Abstract
Background
Tumor deposits (TDs) are associated with poor prognosis in rectal cancer (RC). This study aims to develop and validate a deep learning (DL) model incorporating T2-MR image and clinical factors for the preoperative prediction of TDs in RC patients.
Methods and methods
A total of 327 RC patients with pathologically confirmed TDs status from January 2016 to December 2019 were retrospectively recruited, and the T2-MR images and clinical variables were collected. Patients were randomly split into a development dataset (n = 246) and an independent testing dataset (n = 81). A single-channel DL model, a multi-channel DL model, a hybrid DL model, and a clinical model were constructed. The performance of these predictive models was assessed by using receiver operating characteristics (ROC) analysis and decision curve analysis (DCA).
Results
The areas under the curves (AUCs) of the clinical, single-DL, multi-DL, and hybrid-DL models were 0.734 (95% CI, 0.674–0.788), 0.710 (95% CI, 0.649–0.766), 0.767 (95% CI, 0.710–0.819), and 0.857 (95% CI, 0.807–0.898) in the development dataset. The AUC of the hybrid-DL model was significantly higher than the single-DL and multi-DL models (both p < 0.001) in the development dataset, and the single-DL model (p = 0.028) in the testing dataset. Decision curve analysis demonstrated the hybrid-DL model had higher net benefit than other models across the majority range of threshold probabilities.
Conclusions
The proposed hybrid-DL model achieved good predictive efficacy and could be used to predict tumor deposits in rectal cancer.
Critical relevance statement
The proposed hybrid-DL model achieved good predictive efficacy and could be used to predict tumor deposits in rectal cancer.
Key points
• Preoperative non-invasive identification of TDs is of great clinical significance.
• The combined hybrid-DL model achieved good predictive efficacy and could be used to predict tumor deposits in rectal cancer.
• A preoperative nomogram provides gastroenterologist with an accurate and effective tool.
Graphical Abstract

Similar content being viewed by others
Introduction
As one of the most prevalent malignancies worldwide, colorectal cancer (CRC) is the leading cause of cancer-related death and a serious threat to people’s health and life quality [1]. According to the research results of International Agency for Research on Cancer(IARC), by 2040, the burden of colorectal cancer will increase to 3.2 million new cases per year (an increase of 63%) and 1.6 million deaths per year (an increase of 73%), increasing incidence rates are observed in younger adults and in countries that are undergoing economic transition [2]. Tumor deposits (TDs) are defined as discrete tumor foci located in the pericolonic or perirectal fat without histological evidence of residual lymph nodes or identifiable vascular or neural structures [3]. TDs are an independent risk factor for the prognosis of rectal cancel [4]. Tumors with positive TDs showed more aggressive biological behavior and are prone to local recurrence and distant metastases [5]. Patients with positive TDs show worse overall survival (OS) and disease-free survival (DFS) than patients with negative TDs [6]. TDs can ascend the N staging of tumor and are associated with patients’ treatment protocols, according to the 8th Tumor Node Metastasis (TNM) staging system of American Joint Committee on Cancer (AJCC), e.g., for any T stage patients, if TDs are positive, the patient will be classified as the stage of N1c and will be recommended for preoperative or postoperative neoadjuvant chemoradiotherapy (nCRT) instead of single surgical treatment, according to European Society of Medical Oncology (ESMO) consensus guidelines recommendation that nCRt is required in high-risk groups [7], and recent researches indeed demonstrated that patients with positive TD status and those who have undergone nCRT have a better survival outcome than those who have not undergone nCRT [8]. To sum up, the preoperative non-invasive identification of TDs is of great clinical significance, which plays a critical role in stage evaluation and treatment planning. However, the diagnosis of TDs primarily relies on postoperative pathological assessment, a method characterized by its inherent delay and invasiveness. The pursuit of non-invasive, precise, accurate preoperative diagnostic approaches is the focal point of our efforts.
