Abstract
Background
AngioJet® is an increasingly used method of percutaneous mechanical thrombectomy for the treatment of patients with arterial and venous thromboses. AngioJet® has been shown to cause intravascular haemoylsis universally. We report the case of a 29 year old patient who underwent AngioJet® thrombectomy and post-procedure developed a stage 3 Acute kidney injury (AKI.) requiring renal replacement therapy (RRT), secondary to intravascular haemolysis. We aim to explore the mechanism and potential risk factors associated with developing AKI in these patients and suggest steps to optimise patient management.
Case presentation
A 29 year old Caucasian male who developed a stage 3 AKI, requiring RRT, following AngioJet® thrombectomy for an occluded femoral vein stent. Urine and laboratory investigations showed evidence of intravascular haemolysis, which was the likely cause of AKI. Following a brief period of RRT he completely recovered renal function.
Conclusions
AKI is an increasingly recognised complication following AngioJet® thrombectomy, but remains underappreciated in clinical practice. AKI results from intravascular haemolysis caused by the device. Up to 13% of patients require RRT, but overall short-term prognosis is good. Pre-procedural risk factors for the development of AKI include recent major surgery. Sodium bicarbonate should be administered to those who develop renal impairment. Renal biopsy is high risk and does not add to management. Increased clinician awareness and vigilance for AKI post-procedure can allow for early recognition and referral to nephrology services for ongoing management.
Similar content being viewed by others
Background
Arterial and deep venous thromboses (DVT) are common and can cause significant morbidity and mortality. The mainstay of treatment most commonly involves the administration of antiplatelet or anticoagulant medications, respectively. However, for larger burden clots more invasive treatment options are available, to reduce the associated risk of complications, including clot embolisation and post thrombotic syndrome (PTS). Traditional methods for clot removal with catheter directed thrombolysis (CDT) are now being superseded by Percutaneous mechanical thrombectomy (PMT) devices, such as the AngioJet® rheolytic thrombectomy device (Possis Medical, Minneapolis, Minnesota, USA) (henceforth AngioJet®). These are an increasingly used form of endovascular treatment for both arterial and deep vein thromboses, due to the associated reduction in treatment time, intensive care admissions, and overall length of hospital stay compared to CDT techniques [1, 2].
Angiojet® employs multiple high-pressure saline jets which cause fragmentation of targeted clots, whilst simultaneously delivering thrombolytic agent into the clot. A Venturi effect is created by the jets, which allows for aspiration of the clot debris, and prevents clot embolisation [3]. Although effective, the mechanism of action has been shown to cause significant haemolysis and routinely results in post-procedural haemoglobinuria. This in turn can cause acute kidney injury (AKI), which although an increasingly recognized complication of Angiojet®, remains underappreciated in clinical practice. Five previous cases of AKI following Angiojet® have been reported in the literature, one of which was in a child [4,5,6,7,8]. We report the case of a 29 year old male who developed a severe Stage 3 AKI [9], requiring renal replacement therapy (RRT), following AngioJet® thrombectomy of an occluded iliac vein stent. We aim to expand on the possible risk factors for development of AKI in patients undergoing AngioJet®, and suggest steps which could be taken to optimise the management of these patients.
Case presentation
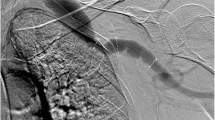
A 29 year old Caucasian male with a known left flank symptomatic venous malformation (VM) (Fig. 1) was admitted with a 2-day history of left leg pain, swelling and discolouration secondary to DVT. There was no history of chest pain, shortness of breath or palpitations. A year prior he had undergone left common-iliac vein stenting for a non-thrombotic iliac vein lesion, to redirect venous return away from the VM. As he remained symptomatic following this procedure, elective surgical excision-and-tie of the main feeder vessel to the VM was performed three weeks prior to this presentation. Bleeding at the time of this operation led to Apixaban, that he was previously on, to be stopped. He had no other past medical history, including no known history of renal impairment, and no family history of renal disease. At the time of presentation cardiorespiratory examination was unremarkable. Examination of the abdomen revealed a firm, palpable mass in the left abdominal wall, consistent with the known VM. The left upper leg was swollen with mottling of the skin, but otherwise soft and non-tender, and peripheral pulses were intact. 7500 units twice daily of low molecular weight heparin (LMWH) were commenced at the time of presentation. Following CT venography (Fig. 1) and duplex ultrasonography, that identified an occluded venous stent, Angiojet thrombectomy and venoplasty were performed under general anaesthetic by the vascular surgical team (Fig. 2). Pre-operative clotting markers were all within normal limits (INR 1.1, APTR 1.1). Intraoperatively, 8000 units of unfractionated heparin were administered, followed by 15000 units of LMWH one hour post-procedure. Successful recanalisation of the thrombosed stent was achieved. In the post-operative period he developed bradycardia and vomiting, and was treated with antiemetic and intravenous fluids. Vomiting settled after 36 hours. He remained haemodynamically stable throughout. Following surgical intervention a continuous intravenous heparin infusion was commenced, to prevent re-occlusion of the stent.
