Abstract
Background
Pulmonary surfactant is a complex mixture of lipids and proteins. Mutations in surfactant protein-C, surfactant protein-D, and adenosine triphosphate-binding cassette subfamily A member 3 have been related to surfactant dysfunction and neonatal respiratory failure in full-term babies. Adenosine triphosphate-binding cassette subfamily A member 3 facilitates the transfer of lipids to lamellar bodies. We report the case of patient with a homozygous intronic ABCA3 mutation.
Case presentation
We describe a newborn full-term Colombian baby boy who was the son of non-consanguineous parents of mixed race ancestry (Mestizo), who was delivered with severe respiratory depression. Invasive treatment was unsuccessful and diagnosis was uncertain. Exons 4 and 5 of the SP-C gene showed heterozygous Thr138Asn polymorphism and homozygous Asn186Asn polymorphism respectively. At intron 25 at position –98 from exon 26 a homozygous C>T transition mutation was detected in ABCA3 gene.
Conclusions
The clinical presentation and the histopathological findings of this case are consistent with a case of neonatal respiratory failure due to surfactant deficiency. Analysis of the five coding SP-C exons does not support surfactant deficiency. An analysis of the mutation IVS25-98 T was performed and a homozygous mutation responsible for our case’s neonatal respiratory failure was detected. The findings suggest an autosomic recessive pattern of inheritance. Genetic counseling was provided and the relatives are now informed of the recurrence risks and treatment options.
Similar content being viewed by others
Background
Pulmonary surfactant is a complex mixture of lipids, primarily dipalmitoylphosphatidylcholine, surfactant proteins (SP-A, SP-B, SP-C, and SP-D), and the protein adenosine triphosphate-binding cassette subfamily A member 3 (ABCA3), produced by type II pneumocytes. Pulmonary surfactant is essential for lowering surface tension at the air–liquid interface to prevent end-expiratory alveolar collapse. Lamellar bodies are dense multilayer secretory organelles found in pneumocytes II which store the surfactant [1]. Mutations in SP-C, SP-D, and ABCA3 have been related to surfactant dysfunction and neonatal respiratory failure (NRF) in full-term babies and interstitial lung disease (ILD) in older children and adults [2].
ABCA3 is a protein expressed predominantly in the lung, localized to the limiting membrane of lamellar bodies of type II pneumocytes. The ABCA3 protein is codified by a single gene located in chromosome 16 which consists of 33 exons [3]. It has been demonstrated that ABCA3 selectively facilitates the transfer of phosphatidylcholine, sphingomyelin, and cholesterol to lamellar bodies [4].
Autosomic recessive mutations in the ABCA3 gene have been frequently involved in NRF due to surfactant deficiency and some forms of ILD in older children. The majority of these identified mutations are located in the exons or the immediate intron–exon boundaries. A recent article identified an intronic ABCA3 mutation in one allele and a known disease causing mutation in the other as responsible for NRF in a full-term newborn [5]. We report the case of a full-term baby boy with a homozygous intronic ABCA3 mutation as the cause of his fatal respiratory disease.
Case presentation
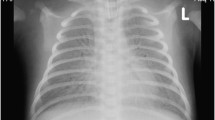
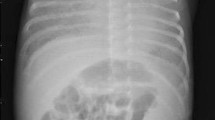
We describe the case of a full-term Colombian newborn baby boy who was the product of a primigravid mother, and non-consanguineous parents of mixed race ancestry (Mestizo). Fetal monitoring at the 37th week gestational age showed continuous decelerations. A caesarean section was performed and he was delivered with severe respiratory depression. Management with noninvasive positive-pressure ventilation was started without success. He was transferred to our intensive care unit and intubation was performed. An echocardiogram showed moderate pulmonary hypertension. Chest X-rays showed complete bilateral opacity of both lungs. Initial treatment with artificial surfactant was offered without success (Fig. 1).
