Abstract
Background
Hodgkin lymphoma usually presents with sequential enlargement of peripheral lymph nodes, and bone marrow invasion rarely occurs (approximately 3–5%). However, several cases have been reported as “primary” bone marrow Hodgkin lymphoma, especially among patients with human immunodeficiency virus and the elderly. This type of Hodgkin lymphoma is characterized by no peripheral lymphadenopathies and has been reported to have poorer prognosis.
Case presentation
A 38-year-old Japanese man was admitted to our hospital because of fever of unknown origin and pancytopenia without lymphadenopathies. Bone marrow examination revealed Hodgkin cells mimicking abnormal cells. These were positive for CD30, EBER-1, CD15, PAX-5, and Bob-1 and negative for Oct-2, CD3, CD20, surface immunoglobulin, CD56. On the basis of systemic evaluation and bone marrow examination, he was diagnosed with primary bone marrow Hodgkin lymphoma. We initiated therapy with DeVIC (dexamethasone, etoposide, ifosfamide, and carboplatin) therapy, but remission was not achieved. Then, the patient was treated with brentuximab vedotin combined with systemic chemotherapy (Adriamycin, vinblastine and dacarbazine), which was effective.
Conclusions
There is no established treatment strategy for Hodgkin lymphoma, and therapeutic outcomes using ABVD (Adriamycin, bleomycin, vinblastine and dacarbazine)-like or CHOP (cyclophosphamide, Adriamycin, vincristine, and prednisone)-like regimens are reportedly poor. Only a few patients have been reported to achieve long-term remission. Through this case report, we suggest an alternative therapeutic option for primary bone marrow Hodgkin lymphoma.
Similar content being viewed by others
Background
Hodgkin lymphoma (HL), one of the most common lymphoproliferative diseases, characteristically presents with progressive and sequential enlargement of peripheral lymph nodes [1]. Bone marrow (BM) invasion rarely occurs in patients with HL (approximately 3–5%) and typically only in those with advanced disease [2]. However, Shah et al. [3] reported a rare case of a patient with “primary bone marrow” Hodgkin lymphoma (PBMHL) with human immunodeficiency virus (HIV). PBMHL has also been reported in both HIV-positive and HIV-negative patients.
Epstein-Barr virus (EBV) is believed to play a causative role in HIV-associated HL [4]. Use of in situ hybridization or immunohistochemical staining has revealed that approximately 40% of patients with non-HIV-associated HL [5] and 75–78% of patients with HIV-associated HL are EBV-positive [4, 6]. The precise mechanism by which EBV contributes to development of HL remains unclear. EBV infection has no influence on the prognosis of children with HL [7]. However, some researchers have reported a higher relapse rate after primary treatment in middle-aged patients with EBV-associated HL than in those with non-EBV-associated HL [8]. In patients with refractory HL, dose-dense systemic chemotherapy, brentuximab vedotin (BV), and autologous hematopoietic stem cell transplant are considered salvage treatments. BV, which is a CD30-directed antibody conjugated with monomethyl auristatin E, is an approved treatment for patients with relapsed or refractory HL [9], and it has safely been combined with systemic chemotherapy [10]. In this report, we present a case of a patient with EBV-associated PBMHL who was successfully treated with BV-containing combination chemotherapy.
Case presentation
A 38-year-old Japanese man was admitted to our hospital because of progressive fever and thrombocytopenia for more than 1 month. His medical history included Burkitt lymphoma (negative for EBV-encoded small ribonucleic acid (RNA)), and he had been treated with hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) 12 years before admission. He had achieved complete remission. He was a daily smoker (18 pack-years) and took no daily medications. He had a family history of hypertension and denied having any malignancies.
The patient’s physical examination findings were normal except for small papules on his upper back. Laboratory tests showed pancytopenia, high C-reactive protein, and a negative result for HIV (Table 1). Computed tomography (CT) revealed moderate pulmonary emphysema with no evidence of infection or inflammation. Positron emission tomography/CT was unavailable for financial reasons. A skin biopsy of his rash revealed no malignant change. BM examination revealed hemophagocytosis without abnormal cells. Plasma viral deoxynucleic acid (DNA) was investigated to identify possible causes of hemophagocytosis, and the patient was found to be EBV DNA-positive (170,000 copies/ml).