His renal function was noted to decline immediately postop, from a baseline serum creatinine of 77 µmol/L to 168 µmol/L (Fig. 3). The patient passed dark red urine, which on urine dipstick tested positive for blood. Renal function continued to decline over the coming 48 hours (Fig. 3). Laboratory investigations demonstrated serum lactate dehydrogenase (LDH) to be elevated at 1148 U/L and haptoglobin level low at 0.3 g/L, haemoglobin fell post-procedure from 145 to 86 g/L (Table 1). Direct antiglobulin test was negative. Blood tests performed pre-procedure and within 72 hours post-procedure are shown in Table 1. Acute renal screen blood tests and virology were all negative. An ultrasound of the kidneys and urinary tract demonstrated normal sized (right 12.5 cm, left 11.9 cm), unobstructed kidneys bilaterally, with a diffuse increase in renal echogenicity and loss of corticomedullary differentiation. Incidentally the spleen was noted to be enlarged at 13 cm. A duplex ultrasound confirmed patent renal vasculature, and good perfusion of both kidneys.
The patient was transferred to the renal ward 72 h post-procedure due to an ongoing decline in renal function and fall in urine output (Fig. 2). He was initially managed with intravenous 1.26% sodium bicarbonate and 0.9% sodium chloride solutions, to maintain a positive fluid balance. However urine output continued to fall and he started to develop evidence of fluid overload. After a further 48 hours, intermittent haemodialysis (HD) was commenced via a right internal jugular vein vascath. Four sessions of HD were completed in total (Fig. 3). He subsequently started to show signs of renal recovery with polyuria, passing over 3 litre of clear urine per day. A decision was made not to perform a renal biopsy in view of the high risk of bleeding given the concomitant heparin infusion. He was discharged with a falling creatinine and once loaded on warfarin. At the time of writing the patient’s renal function had improved to near baseline, with a serum creatinine of 90 µmol/L (Fig. 3).
Discussion and conclusions
Haemolysis is a well-documented cause of AKI in many conditions, including autoimmune haemolysis, paroxysmal nocturnal haemoglobinuria and haemolysis secondary to prosthetic cardiac valves [10]. AngioJet® has previously been shown to universally result in post-procedural gross haematuria, following intravascular haemolysis caused by the high-pressure saline jets [11]. Furthermore, previous cases of AKI secondary to AngioJet®-induced intravascular haemolysis have also been reported [4,5,6,7,8]. The occurrence of haemolysis in the case presented, as evidenced by the passage of dark red urine post-procedure, fall in haemoglobin and haptoglobin, and rise in serum LDH, was an anticipated consequence of the procedure. Given the patients’ young age and absence of other risk factors, deterioration in renal function to the point of requiring RRT (Fig. 3), was not anticipated. The patient had a small volume of contrast intra-operatively and significant vomiting post-operatively, both of which could have contributed to AKI. The severity of AKI with need for RRT, despite aggressive fluid replacement, suggests the cause of deterioration in renal function was likely haemolysis, as previously reported.
