female with no known medical illness presented with double vision and body imbalances for the past 2 days. She described that the diplopia was of sudden onset, painless, and did not occur on looking in any specific direction. Regarding the body imbalance, she showed a tendency to sway to the right side. On further questioning, she also had history of preceding fever with mild flu symptoms for the past 5 days.
She also complained of numbness and cramping sensation over the hands and feet bilaterally, 1 day before presentation. Otherwise, there was no neck stiffness, no upper and lower limb weakness, no slurring of speech, no dysphagia, no dyspnea, and no history of recent accident or trauma. Upon arrival to the emergency department, she required assistance for ambulation as she has difficulty in maintaining balance. Her vital signs were stable with blood pressure of 124/78 mmHg, pulse rate of 80 beats per minute, regular rhythm and no collapsing character, afebrile, and not tachypneic. On physical examination, she had diplopia over the lateral gaze bilaterally, but otherwise there was no nystagmus, no dysdiadochokinesia, no dysmetria, and no facial weakness, and examination of the rest of the cranial nerves, upper limbs, and lower limbs revealed no abnormal findings. Romberg’s test was negative, but sharpened Romberg’s test was positive. Her abdomen was soft, not tender, and there was no palpable mass or organomegaly. Examination of the cardiorespiratory system revealed no abnormal findings. Initial blood investigation was normal (Table 1).
Blood investigations taken upon arrival to the hospital showed normal cell counts. There was no electrolyte abnormality, and the renal profile and liver function were also normal.
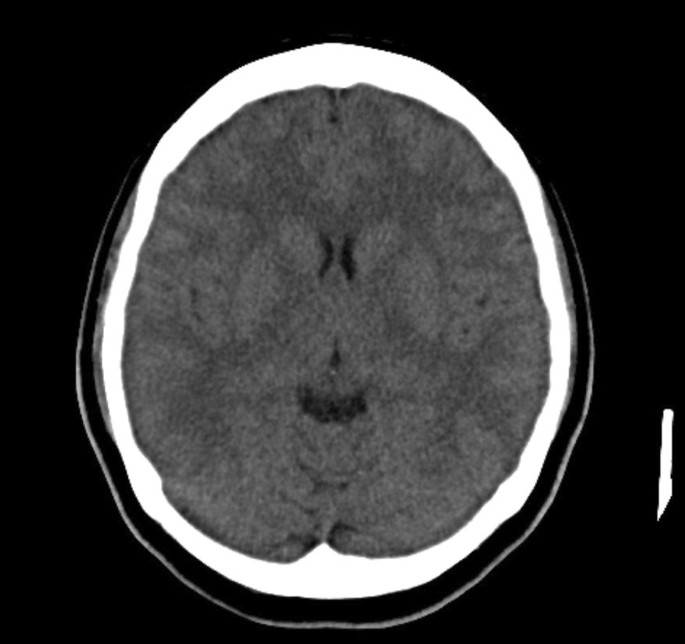
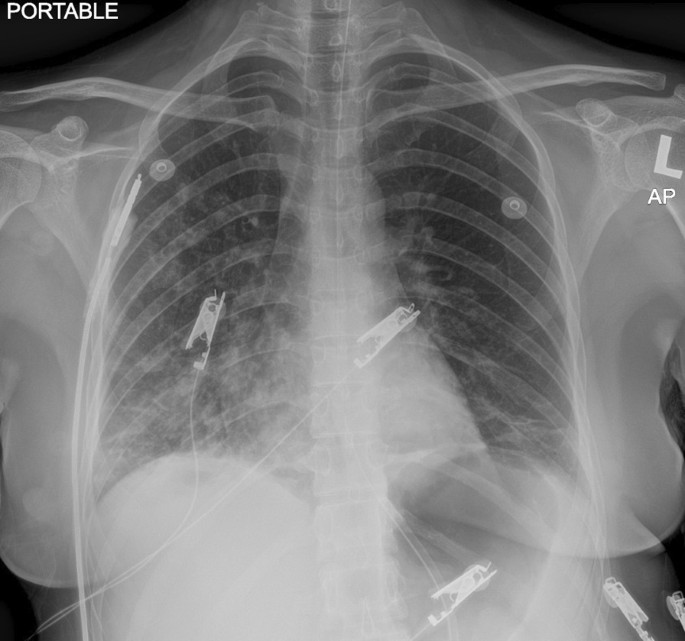
Computed tomographic scan of the brain revealed no intracranial bleeding and no space-occupying lesion (Fig. 1). Nerve conduction study revealed mild sensory axonal polyneuropathy with segmental demyelination. H reflex was absent bilaterally. Lumbar puncture for cerebrospinal fluid analysis was done and showed normal cell counts and biochemistry profile and no abnormal cells, and was negative for bacterial culture, multiple viral antibody panels, and cryptococcal antigen test. In view of the presence of ataxia and diplopia, the Miller Fischer syndrome variant of Guillain–Barré syndrome was suspected and later confirmed with the presence of serum anti-GQ1b autoantibody. Intravenous immunoglobulin (IVIg) 0.4 gm/kg once daily was started and planned to complete for 5 days. On the second day of admission, she developed worsening cough and shortness of breath. Radiographic imaging of the chest revealed homogeneous opacity over the lower right zone (Fig. 2). She was initially started on intravenous (IV) co-amoxiclav 1.2 g three times daily, and was escalated to IV piperacillin–tazobactam 4.5 gm three times daily after 2 days after she failed to show any improvement. As atypical pneumonia was suspected by the treating physician in view of the associated neurological symptoms, Mycoplasma pneumoniae antigen was taken and came back positive with 1 over 2560 titer of total mycoplasma antibody and presence of IgM antibody, suggestive of an acute infection. She was started with macrolide antibiotic (oral azithromycin 500 mg once daily for 5 days) on top of the previous IV antibiotic and IVIg treatment. She was also subjected to inpatient physiotherapy for a few days, then discharged well.