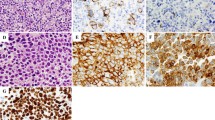
As recommended by a previous report, the patient’s EBV-associated hemophagocytic lymphohistiocytosis (HLH) was treated with chemotherapy comprising etoposide and dexamethasone [11]. Shortly after initiation of chemotherapy, his white blood cell count recovered to within the normal range, and his plasma EBV DNA became undetectable. However, his EBV DNA turned positive (290 copies/ml), and his white blood cell count declined again 7–9 weeks later. A second BM sample showed infiltration with Reed-Sternberg (RS)-like cells (Fig. 1a, b) that were positive for CD30 (Fig. 1c), EBER-1 (Epstein-Barr encoding region 1) (Fig. 1d), CD15 (Fig. 1e), Bob-1 (Fig. 1d), and PAX-5 (Fig. 1e) but negative for Oct-2, CD3, CD20, surface immunoglobulin, and CD56. Another CT examination showed neither lymphadenopathy nor hepatosplenomegaly. The patient was eventually diagnosed with PBMHL (Ann Arbor stage IVB, International Prognostic Index high-intermediate risk). Previous reports have indicated that PBMHL progresses rapidly and that combination chemotherapy (Adriamycin, bleomycin, vinblastine and dacarbazine [ABVD]-like or cyclophosphamide, Adriamycin, vincristine and prednisone [CHOP]-like regimens) is ineffective, as shown in Table 2. Because some patients with EBV-associated lymphoproliferative disease express P-glycoprotein, which is a multidrug resistance 1 (MDR1) gene product [12], the poor prognosis of PBMHL may be related to the presence of the MDR1 gene. DeVIC (dexamethasone, etoposide, ifosfamide, and carboplatin) includes ifosfamide and carboplatin, which are MDR-unrelated anticancer agents.
Histopathological findings in bone marrow on presentation. Representative photomicrographs of hematoxylin and eosin (HE) and immunohistochemically stained sections of bone marrow. Rectangular area in (a) (HE, × 40) is shown at higher magnification in (b) (HE, original magnification × 400). Reed-Sternberg cell-like cells are present (arrowhead). These cells are positive for CD30 (c, original magnification × 400), Epstein-Barr encoding region in situ hybridization (EBER-ISH) (d, original magnification × 400), CD15 (e, original magnification × 400), Bob-1 (f, original magnification × 400), and PAX-5 (g, original magnification × 400). The small rectangulars in d–g show expression of CD30 in the same field. Arrowheads in f and g identify the CD30 positive malignant cells
In addition, because of coexisting pulmonary emphysema, DeVIC therapy was initiated at 12 weeks from onset. Although DeVIC therapy induced transient recovery of pancytopenia, it recurred with high plasma EBV DNA titers (2300 copies/ml) after the second course of DeVIC therapy (19–20 weeks from onset). Because a third BM examination revealed residual RS cells, the patient’s disease was considered refractory to DeVIC therapy. After giving informed consent, he was further treated with BV (1.2 mg/kg) and AVD (Adriamycin, vinblastine, and dacarbazine) as described in a previous report [12]. His peripheral blood cell count recovered without support of medication, and the RS cells disappeared. After four courses of combined chemotherapy, BV monotherapy was continued for 8 months, during which both soluble interleukin (IL)-2 receptor and plasma EBV DNA titers remained within the normal range. The patient declined autologous hematopoietic stem cell transplant. To date, no evidence of relapse has been detected.
Discussion
The overall incidence of BM involvement in HL is reportedly 5% [2]. However, approximately 40–50% of patients with HIV-associated HL have BM invasion, and these patients commonly present with an advanced stage of disease (mainly stage IV). PBMHL is characterized by solitary BM invasion with HL. PBMHL has been reported in patients with and without HIV infection (Table 2). Our review of published reports revealed that patients with acquired immunodeficiency syndrome or older patients are more likely to develop PBMHL. Although our patient was HIV-negative, his number of CD4+ T cells was low (200/μl). As shown in Table 2, low CD4+ T-cell counts (or lymphocytopenia) are common in patients with PBMHL, especially in those with HIV-associated PBMHL, in whom CD4+ T-cell counts are reportedly 133 ± 130/μl (range 14–549/μl, median 103/μl).