The T2-weighted magnetic resonance imaging (T2WI-MRI) is the first-choice imaging modality for local staging in RC patients and can be used to evaluate the risk factors of rectal cancer, such as circumferential resection margin (CRM) status and the extramural vascular invasion (EMVI) [9], e.g., Yang et al. [10]. developed two nomograms through the integration of clinical risk factors, high-resolution magnetic resonance imaging (HRMRI) findings, and the research results demonstrated that the nomograms based on HRMRI and radiomics exhibited good predictive performance, with AUC values of 0.90 (95% confidence interval [CI] = 0.83–0.96) for LNM and 0.80 (95% CI = 0.69–0.92) for TDs, respectively. And Atre [11] undertook a retrospective analysis of the MRI imaging performance in 40 patients with pathologically confirmed TDs and malignant lymph nodes, and the research findings demonstrated that the morphology of lesions observed on MR imaging can serve as a valuable predictive factor for distinguishing TDs from positive lymph nodes (LNs) in patients with rectal cancer. Furthermore, when morphology information was combined with the MR texture parameter of skewness, diagnostic accuracy was significantly enhanced. However, the radiomics features defined by manually outlining the target region are pre-defined and highly dependent on the accurate segmentation of regions of interest (ROI); the inter-observer variability might challenge the reliability of the result [12]. In addition, the stability of texture features varies among different image acquisition and preprocessing, which could also limit its practical application [13].
In recent years, deep learning (DL), especially the convolutional neural network (CNN), has provided a new approach to medical image analysis and raised much attention in clinical practice. Compared with conventional machine learning, deep learning can automatically extract features from an image without the necessity of feature predefinition and is suitable for mining the most relevant feature representations [14,15,16,17]. TDs are scattered and distributed within the peritumoral adipose tissue, and our analysis focuses on the adipose tissue surrounding the tumor, for which deep learning methods are well-suited for the study of the imaging area. Moreover, there were researches about the application of DL models on MR imaging in predicting microsatellite instability and nCRT outcome [18, 19]; whether the DL algorithms could help to characterize the TDs status in RC patients has not been reported yet. So this study aims to construct a DL model incorporating both T2-MR images and clinical risk factors and assess its diagnostic accuracy for predicting TDs in RC patients.
Methods
Patient enrollment and study design
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Biomedical Research Ethics Committee of Qujing First People’s Hospital of Kunming Medical University (202201), and the requirement for informed consent was waived.
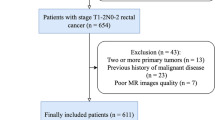
Rectal cancer patients from January 2016 to December 2019 who underwent MR examination were retrospectively collected. The inclusion criteria were as follows: (1) patients who were pathologically diagnosed with rectal adenocarcinoma and (2) patients who underwent MR examination 1 week before surgery. The exclusion criteria were as follows: (1) MR images with motion artifact or metal artifact, (2) patients with other malignant tumors at the same time, (3) lack of pre-operative laboratory test results, (4) tumor deposits status was not tested. Finally, a total of 137 RC patients with TDs and 190 RC patients without TDs were enrolled in this study.
A pseudorandom number generator was applied to give a random number between 0 and 1 for each patient, and the patients with a random number less than 0.25 were selected as the testing dataset while the rest were used as the development dataset. Finally, the patients were divided into a development dataset (143 non-TDs and 103 TDs) and an independent testing set (47 non-TDs and 34 TDs). The predictive models were trained only in the development dataset, and the independent dataset was used as a held-out dataset for evaluating the model performance. The flowchart of patient enrollment is shown in Fig. 1.
MRI examination protocol
All patients underwent MR examinations with 3.0-Tesla (T) scanners (MAGENTOM Skyra, Siemens Healthcare Sector, Germany; SIGNA Architect, GE Medical System, America; Ingenia Elition X, Philips, the Netherlands). Patients underwent some special bowel preparations before the MRI examination, including consuming liquid food on the day before the examination, and were administered a routine cleansing enema to ensure that the rectum was clear and empty 3 to 4 h before the examination. The main MR serial name was fast spin-echo no fat suppression T2-weighted imaging (FSE-T2WI). The parameters were set up as follows: repetition/echo time (TR/TE), 6890/100 ms; slice thickness, 3 mm; field of view (FOV), 220 × 220 mm; echo train length, 25; number of averages, 3; pacing between slices, 3.9 mm; flip angle, 150°; voxel size, 0.7 mm × 0.7 mm × 4.0 mm.