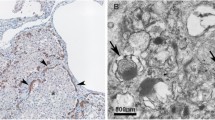
Blood tests showed 38,900 leukocytes with 90 % neutrophils, but the results of all the cultures and the C-reactive protein were negative. A lung biopsy was performed. Right apical pneumothorax appeared as a complication of the procedure and was treated with a chest tube. The lung biopsy showed minimal interstitial changes, preserved alveolar architecture, hyperplasia of the alveolar epithelium (pneumocytes type 2) and a thickened septum full of mesenchymal immature cells and few inflammatory cells (some eosinophils, neutrophils, and leukocytes; Fig. 2).
During his hospitalization, his fever persisted; his leukocytosis was treated with vancomycin and meropenem without any bacteriological finding. He died on day 60 due to respiratory failure and the diagnosis was still uncertain.
Based on the suspicion of a SP deficiency, the genes for SP-B and exon 9 for ABCA3 gene were analyzed without finding any abnormalities. Exon 4 of the SP-C gene showed the polymorphism Thr138Asn in the heterozygous form (ACT/AAT) and exon 5 the Asn186Asn polymorphism in the homozygous form (AAC/AAC).
Due to the uncertainty of the diagnosis a literature review was performed and experts were asked for advice. A search for a specific mutation in the intronic region of the ABCA3 gene was performed. In intron 25 at position –98 from exon 26 a homozygous C>T transition mutation was detected. This mutation changes the intronic sequence, creates a new donor splice site and leads to aberrant ABCA3 proteins and is the cause of our patient’s fatal respiratory disease (Fig. 3).
To confirm the mechanism of inheritance of the disease and to be able to perform proper genetic counseling, genetic sequencing for the specific gene was done on both parents and both are carriers of the mutation IVS25-98 T.
Discussion
The most common presentation of a baby with ABCA3 mutation that leads to NRF is a full-term baby with moderate to severe respiratory distress and signs of diffuse lung disease without satisfactory history or laboratory findings. The disease is often progressive and fatal within the first 3 months of life even with proper therapy as occurred in this case [6–14]. Some cases of older children with ILD and ABCA3 mutations have been reported. Out of the four reported cases of patients with a IVS25-98 T mutation in one allele, two died, one of them received a lung transplant and the other one is still alive but has ILD (see Table 1) [5, 7–14].
The histopathological findings in patients with NRF due to surfactant deficiency consist of type II pneumocytes hyperplasia, interstitial thickening, and prominent foamy macrophages in the airspaces, often embedded in variable amounts of proteinaceous material as found in the biopsy of our case. These findings are the result of an inborn error disrupting surfactant metabolism and function and are nonspecific for any of the SP-B, SP-C and ABCA3 mutations. A molecular diagnosis is needed to determine the specific mutation affecting each case [15, 16].
In exon 4 our patient shows the polymorphism Thr138Asn in the heterozygous form (ACT/AAT) and in exon 5 the Asn186Asn polymorphism in the homozygous form (AAC/AAC). These findings have been associated with risk of perinatal NRF in preterm male newborns. However, our patient was born at term and these polymorphisms have been frequently found in healthy people. Therefore, one may conclude that the analysis of the five coding SP-C exons does not support surfactant deficiency or a fatal malfunction of surfactant transport [17].
Definitive diagnosis required examination of DNA for ABCA3 intronic mutations. The analysis of the mutation IVS25-98 T was performed and a homozygous mutation was detected. This intronic mutation has been previously reported in heterozygous patients with severe NRF. The genetic analysis of such patients showed one allele with the intronic mutation and an exonic mutation in the other one. It is known that the IVS-98 T is a NRF-causing ABCA3 mutation since the intronic sequence creates a new donor splice site which leads to aberrantly spliced transcripts. It is suggested that the additional amino acids added to the ABCA3 protein alter its intracellular routing, stability, and function [5]. Recently, intronic mutations have been found in cystic fibrosis as disease-causing mutations in patients without a previously identified exonic mutation [18].