Previous reports have demonstrated an increased risk of complications following native renal biopsy in hospital in-patients who develop AKI, compared to outpatients [12]. Given this and the concomitant Heparin infusion, our patient was started on post AngioJet®, the decision was made not to perform a renal biopsy to further investigate the cause for AKI. It was felt that there was sufficient evidence of haemolysis (as previously discussed) as a cause for AKI, and that a biopsy would add little to guide further management. One previous study reports on renal biopsy findings in a patient who developed AKI post AngioJet®. This study reported findings including acute tubular injury, red blood cell debris within tubules, and tubular epithelial cells and podocytes staining for ferritin and haemo-oxygenase-1 (HO-1) [7]. These findings lend support to numerous studies which suggest the mechanism of AKI following haemolysis is likely related to a complex interplay of cytotoxic inflammatory mediators, activated in response to the increased iron and haemprotein load from lysed red blood cells. Filtered haemoproteins induce the release of ferritin and HO-1, which protect against oxidative-stress by scavenging free haem and iron. When these protective mechanisms are overwhelmed however, haem and iron can have direct toxic effects on glomeruli and tubular cells, resulting in renal dysfunction [13].
The ‘Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths’ (PEARL) registry only briefly mention the association between AngioJet® and the development of AKI. PEARL made no comment on the incidence of AKI not requiring RRT, and quoted that 5% of patients required RRT at 12 months post-procedure. They did not however expand on the indication for RRT, nor resolution and prevention of AKI in this group [14]. Subsequent studies have reported on the risk of AKI associated with AngioJet®. Morrow et al. observed the incidence of AKI in patients with arterial and venous thromboses, undergoing PMT with AngioJet®. They found the incidence of renal dysfunction to be significantly higher in the PMT group compared to CDT controls, 21% and 0% (p = 0.033), respectively. None of the PMT patients however required RRT [15]. Similarly, Escobar et al. found AngioJet® to be an independent risk factor for the development of AKI (odds ratio 8.22, p = 0.004) [16]. Shen et al. also reported a significantly increased risk of AKI in patients undergoing AngioJet® for iliofemoral DVT compared to CDT, 22.8% and 9.2% (p = 0.013), respectively. Furthermore, they demonstrated major surgery within 3 months prior to vascular intervention, to be a risk factor for the development in AKI post AngioJet® (odds ratio 8.51, p < 0.01) [11]. Our patient underwent excision-and-tie of the VM within 3 months prior to AngioJet®, potentially placing him at increased risk of developing AKI. Other than major surgery performed within 3 months of vascular intervention [11], none of the studies identified any pre-procedural risk factors for the development of AKI, including traditional risk factors for AKI. Both Escobar et al. and Shen et al. reported 2 patients requiring a period of RRT, 11% and 13%, respectively [11, 16].
The case presented appears typical when compared to other reported cases of significant AKI following AngioJet® [4,5,6,7,8]. Our patient developed AKI immediately post-procedure with associated haematuria and evidence of haemolysis, despite aggressive intravenous rehydration. After a brief period of HD there was evidence of renal recovery with increased urine output and improvement in serum creatinine (Fig. 3). It remains unclear as to whether the presence of a VM contributed to the development of AKI in our patient. The presence of a VM meant there was a larger burden of thrombus present, which in turn would require a more prolonged procedure to clear. It is conceivable that the increased clot burden allowed for a greater degree of haemolysis and therefore an increased risk of AKI in this patient. Prevention and management of AKI associated with haemolysis is an area which remains under investigation. There is some evidence to suggest the use of sodium bicarbonate may be beneficial through the effect of alkalinisation, reduction of free radical generation and attenuation of the effects of oxidative stress on renal tubules [13] (Table 2). In some individuals however, these conservative measures are unsuccessful and the need for RRT may be inevitable. Timely referral to nephrology services allows for advice regarding fluid resuscitation and commencement of RRT, potentially without the need for admission to an intensive care unit. This case, along with previous reports, would suggest that the short-term prognosis in patients who develop AKI post AngioJet® is good, with good recovery of renal function in most. Further studies are needed however to determine the potential long-term implications of AKI following AngioJet®, including the long-term risk of needing RRT.