MR image evaluation
Two professional radiologists (with 10 and 15 years of experience in RC diagnoses), who were blinded to patients’ information, reviewed the MR images. Lesions classified as T1 (the submucosa of the rectum was invaded) and T2 (the muscularis propria of the rectum was invaded) stages on MR images were integrated to form the MRI T1–2 cohorts, according to AJCC 8th edition of the TNM staging system. Nodules larger than 3 mm in diameter were considered positive lymph nodes on MR images. Apparent diffusion coefficient (ADC) value was measured with ADC mapping. CRM was considered positive if the distance between the mesorectal fascia and the outermost edge of the tumor was less than 1 mm. EMVI was considered positive if dense blood vessels were present around the tumor or vascular emptying signals were absent. The tumor location was measured based on the distance between the anal verge and lower pole of the tumor (bottom, ≤ 5 cm; middle, 5–10 cm; top, ≥ 10 cm). Differences of opinion were resolved through consultation during the image review. The baseline features of MR images of study patients are shown in Table 1.
Pathological diagnosis of tumor deposits
Pathological reports recording pathological information of patients enrolled in this study were searched and downloaded from the Department of Pathology, West Chinese Hospital. The TD status (positive or negative) was described in the pathology reports, according to the standard of the AJCC 8th edition of the TNM staging system.
Lesion segmentation
Two types of lesions (the tumor regions and the peri-tumoral regions) were manually annotated by two radiologists with 10 and 15 years of diagnostic experience on the MR images. Both the tumor regions and the peri-tumoral regions were manually labeled within the border of the ROI by using the ITK-SNAP software (v3.8.0, http://www.itksnap.org).
The peri-tumoral regions refer to the mesorectal fat surrounding the tumor, which fills the area between the tumor's edge and the mesorectal fascia. As recent studies [20, 21] had reported that peritumoral mesorectal fat played an important role in understanding the tumor microenvironment, predicting tumor recurrence, and assessing the aggressiveness and heterogeneity of rectal cancer, so we included peritumoral adipose tissue in analysis. We map the entire area between the tumor margin and the rectal fascia, instead of defining a 1- or 5-mm distance around the tumor, as the precise definition of a 1- or 5-mm distance was difficult and would increase the workload. An example of the manually segmented tumor region and corresponding peri-tumoral region is shown in Supplementary Fig. 1.
Both radiologists were blinded to the pathological reports. After the completion of the three-dimensional segmentation, 20 patients were randomly selected for evaluating feature stability. For the intra-class correlation analysis, radiologist A (with 10-year experience) drew ROI of those patients once again (1 month apart). For the inter-class correlation analysis, the correlation coefficient was calculated by comparing ROIs of radiologist A (first time) and radiologist B (with 15-year experience). The features with intra and inter-class correlation coefficient (ICC) ≤ 0.75 were excluded, according to commonly admitted rule that ICC > 0.75 = good or excellent reliability, < 0.5 = poor reliability, and 0.5–0.75 = moderate reliability [22].
Data pretreatment
The clinical variables were recorded in relation to the TD diagnosis as follows:
(1) age, an actual variable; (2) gender, a dichotomous variable (female = 0, male = 1); (3) MR T stage, a polytomous variable (T1/T2 = 0, T3 = 1, T4 = 2); (4) MR N stage, a polytomous variable (N0 = 0, N1 = 1, N2 = 2); (5) MR M stage, a dichotomous variable (M0 = 0, M1 = 1); (6) lesion location, a polytomous variable (bottom = 0, middle = 1, top = 2, bottom/middle = 3, middle/top = 4, bottom/middle/top = 5); (7) tumor type, a dichotomous variable (mass = 0, infiltrative = 1); (8) CRM a dichotomous variable (negative = 0, positive = 1); (9) EMVI a dichotomous variable (negative = 0, positive = 1); (10) ADC value, an actual variable; (11) serum carcinoembryonic antigen (CEA) level, a dichotomous variable {negative = 0, positive = 1(> 5 ng/ml)}; (12) serum CA19-9 level, a dichotomous variable {negative = 0, positive = 1(> 35 u/ml)}; (13) serum carbohydrate antigen (CA)125 level, a dichotomous variable {negative = 0, positive = 1(> 35 u/ml)}.
Intensity normalization was first applied on the T2WI images to rescale the intensities to 0.255, and then the manually labeled ROIs on MR images were transformed and defined as follows before model development: (1) a three-dimensional (3D) patch containing the cropped ROI, which the size was determined on the largest ROI (64pixel × 64pixel × 10pixel for tumor regions, 128pixel × 128pixel × 10pixel for peri-tumoral regions); (2) manually labeled pixel-wise ROI masks, in which non-lesion areas were padded with zero; (3) the label of tumor deposits which was pathologically identified.