Discussion
Mycoplasma pneumoniae (MP) is a common respiratory pathogen. This short, rod-shaped bacterium lacking a cell wall commonly causes respiratory infections such as bronchitis and pneumonia. It is often considered to be an atypical organism as it is not visible on the usual Gram stain and cannot be cultured using standard methods [1]. This organism is also not susceptible to the broad-spectrum beta-lactam antibiotics normally used as first-line treatment for community-acquired pneumonia. MP is one of the causative organisms of “atypical” bacterial pneumonia because the respiratory manifestations are predominated by a complex of constitutional symptoms such as low-grade fever, headache, and malaise, usually associated with extrapulmonary manifestations such as hemolytic anemia, myocarditis, myringitis, encephalitis, and many others that can occur alongside or independent of the respiratory symptoms [2]. Miller Fisher syndrome (MFS) is one of the rare extrapulmonary manifestations of MP infection.
MFS is considered a rare variant of Guillain–Barré syndrome, which is an acute idiopathic, immune-mediated inflammatory polyradiculopathy. The pathophysiology of this condition is still not well understood, but autoantibody-mediated neuritis, a condition in which the immune system attacks the nerves as a result of molecular mimicry that can be triggered by various agents, is a possibility. Among the common pathogens are Campylobacter jejuni, Haemophilus influenzae, and cytomegalovirus. However, MP is a rare pathogen also found to be associated with MFS. MFS classically presents with a triad of ataxia, ophthalmoplegia, and areflexia. However, the clinical manifestations of MFS can vary widely. The less common presentations include limb dysesthesia, ptosis, facial and bulbar palsies, mild muscle weakness, and urinary incontinence [3]. MFS is two times more common in men and can affect people of all ages, with median age of onset in the fifth decade [4].
The diagnosis of MFS is made clinically. However, serological confirmation with the presence of Anti-GQ1b antibodies is often made to support the diagnosis. This antibody acts on GQ1b, which is a ganglioside found abundantly in the paranodal region of the extramedullary portion of the oculomotor, abducens, and trochlear nerves. These areas are usually those most affected by self-reactive Anti-GQ1b antibodies, blocking release of acetylcholine from the motor nerve ending [4]. Although this antibody is not unique to MFS, it can help support the diagnosis in cases of uncertainty. Moreover, it can also determine the severity of the disease, as the level relates to the disease activity.
MFS is a self-limiting condition, and gradual improvement marks its recovery period and often the resolution of symptoms. Rarely, serious complications such as cardiac arrhythmia or respiratory failure have been reported [5]. Ataxia and ophthalmoplegia typically resolve within 1–3 months after onset, and near-complete recovery is expected within 6 months [5]. Though areflexia may persist, it is not associated with functional disability. Although MFS follows a self-limiting course, immunomodulatory therapies including intravenous immune globulin and plasmapheresis have been used to hasten disease recovery and perhaps decrease the likelihood of progression to more severe conditions such as GBS [6]. Based on the observational data available, complete resolution of ataxia in 1 month and resolution of ophthalmoplegia within 3 months would be acceptable outcome measures [5].
In this report, our patient presented initially with ataxia and diplopia, suggestive of Miller Fischer syndrome that was later confirmed with the presence of anti-GQ1b antibody in serum. She was then treated with IVIg 0.4 g/kg once daily for a total of 5 days. However, she developed worsening respiratory symptoms later on during inpatient immunoglobulin therapy. She did not respond to first-line beta-lactam antibiotics and was investigated for atypical pneumonia, which was later confirmed with the presence of Mycoplasma pneumoniae antibody titer in serum and clinically response to macrolide antibiotic. She recovered well and is currently under regular outpatient clinic and physiotherapy follow-up.
Conclusion
The aim of this case report is to increase the level of suspicion among clinicians regarding the possibility of an association between Miller Fischer syndrome and Mycoplasma pneumoniae infection, which should be considered in any cases of pneumonia that develop neurological manifestations such as ophthalmoplegia and ataxia.
Availability of data and materials
Not applicable.
References
Baum SG. Mycoplasma pneumoniae infection in adults. UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA. 2012.
Ran Nir-Paz. Atypical pneumonia. BMJ Best practice. Dec 2019.
Lo YL. Clinical and immunological spectrum of the Miller Fisher syndrome. Muscle Nerve. 2007;36(5):615–27.
Sumera B, Taboada J. A case of Miller Fisher syndrome and literature review. Cureus. 2017;9(2).
Gupta SK, Jha KK, Chalati MD, Alashi LT. Miller Fisher syndrome. Case Reports. 2016;13(2016):2016217085.
Yepishin IV, Allison RZ, Kaminskas DA, Zagorski NM, Liow KK. Miller Fisher syndrome: a case report highlighting heterogeneity of clinical features and focused differential diagnosis. Hawai’i J Med Public Health. 2016;75(7):196.
Acknowledgements
The authors would like to thank the Director General of Ministry of Health of Malaysia for his permission to publish this article.
Funding
No funding was received for the writing of this article.
Author information
Authors and Affiliations
Contributions
AOP was a major contributor to the writing of this manuscript. CC analyzed and interpreted the patient data. RH helped in data collection and contributed to writing the manuscript. WNNWY is the advisor and helped with writing and final editing of the manuscript. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Payus, A.O., Clarence, C., Nee, T. et al. Atypical presentation of an atypical pneumonia: a case report. J Med Case Reports 16, 105 (2022). https://doi.org/10.1186/s13256-022-03320-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-022-03320-y