Our patient developed HLH as the first symptom of HL. Although HL-associated HLH is rare, a previous retrospective study showed that EBV was frequently detected in patients with HL-associated HLH [13]. Patients with HIV-associated HL, in whom HLH is more common, also exhibit a high prevalence of EBV. These findings suggest that patients with HL-associated HLH might have an unclear underlying immune disturbance for EBV. Our patient’s case indicates that clinicians should perform BM biopsies to check for PBMHL in patients with (1) pancytopenia, (2) low CD4+ T-cell counts (or lymphocytopenia), and (3) EBV DNA positivity.
As for the prognosis of PBMHL, only a few patients achieve long-term remission (Table 2). When our patient’s PBMHL proved refractory, we selected BV with AVD as salvage therapy for the following reasons. First, because of its rarity, there is no established treatment strategy for this disease, and therapeutic outcomes using ABVD- or CHOP-like regimens are reportedly poor (as shown in Table 2). Second, bleomycin was contraindicated for our patient because of his coexisting moderate emphysema. Third, a combination of BV with AVD was significantly superior to BV with ABVD [10]. Fortunately, our patient completed his planned therapy without relapse. To the best of our knowledge, this is the first reported case of successful treatment of HIV-negative PBMHL with BV.
The association of EBV and HL has been investigated; however, the exact mechanism involved remains unclear. Approximately 40% of patients with non-HIV-associated HL are EBV-positive [5]; the rate of EBV positivity is much higher in patients with HIV-associated HL [4, 6]. In addition to in situ hybridization and histopathological staining, plasma EBV DNA titers are also a useful biomarker for monitoring prognosis in patients with EBV-associated HL [14]. In our patient, an increase in EBV DNA titer preceded recurrence of pancytopenia and occurred earlier than the increase in soluble IL-2 receptor titer. Plasma EBV DNA titers may be helpful for early detection of recurrence of PBMHL.
Conclusions
We present a rare case of a patient with PBMHL without HIV infection. More experience is needed to establish the optimum treatment for this disease. On the basis of our patient’s progress, we propose that combined therapy with BV and AVD could be a therapeutic option for PBMHL.
Abbreviations
- ABVD:
-
Adriamycin, bleomycin, vinblastine and dacarbazine
- AIDS:
-
Acquired immunodeficiency syndrome
- Alb:
-
Albumin
- ALT:
-
Alanine aminotransferase
- APTT:
-
Activated partial thromboplastin time
- AST:
-
Aspartate aminotransferase
- Aty lym:
-
Atypical lymphocytes
- AVD:
-
Adriamycin, vinblastine, and dacarbazine
- Bas:
-
Basophils
- BM:
-
Bone marrow
- BUN:
-
Blood urea nitrogen
- BV:
-
Brentuximab vedotin
- C7-HRP:
-
Cytomegalovirus antigenemia
- CHOP:
-
Cyclophosphamide, Adriamycin, vincristine, and prednisone
- Cre:
-
Creatinine
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- DeVIC:
-
Dexamethasone, etoposide, ifosfamide, and carboplatin
- EB:
-
Epstein-Barr virus
- EB EA IgG:
-
Epstein-Barr virus early antigen immunoglobulin G
- EB VCA:
-
Epstein-Barr virus viral capsid antigen immunoglobulin M
- EBER:
-
Epstein-Barr-encoding region
- EBER1:
-
Epstein-Barr virus-encoded RNA 1