In order to avoid overfitting, data augmentation was applied during the training process of the deep learning models [23]. Flipping (at the x and y axis), random brightness contrast adjustment (80%, 90%, 110%, and 120%), and random rotation (90, 180, and 270° at the z axis) were performed. Finally, the sample size was increased to 2460 in the development dataset for the construction of the deep learning models.
Development of the clinical model
To select the tumor deposits associated with clinical variables, both univariate regression analysis and multivariate regression analysis were carried out in the development dataset. The back stepwise selection was applied in multivariate regression analysis and only the clinical factors with a p-value less than 0.05 were selected for model development. A clinical model was constructed based on the selected clinical variables by applying logistic regression with the scikit-learn toolkit [24]. Liblinear was used as the default solver and an L2 regularization of C = 1 was also applied. The tolerance was set to 0.0001, other parameters were set by default.
Design of the DL models
Based on whether integrating the peri-tumoral ROI images or clinical variables, three deep learning models were developed for our purpose: a single-channel deep learning (single-DL) model using only the tumor ROI as input, a multi-channel deep learning (multi-DL) model using both the tumor ROI image and peri-tumoral ROI image as input, and a hybrid deep learning (hybrid-DL) model that incorporating both tumor ROI and peri-tumoral ROI images as well as the selected clinical variables. For the single-DL model, ResNet50 was used as the backbone of the neural network for the extraction of high-dimension features from the tumor ROI images. The extracted image features were converted into a 1280-bit vector followed by a soft-max regression for the binary classification of TDs or non-TDs. For the multi-DL model, two ResNet50-based networks with fully connected layers were used for extracting high-dimension features from the ROIs containing tumor and peri-tumoral regions, respectively. These features were converted into 1280-bit vectors and further concatenated to 2560-bit vectors, and then the probability of TDs was predicted by the soft-max layer. The conceptual architecture of our single-DL and multi-DL models is shown in Supplementary Fig. 2.
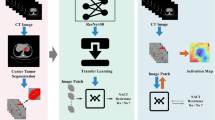
For the hybrid-DL model, the selected clinical variables of each patient were first transformed into a 512-bit vector by using a fully connected layer and were concatenated with the transformed vectors from the tumor ROI as well as the peri-tumoral ROI. Finally, the concatenated 3072-bit vector integrating both MR image and clinical variables was used for assessing the probability of TDs in each patient (Fig. 2).
Training of the DL models
To improve the adversarial robustness and transferability of the DL models, the transfer learning method was applied in this study. The neural networks were pretrained on the natural images from the ImageNet dataset and the medical images of multiple dataset from The Cancer Imaging Archive (TCIA) database [25, 26].
The weighted-balanced binary cross-entropy loss function was used to penalize errors of the majority class and value the minority class more positively, as class weights were inversely proportional to their frequency in the training data. The Mini-Batch Gradient Descent (MBGD) algorithm with a learning rate of 1e − 4 was used to optimize the parameters of the model and contribute to speeding up the convergence rate. The minibatch size and the dropout rate were set to 16 and 0.6, respectively. The other parameters were set as default. The training process was stopped when the loss in the validation set became stable, and the average number of epochs for the DL models varied from 60 to 80. The development of the proposed deep learning models was performed on the InferScholar platform (InferVision, China).
Calibration and decision curve analysis
The calibration curve analysis was applied for the evaluation of consistency between the predicted TD probability and the actual observed rate, and the calibration curve was plotted by using 1000 bootstrapping resamples method. In addition, the goodness-of-fit of the predictive models was assessed with the Hosmer–Lemeshow test in the independent testing dataset [27]. Decision curve analysis (DCA) was also used to evaluate and compare the clinical usefulness of different models by calculating the net benefit across the reasonable range of threshold probabilities [28].
Model interpretability
As the deep learning was “black-box” models, the Gradient-weighted Class Activation Mapping (Grad-CAM) approach was applied to better comprehend the DL models by highlighting the critical response areas on the T2WI images for the deep learning algorithms in predicting TDs [29]. For the single-DL and multi-DL models, the saliency maps were generated by applying Grad-CAM on the last convolutional layer of the deep learning models. In addition, beside the saliency map of the T2WI images, the relative weights of the selected clinical variables for each patient were also presented for the hybrid-DL model.