The literature reports four additional cases homozygous for ABCA3 IVS25-98 T. The four babies were unrelated, no history of consanguinity was identified, but all of them came from South America [5]. Although the mechanism of inheritance is still unclear and isodisomic uniparental disomy has been reported for ABCA3 deficiency, we speculate through our findings that the mechanism of inheritance could be autosomic recessive and the ethnical similarities of the cases could suggest a possible founder effect for this population [6]. Further larger population-based studies are required to determine the real frequency of IVS25-98 T in this population.
In spite of the fact that establishing the diagnosis did not alter the outcome of the patient and usually the diagnosis is established after the decease of the patient, it is essential to adequately counsel the parents and family members of the recurrence risk. Our patient’s parents were encouraged to have their ABCA3 gene screened for the IVS25-98 T mutation. Both parents of our patient are heterozygous carriers of the IVS25-98 T mutation in the ABCA3 gene. Therefore, children have a chance of 25 % of being affected by lethal ABCA3 deficiency and 50 % of being carriers. Two sisters of our patient’s father are heterozygous carriers of the IVS25-98 T mutation in the ABCA3 gene. Their partners do not carry the IVS25-98 T mutation, thus children of both couples have a statistical chance of 25 % of being heterozygous carriers of the mutation and none of them will be affected.
Conclusions
The clinical presentation of this case is consistent with a case of NRF due to surfactant deficiency. The histopathological findings in patients with NRF due to surfactant deficiency are nonspecific for any of the SP-B, SP-C and ABCA3 mutations and a molecular diagnosis is needed. The analysis of the five coding SP-C exons does not support surfactant deficiency or a fatal malfunction of surfactant transport due to SP-C mutations as the cause for our patient’s symptoms. Without evidence of a previously identified ABCA3 mutation in an exon or an immediate intron–exon boundary, we conclude that the homozygous IVS25-98 T mutation is responsible for this case’s NRF. The findings of our patient’s relatives suggest an autosomic recessive pattern of inheritance. Genetic counseling was provided and the relatives are now informed of the recurrence risks and treatment options.
Abbreviations
- ABCA3:
-
Adenosine triphosphate-binding cassette subfamily A member 3
- ILD:
-
Interstitial lung disease
- NRF:
-
Neonatal respiratory failure
- SP:
-
Surfactant protein
References
Batenburg JJ. Surfactant phospholipids: synthesis and storage. Am J Physiol. 1992;262(4 Pt 1):L367–85.
Thomas AQ, Lane K, Phillips J, Prince M, Markin C, Speer M, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165(9):1322–8.
Yamano G, Funahashi H, Kawanami O, Zhao LX, Ban N, Uchida Y, et al. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett. 2001;508(2):221–5.
Cheong N, Madesh M, Gonzales LW, Zhao M, Yu K, Ballard PL, et al. Functional and trafficking defects in ATP binding cassette A3 mutants associated with respiratory distress syndrome. J Biol Chem. 2006;281(14):9791–800.
Agrawal A, Hamvas A, Cole FS, Wambach JA, Wegner D, Coghill C, et al. An intronic ABCA3 mutation that is responsible for respiratory disease. Pediatr Res. 2012;71(6):633–7.
Hamvas A, Nogee LM, Wegner DJ, Depass K, Christodoulou J, Bennetts B, et al. Inherited surfactant deficiency caused by uniparental disomy of rare mutations in the surfactant protein-B and ATP binding cassette, subfamily A, member 3 genes. J Pediatr. 2009;155(6):854–9.
Young LR, Nogee LM, Barnett B, Panos RJ, Colby TV, Deutsch GH. Usual interstitial pneumonia in an adolescent with ABCA3 mutations. Chest. 2008;134:192–5.
Hofmeister J, Bruder E, Aslanidis C, Hammer J, Schmitz G, Bührer C. Swiss Society of Neonatology. January 2008. http://www.neonet.ch/files/2514/2591/5213/january_2008.pdf. Accessed 28 Jul 2016.