- EBNA:
-
Epstein-Barr nuclear antigen
- EBV:
-
Epstein-Barr virus
- EIA:
-
Enzyme immunoassay
- Eos:
-
Eosinophils
- FDP:
-
Fibrinogen degradation product
- γ-GTP:
-
γ-Glutamyltransferase
- Hb:
-
Hemoglobin
- Hct:
-
Hematocrit
- HHV-6:
-
Human herpesvirus 6
- HIV:
-
Human immunodeficiency virus
- HL:
-
Hodgkin lymphoma
- HLH:
-
Hemophagocytic lymphohistiocytosis
- IFN-γ:
-
Interferon-γ
- IgA:
-
Immunoglobulin A
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- IL-6:
-
Interleukin 6
- LDH:
-
Lactate dehydrogenase
- LMP1:
-
Latent membrane protein 1
- Lym:
-
Lymphocytes
- MCV:
-
Mean corpuscular volume
- MDR1:
-
Multidrug resistance 1
- β2-MG:
-
β2-microglobulin
- Mo:
-
Monocytes
- MOPP:
-
Mechlorethamine, vincristine, procarbazine, and prednisone
- ND:
-
No data
- Neu:
-
Neutrophils
- PBMHL:
-
Primary bone marrow Hodgkin lymphoma
- Plt:
-
Platelets
- PT:
-
Prothrombin time
- RBC:
-
Red blood cells
- RS:
-
Reed-Sternberg
- sIL-2:
-
Soluble interleukin 2
- T-Bil:
-
Total bilirubin
- THP-COP:
-
Pirarubicin, cyclophosphamide, vincristine, and prednisolone
- TNF-α:
-
Tumor necrosis factor-α
- TP:
-
Thymidine phosphorylase
- WBC:
-
White blood cells
References
Gobbi PG, Ferreri AJM, Ponzoni M, Levis A. Hodgkin lymphoma. Crit Rev Oncol Hematol. 2013;85(2):216–37.
Franco V, Tripodo C, Rizzo A, Stella M, Florena AM. Bone marrow biopsy in Hodgkin’s lymphoma. Eur J Haematol. 2004;73(3):149–55.
Shah BK, Subramaniam S, Peace D, Garcia C. HIV-associated primary bone marrow Hodgkin’s lymphoma: a distinct entity? J Clin Oncol. 2010;28(27):e459–60.
Yotsumoto M, Hagiwara S, Ajisawa A, Tanuma J, Uehira T, Nagai H, et al. Clinical characteristics of human immunodeficiency virus-associated Hodgkin lymphoma patients in Japan. Int J Hematol. 2012;96(2):247–53.
Chang KL, Kamel OW, Arber DA, Horyd ID, Weiss LM. Pathologic features of nodular lymphocyte predominance Hodgkin’s disease in extranodal sites. Am J Surg Pathol. 1995;19(11):1313–24.
Westmoreland KD, Stanley CC, Montgomery ND, Kaimila B, Kasonkanji E, El-Mallawany NK, et al. Hodgkin lymphoma, HIV, and Epstein-Barr virus in Malawi: longitudinal results from the Kamuzu Central Hospital Lymphoma study. Pediatr Blood Cancer. 2017;64(5):e26302.
Claviez A, Tiemann M, Luders H, Krams M, Parwaresch R, Schellong G, et al. Impact of latent Epstein-Barr virus infection on outcome in children and adolescents with Hodgkin’s lymphoma. J Clin Oncol. 2005;23(18):4048–56.
Diepstra A, van Imhoff GW, Schaapveld M, Karim-Kos H, van den Berg A, Vellenga E, et al. Latent Epstein-Barr virus infection of tumor cells in classical Hodgkin’s lymphoma predicts adverse outcome in older adult patients. J Clin Oncol. 2009;27(23):3815–21.
Zinzani PL, Viviani S, Anastasia A, Vitolo U, Luminari S, Zaja F, et al. Brentuximab vedotin in relapsed/refractory Hodgkin’s lymphoma: the Italian experience and results of its use in daily clinical practice outside clinical trials. Haematologica. 2013;98(8):1232.
Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2017;378(4):331–44.
Henter J-I, Samuelsson-Horne A, Aricò M, Egeler RM, Elinder G, Filipovich AH, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367.
Younes A, Connors JM, Park SI, Fanale M, O’Meara MM, Hunder NN, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14(13):1348–56.