Statistical analysis
All statistical analyses were performed with the MedCalc software (version 20.0) and the SPSS software (version 23.0). The continuous and the categorical variables were compared by the Mann–Whitney U test and the chi-square test, respectively. The discriminative capability of the predictive models was evaluated through the ROC analysis by assessing the AUC, and the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each model were also calculated according to the optimal threshold which was determined by the maximum Youden index. The Delong’s test was used to compare the difference between two AUCs [30]. The calibration curve and the decision curve were generated with R language (version 3.6.4) by using the “rms” package and the “rmda” package, respectively. A two-sided p < 0.05 was considered statistically significant.
Results
Patient characteristics
The TD patients showed an improved MR N-stage, increased mass type and EMVI prevalence, and higher serum CA19-9 level than the non-TD patients in both development and independent testing datasets. There was no significant difference in the prevalence of TDs (p = 0.987) between the development dataset (42%, 103/246) and the independent testing dataset (42%, 34/81), as summarized in Table 1. There was no significant difference in gender, age, MR M stage, ADC value, serum CEA level, and serum CA125 level between the TD patients and non-TD patients in both development and independent testing datasets.
Selection of the clinical variables
The clinical variables were analyzed before model development. After multivariate regression analysis, only the MR N-stage, EMVI, tumor location, CRM, and serum CA19-9 level showed a p-value less than 0.05 and were selected for model development (Table 2).
Model performance comparison
Based on the manually segmented tumor ROI, peri-tumoral ROI, and selected clinical variables, the clinical model, single-DL model, multi-DL model, and hybrid DL model were constructed in the development dataset and evaluated in the independent testing dataset.
The diagnostic performance of these predictive models was compared through ROC analysis (Fig. 3). The clinical model showed AUCs of 0.734 (95% CI, 0.674–0.788) and 0.726 (95% CI, 0.615–0.819) in the development and the independent testing datasets, respectively. The single-DL model based on tumor ROI images had achieved AUCs of 0.710 (95% CI, 0.649–0.766) and 0.676 (95% CI, 0.563–0.776) in the development and the independent testing datasets, respectively. After incorporating peri-tumoral ROI images, the AUCs of the multi-DL model were improved to 0.767 (95% CI, 0.710–0.819) in the development dataset (p < 0.001) and 0.738 (95% CI, 0.628–0.829) in the independent testing dataset (p = 0.348). The results indicate that the peri-tumoral ROI could benefit TD diagnosis.
The hybrid-DL model showed favorable discrimination capability in the development dataset, with AUCs yielding 0.857 (95% CI, 0.807–0.898), and outperformed the clinical model (p = 0.068), single-DL model (p < 0.001), and multi-DL model (p < 0.001). Similar results were obtained in the independent testing dataset, although not statistically significant, the hybrid-DL model had achieved higher AUC (0.839 [95% CI, 0.741–0.911]) than that of the clinical model (p = 0.123), single-DL model (p = 0.028), and multi-DL model (p = 0.066). The sensitivity, specificity, PPV, and NPV of each model in the development and independent testing datasets are listed in Table 3.
Clinical utility of the predictive models
All the predictive models showed good consistency between the actual observed rate and the predicted TD probability (Fig. 4). The Hosmer–Lemeshow test showed that the non-significant statistic was 0.919, 0.163, 0.941, and 0.092 for the clinical model, single-DL model, multi-DL model, and hybrid-DL model, respectively, which indicated no significant deviation of the models from an ideal fitting.
The DCA in the independent testing dataset demonstrated that the hybrid-DL model had higher net benefit than the other three models across almost the entire range of the threshold probabilities, which suggested that the hybrid-DL model was superior to other models in terms of clinical usefulness (Fig. 5).
Interpretation of the DL models
The saliency maps of two representative cases are presented in Fig. 6. The distribution of the highlighted attentional regions was consistent with the manually labeled ROIs, and both tumor and peri-tumoral regions could contribute to TDs diagnosis in the multi-DL model and hybrid-DL model. In addition, the relative weights of the selected clinical variables changed among patients, indicating the personalized diagnosis mechanism of the Hybrid-DL model.
Discussion
In the present study, we developed three DL models and a clinical model to predict TDs in RC patients. In the independent testing dataset, the hybrid DL model showed superior diagnostic capability compared to other models (AUC = 0.839; sensitivity = 77%; specificity = 85%). To our knowledge, this is the first study assessing the feasibility of DL on MR imaging to predict TDs in RC patients preoperatively.