AKI is an increasingly reported complication following AngioJet® thrombectomy, but remains underappreciated in day-to-day clinical practice. AKI can be severe and in up to 13% of cases require RRT, but short-term outcomes are good. Routine risk factors for the development of AKI in hospital inpatients, are not associated with AngioJet®. Undergoing major surgery within 3 months of AngioJet® is the only pre-procedural risk factor reported to be associated with the development of AKI. The use of CDT over AngioJet® may therefore need to be considered in these potentially at risk patients. Measures to prevent AKI following haemolysis remain under investigation, however the administration of sodium bicarbonate may be beneficial. Performing a renal biopsy to investigate these patients is high risk and we feel does not offer any clinical benefit. Clinicians should be mindful of the risk of AKI associated with AngioJet® thrombectomy in order to allow for; appropriate counselling and consent pre-procedure; post-procedural vigilance for deterioration in renal function; and timely referral to nephrology services in the event of developing AKI (Table 2).
Availability of data and materials
Not applicable.
Abbreviations
- AKI:
-
Acute kidney injury
- CDT:
-
Catheter directed thrombolysis
- DVT:
-
Deep vein thromboses
- HD:
-
Haemodialysis
- LDH:
-
Lactate dehydrogenase
- LMWH:
-
Low molecular weight heparin
- PEARL:
-
Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths
- PTS:
-
Post thrombotic syndrome
- RRT:
-
Renal replacement therapy
- VM:
-
Venous melaformation
References
Garcia MJ, et al. Endovascular management of deep vein thrombosis with rheolytic thrombectomy: final report of the prospective multicenter PEARL (Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths) Registry. J Vasc Interv Radiol. 2015;26(6):777–85 (quiz 786).
Lin PH, et al. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg. 2006;192(6):782–8.
Sharafuddin MJA, Hicks ME. Current status of percutaneous mechanical thrombectomy part ii devices and mechanisms of action. J Vasc Int Radiol. 1998;9(1):15–31.
Arslan B, Turba UC, Matsumoto AH. Acute renal failure associated with percutaneous mechanical thrombectomy for iliocaval venous thrombosis. Semin Intervent Radiol. 2007;24(3):288–95.
Dukkipati R, et al. Acute kidney injury caused by intravascular hemolysis after mechanical thrombectomy. Nat Clin Pract Nephrol. 2009;5(2):112–6.
Bedi P, et al. Acute kidney injury requiring renal replacement therapy due to severe hemolysis after mechanical thrombectomy. Case Rep Intern Med. 2016;3(4):87–90.
Esteras R, et al. Podocyte and tubular involvement in AngioJet-induced kidney injury. Clin Kidney J. 2019;14:424.
Hultin S. AngioJetTM rheolytic thrombectomy induced intravascular haemolysis leading to Acute Kidney Injury requiring Dialysis. J Clin Nephrol. 2018;2:025–8.
Mehta RL, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31.
Dvanajscak Z, et al. Hemolysis-associated hemoglobin cast nephropathy results from a range of clinicopathologic disorders. Kidney Int. 2019;96(6):1400–7.
Shen Y, et al. Increased risk of acute kidney injury with percutaneous mechanical thrombectomy using AngioJet compared with catheter-directed thrombolysis. J Vasc Surg Venous Lymphat Disord. 2019;7(1):29–37.
Moledina DG, et al. Kidney biopsy-related complications in hospitalized patients with acute kidney disease. Clin J Am Soc Nephrol. 2018;13(11):1633–40.
Van Avondt K, Nur E, Zeerleder S. Mechanisms of haemolysis-induced kidney injury. Nat Rev Nephrol. 2019;15(11):671–92.
Leung DA, et al. Rheolytic pharmacomechanical thrombectomy for the management of acute limb ischemia: results from the PEARL registry. J Endovasc Ther. 2015;22(4):546–57.
Morrow KL, et al. Increased risk of renal dysfunction with percutaneous mechanical thrombectomy compared with catheter-directed thrombolysis. J Vasc Surg. 2017;65(5):1460–6.
Escobar GA, et al. Risk of acute kidney injury after percutaneous pharmacomechanical thrombectomy using AngioJet in venous and arterial thrombosis. Ann Vasc Surg. 2017;42:238–45.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MA wrote the introduction section. TR wrote the case presentation and discussion sections. PS contributed to the case presentation. MA, PS, CB and DG reviewed and approved the final text. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Roper, T., Amaran, M., Saha, P. et al. Unclogging the effects of the Angiojet® thrombectomy system on kidney function: a case report. J Med Case Reports 15, 459 (2021). https://doi.org/10.1186/s13256-021-03062-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-021-03062-3