Mánard F, Besson C, Rincá P, Lambotte O, Lazure T, Canioni D, et al. Hodgkin lymphoma–associated hemophagocytic syndrome: a disorder strongly correlated with Epstein-Barr virus. Clin Infect Dis. 2008;47(4):531–4.
Gandhi MK, Lambley E, Burrows J, Dua U, Elliott S, Shaw PJ, et al. Plasma Epstein-Barr virus (EBV) DNA is a biomarker for EBV-positive Hodgkin’s lymphoma. Clin Cancer Res. 2006;12(2):460–4.
Meadows LM, Rosse WR, Moore JO, Crawford J, Laszlo J, Kaufman RE. Hodgkin’s disease presenting as myelofibrosis. Cancer. 1989;64(8):1720–6.
Kojima H, Takei N, Mukai Y, Hasegawa Y, Suzukawa K, Nagata M, et al. Hemophagocytic syndrome as the primary clinical symptom of Hodgkin’s disease. Ann Hematol. 2003;82(1):53–6.
Ponzoni M, Ciceri F, Crocchiolo R, Famoso G, Doglioni C. Isolated bone marrow occurrence of classic Hodgkin’s lymphoma in an HIV-negative patient. Haematologica. 2006;91(3):Ecr04.
Cacoub L, Touati S, Yver M, Frayfer J, Abarah W, Andre-Kerneis E, et al. Isolated bone marrow Hodgkin lymphoma in a human immunodeficiency virus-negative patient: a second case. Leuk Lymphoma. 2014;55(7):1675–7.
Dholaria B, Alapat D, Arnaoutakis K. Primary bone marrow Hodgkin lymphoma in an HIV-negative patient. Int J Hematol. 2014;99(4):503–7.
Morita Y, Emoto M, Serizawa K, Rai S, Hirase C, Kanai Y, et al. HIV-negative primary bone marrow Hodgkin lymphoma manifesting with a high fever associated with hemophagocytosis as the initial symptom: a case report and review of the previous literature. Intern Med. 2015;54(11):1393–6.
Suzuki T, Kusumoto S, Masaki A, Ishida T, Inagaki H, Iida S, et al. CD30-positive primary bone marrow lymphoma mimicking Hodgkin lymphoma. Int J Hematol. 2015;101(2):109–11.
Ponzoni M, Fumagalli L, Rossi G, Freschi M, Re A, Vigano MG, et al. Isolated bone marrow manifestation of HIV-associated Hodgkin lymphoma. Mod Pathol. 2002;15(12):1273–8.
Gerard L, Oksenhendler E. Hodgkin’s lymphoma as a cause of fever of unknown origin in HIV infection. AIDS Patient Care STDS. 2003;17(10):495–9.
Salama ME, Perkins SL, Mariappan MR. Images in HIV/AIDS. Primary bone marrow presentation of Epstein-Barr virus-driven HIV-associated Hodgkin lymphoma. AIDS Read. 2007;17(12):604–5.
Corti M, Villafane M, Minue G, Campitelli A, Narbaitz M, Gilardi L. Clinical features of AIDS patients with Hodgkin’s lymphoma with isolated bone marrow involvement: report of 12 cases at a single institution. Cancer Biol Med. 2015;12(1):41–5.
Acknowledgements
We thank Dr. Trish Reynolds, MBBS, FRACP, of Edanz Group (www.edanzediting.com/ac) for editing a draft of the manuscript.
Author information
Authors and Affiliations
Contributions
KN, YK, TY, RI, and KK were responsible for the clinical management of the patient. MI evaluated the pathological examinations. MM and NK supervised the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This case report was approved by the Suzuka General Hospital Ethics Committee on 30 Nov 2017 (approval number 176).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Nagaharu, K., Masuya, M., Kageyama, Y. et al. Successful treatment of primary bone marrow Hodgkin lymphoma with brentuximab vedotin: a case report and review of the literature. J Med Case Reports 12, 151 (2018). https://doi.org/10.1186/s13256-018-1693-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-018-1693-0