As an important prognostic factor in RC, the presence of TDs is associated with perineural, lymphatic, or vascular invasion [31]. More importantly, TDs are correlated with the N stage of the tumor and have implications for the selection of treatment strategies, e.g., Nagtegaal et al. [32] demonstrated that patients with TDs had the worst outcome whether there were LNMs or not. Goldstein et al. also reported that the patients with TDs always demonstrated a worse survival when their numbers of metastatic LNs differed [33]. However, a major obstacle to TD classification is that no reliable diagnostic method of TDs on the traditional imaging by gross eye has been established [34]. Hence, an objective and accurate assessment method for predicting TDs in RC patients before surgery is urgently required and of great clinical significance. If we can predict the presence of TDs preoperatively, we can avoid overestimating patients’ prognoses and refraining from forgoing nCRT, which has the potential to significantly enhance patient outcomes, and improve patients’ survival rates. Although identification of TDs in RC patients through radiomics analysis on MR, CT, and US images had been reported in previous literature [35, 36], radiomic feature quantification was sensitive to acquisition modes and matrix sizes, and the delineation of tumor volumes was also crucial for the assessment of radiomics features [37]. On the other hand, many radiomics features were found to be unstable between two scans acquired within weeks or even 15 min of each other [38]. As a special type of artificial neural network, deep learning can automatically extract high-level features directly from images and has achieved impressive success in medical analysis for its image-based pattern recognition [39]. Compared with previous radiomics analysis, several advantages of the DL model in this study could be highlighted. First, our study has enrolled more patients than previous studies (327 vs 40 ~ 254) and the larger sample size could lead to lower bias and better model reliability, which is confirmed by the similar performance of the hybrid-DL model between development and independent testing datasets. Second, more risk factors including clinical characteristics, MR findings, and serum biomarkers were used in our study, which could provide more valuable information and improve model performance. Third, our DL models are trained end-to-end supervised method, avoiding the limitation of pre-defined hand-crafted radiomics features [40].
Interestingly, in this study, we found that the incorporation of peri-tumoral fat regions could benefit DL-based TD diagnosis, as the multi-DL model achieved higher AUC than that of the single-DL model in both development and independent testing datasets. The potential mechanisms may be that TDs via neurotrophic extravascular migratory and perineural invasion, which could lead to radiographic changes around the tumor regions [41, 42], so the analysis of peritumoral adipose tissue could contribute to the detection of TDs. Previous studies [20, 21] had reported that peritumoral mesorectal fat played an important role in understanding the tumor microenvironment, predicting tumor recurrence, assessing the aggressiveness and heterogeneity of rectal cancer, and our finding was consistent with previous studies.
It should be noted that there were several limitations in this study. Firstly, as a single-center retrospective analysis, only RC patients confirmed by surgical pathology were enrolled in this study, and selection bias might be unavoidable, and the generalizability of our findings to a broader patient population may be compromised. To address this limitation, a multicenter study with more enrolled patients should be conducted to enhance the robustness and external validity of our conclusions. Secondly, the DL model was built on T2-weighted MR images. Multi-MR sequences included in the building of the model may potentially improve the reliability and applicability of the model to other different image datasets. Therefore, model incorporating other MR sequences such as diffusion-weighted imaging need to be investigated in the future. Third, the inability to automatically delineate the target region and issues may weaken the reproducibility of the model, and a method that can automatically delineate the target region and issues needs to be used in the future.
Conclusions
The presence of tumor deposits has been found to be associated with both the tumor stage and the treatment strategy in patients with rectal cancer. The non-invasive identification of TDs holds significant clinical implications. In this study, we developed four distinct models, comparing their diagnostic performance with the aim of furnishing a valuable predictive tool for TD status. Furthermore, our investigation revealed that the model utilizing deep learning techniques exhibited superior performance compared to either a single radiomics or clinical model. Specifically, hybrid DL model integrating T2-MR images and clinical risk factors demonstrated robust predictive capabilities and holds promise as an asset in predicting TDs in rectal cancer cases, and the application of deep learning methodologies in clinical settings exhibits promising prospects.