Thavagnanam S, Cutz E, Manson D, Nogee LM, Dell SD. Variable clinical outcome of ABCA3 deficiency in two siblings. Pediatr Pulmonol. 2013;48(10):1035–8.
Gonçalves JP, Pinheiro L, Costa M, Silva A, Gonçalves A, Pereira A. Novel ABCA3 mutations as a cause of respiratory distress in a term newborn. Gene. 2014;534(2):417–20. doi:10.1016/j.gene.2013.11.015. Epub 2013 Nov 20.
Panigrahy N, Poddutoor PK, Chirla DK. ATP-binding cassette transporter A3 (ABCA3) mutation in a late preterm with respiratory distress syndrome. Indian Pediatr. 2014;51(7):579–80.
Malý J, Navrátilová M, Hornychová H, Looman AC. Respiratory failure in a term newborn due to compound heterozygous ABCA3 mutation: the case report of another lethal variant. J Perinatol. 2014;34(12):951–3. doi:10.1038/jp.2014.132.
Rezaei F, Shafiei M, Shariati G, Dehdashtian A, Mohebbi M, Galehdari H. Novel Mutation in the ATP-Binding Cassette Transporter A3 (ABCA3) Encoding Gene Causes Respiratory Distress Syndrome in A Term Newborn in Southwest Iran. Iran J Pediatr. 2016;26(2):e2493.
Ota C, Kimura M, Kure S. ABCA3 mutations led to pulmonary fibrosis and emphysema with pulmonary hypertension in an 8-year-old girl. Pediatr Pulmonol. 2016;51:E21–3. doi:10.1002/ppul.23379.
Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med. 2004;350(13):1296–303.
Doan ML, Guillerman RP, Dishop MK, et al. Clinical, radiological and pathological features of ABCA3 mutations in children. Thorax. 2008;63(4):366–73.
Lahti M, Marttila R, Hallman M. Surfactant protein C gene variation in the Finnish population – association with perinatal respiratory disease. Eur J Hum Genet. 2004;12(4):312–20.
Groman JD, Meyer ME, Wilmott RW, Zeitlin PL, Cutting GR. Variant cystic fibrosis phenotypes in the absence of CFTR mutations. N Engl J Med. 2002;347(6):401–7.
Acknowledgements
We thank the team at the Centro de Investigación en Anomalías Congénitas y Enfermedades Raras (CIACER), of the Universidad Icesi; and the neonatology team of Fundación Valle del Lili.
Authors’ contributions
HP, LM, and FR did the morphologic assessment, analyzed and interpreted the patient data regarding the genetics findings. VD and DD analyzed and interpreted the patient radiographs and AB, IP, and DD did the clinical assessment of the patient. All authors have been involved in drafting the manuscript or revising it critically for important intellectual content; have given final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Authors’ information
HP: Geneticist at Fundación Valle del Lili, Director of Centro de Investigación en Anomalías Congénitas y Enfermedades Raras (CIACER), full-time professor Universidad Icesi.
FR: General physician, Master’s student in basic biomedical sciences with emphasis on clinical genetics, researcher at Centro de Investigación en Anomalías Congénitas y Enfermedades Raras (CIACER), professor Universidad Icesi.
LM: Plastic surgery resident Universidad del Valle.
VD: Diagnostic radiology resident Universidad Icesi.
AB: Neonatologist Fundación Valle del Lili.
IP: Neonatologist Fundación Valle del Lili.
DD: Neumologist Fundación Valle del Lili.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient’s legal guardian(s) for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Pachajoa, H., Ruiz-Botero, F., Meza-Escobar, L.E. et al. Fatal respiratory disease due to a homozygous intronic ABCA3 mutation: a case report. J Med Case Reports 10, 266 (2016). https://doi.org/10.1186/s13256-016-1027-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-016-1027-z