Availability of data and materials
This is a free-access article. Supplementary Material and the primary article both contain the original data.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AJCC:
-
American Joint Committee on Cancer
- AUC:
-
Area under the curve
- CA19-9:
-
Carbohydrate antigen19-9
- CEA:
-
Carcinoembryonic antigen
- CNN:
-
Convolutional neural network
- CRC:
-
Colorectal cancer
- CRM:
-
Circumferential resection margin
- DCA:
-
Decision curve analysis
- DL:
-
Deep learning
- EMVI:
-
Extramural vascular invasion
- FOV:
-
Field of view
- FSE-T2WI:
-
Fast spin-echo no fat suppression T2-weighted imaging
- IARC:
-
International Agency for Research on Cancer
- LNM:
-
Lymph node metastasis
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristics
- ROI:
-
Regions of interest
- T2WI-MRI:
-
T2-weighted magnetic resonance imaging
- TCIA:
-
Cancer Imaging Archive
- TDs:
-
Tumor deposits
- TR/TE:
-
Repetition/echo time
References
Mattiuzzi C, Sanchis-Gomar F, Lippi G (2019) Concise update on colorectal cancer epidemiology. Ann Transl Med 7:609. https://doi.org/10.21037/atm.2019.07.91
Morgan E, Arnold M, Gini A et al (2023) Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from globocan. Gut 72:338–344. https://doi.org/10.1136/gutjnl-2022-327736
Li M, Xu G, Chen Q, et al (2023) Computed tomography-based radiomics nomogram for the preoperative prediction of tumor deposits and clinical outcomes in colon cancer: a multicenter study. Acad Radiol 1572–1583. https://doi.org/10.1016/j.acra.2022.11.005
Lord AC, Graham Martínez C, D’Souza N et al (2019) The significance of tumour deposits in rectal cancer after neoadjuvant therapy: a systematic review and meta-analysis. Eur J Cancer 11:1–8. https://doi.org/10.1016/j.ejca.2019.08.020
Wang Y, Zhang J, Zhou M, et al (2019) Poor prognostic and staging value of tumor deposit in locally advanced rectal cancer with neoadjuvant chemoradiotherapy. Cancer Med 8:1508–1520. https://doi.org/10.1002/cam4.2034
Fata CR, Gonzalez RS, Liu E et al (2017) Mesenteric tumor deposits in midgut small intestinal neuroendocrine tumors are a stronger indicator than lymph node metastasis for liver metastasis and poor prognosis. Am J Surg Pathol 41:128–133. https://doi.org/10.1097/PAS.0000000000000751
Glynne-Jones R, Wyrwicz L, Tiret E, et al (2018) Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:263. https://doi.org/10.1093/annonc/mdy161.
Schaap DP, Voogt ELK, Burger JWA et al (2021) Prognostic implications of MRI-detected EMVI and tumor deposits and their response to neoadjuvant therapy in cT3 and cT4 rectal cancer. Int J Radiat Oncol Biol Phys 111:816–825. https://doi.org/10.1016/j.ijrobp.2021.06.013
Zhu HT, Zhang XY, Shi YJ et al (2022) The conversion of MRI data with multiple b-values into signature like pictures to predict treatment response for rectal cancer. J Magn Reson Imaging 56:562–569. https://doi.org/10.1002/jmri.28033
Yang YS, Feng F, Qiu YJ, Zheng GH, Ge YQ, Wang YT (2021) High-resolution MRI-based radiomics analysis to predict lymph node metastasis and tumor deposits respectively in rectal cancer. Abdom Radiol (NY) 46:873–884. https://doi.org/10.1007/s00261-020-02733-x
Atre ID, Eurboonyanun K, Noda Y, et al (2021) Utility of texture analysis on T2-weighted MR for differentiating tumor deposits from mesorectal nodes in rectal cancer patients, in a retrospective cohort. Abdom Radiol (NY) 46:459–468. https://doi.org/10.1007/s00261-020-02653-w
Pavic M, Bogowicz M, Würms X, et al (2018) Influence of inter-observer delineation variability on radiomics stability in different tumor sites. Acta Oncol 57:1070–1074. https://doi.org/10.1080/0284186X.2018.1445283
Yang F, Dogan N, Stoyanova R, et al (2018) Evaluation of radiomic texture feature error due to MRI acquisition and reconstruction: a simulation study utilizing ground truth. Phys Med 50:26–36. https://doi.org/10.1016/j.ejmp.2018.05.017
Xu J, Zhang R, Zhou Z, et al (2021) Deep network for the automatic segmentation and quantification of intracranial hemorrhage on CT. Front Neurosci 14:541817. https://doi.org/10.3389/fnins.2020.541817
McKinney SM, Sieniek M, Godbole V, et al (2020) International evaluation of an AI system for breast cancer screening. Nature 586:E19. https://doi.org/10.1038/s41586-020-2679-9
Xu Y, Hosny A, Zeleznik R, et al (2019) Deep learning predicts lung cancer treatment response from serial medical imaging. Clin Cancer Res 25:3266–3275. https://doi.org/10.1158/1078-0432.CCR-18-2495.
Coudray N, Ocampo PS, Sakellaropoulos T, et al (2018) Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat Med 24:1559–1567. https://doi.org/10.1038/s41591-018-0177-5
Zhang W, Huang Z, Zhao J, et al (2021) Development and validation of magnetic resonance imaging-based radiomics models for preoperative prediction of microsatellite instability in rectal cancer. Ann Transl Med 9:134. https://doi.org/10.21037/atm-20-7673
Jang BS, Lim YJ, Song C, et al (2021) Image-based deep learning model for predicting pathological response in rectal cancer using post-chemoradiotherapy magnetic resonance imaging. Radiother Oncol 161:183–190. https://doi.org/10.1016/j.radonc.2021.06.019
Pan AF, Zheng NX, Wang J, et al (2022) Role of perirectal fat in the carcinogenesis and development of early-onset rectal cancer. J Oncol 2022:4061142. https://doi.org/10.1155/2022/4061142
Felsenreich DM, Gachabayov M, Bergamaschi R et al (2023) Does the mesorectal fat area impact the histopathology metrics of the specimen in males undergoing tme for distal rectal cancer? Updates Surg 75:581–588. https://doi.org/10.1007/s13304-022-01429-9
Benchoufi M, Matzner-Lober E, Molinari N, Jannot AS, Soyer P (2020) Interobserver agreement issues in radiology. Diagn Interv Imaging 101:639–641. https://doi.org/10.1016/j.diii.2020.09.001
Shorten C, Khoshgoftaar TM (2021) A survey on image data augmentation for deep learning. J Big Data 55:2351–2377. https://doi.org/10.1007/s10462-021-10066-4
Bac J, Mirkes EM, Gorban AN, Tykin I, Zinovyev A (2011) Scikit-learn: machine learning in Python. Entropy. https://doi.org/10.3390/e23101368
Russakovsky O, Deng J, Su H, et al (2015) ImageNet large scale visual recognition challenge. Int J Comput Vis 37:1904–16. https://doi.org/10.1109/TPAMI.2015.2389824
Clark K, Vendt B, Smith K, et al (2013) The cancer imaging archive (TCIA): Maintaining and operating a public information repository. J Digit Imaging 26:1045–57. https://doi.org/10.1007/s10278-013-9622-7
Finazzi S, Poole D, Luciani D, et al (2011) Calibration belt for quality-of-care assessment based on dichotomous outcomes. PLoS One 6:e16110. https://doi.org/10.1371/journal.pone.0016110
Vickers AJ, Elkin EB (2006) Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 26:565–74. https://doi.org/10.1177/0272989X06295361
Selvaraju RR, Cogswell M, Das A, et al (2022) Grad-CAM: visual explanations from deep networks via gradient-based localization. Int J Comput Vis 132:102382. https://doi.org/10.1016/j.artmed.2022.102382
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–45. 3203132
Kim S, Huh JW, Lee WY et al (2023) Prognostic impact of lymphatic invasion, venous invasion, perineural invasion and tumor budding in rectal cancer treated with neoadjuvant chemoradiotherapy followed by total mesorectal excision. Dis Colon Rectum 66:905–913. https://doi.org/10.1097/DCR.0000000000002266
Nagtegaal ID, Knijn N, Hugen N, et al (2017) Tumor deposits in colorectal cancer: Improving the value of modern staging-a systematic review and meta-analysis. J Clin Oncol 35:1119–1127. https://doi.org/10.1200/JCO.2016.68.9091
Goldstein NS, Turner JR (2000) Pericolonic tumor deposits in patients with T3N+M0 colon adenocarcinomas: markers of reduced disease free survival and intra-abdominal metastases and their implications for TNM classification. Cancer 88:2228–38. 10820343
Chen L Da, Li W, Xian MF, et al (2020) Preoperative prediction of tumour deposits in rectal cancer by an artificial neural network–based US radiomics model. Eur Radiol 30:1969–1979. https://doi.org/10.1007/s00330-019-06558-1
Jin Y, Li M, Zhao Y, et al (2021) Computed tomography-based radiomics for preoperative prediction of tumor deposits in rectal cancer. Front Oncol 11:710248. https://doi.org/10.3389/fonc.2021.710248
Ibrahim A, Primakov S, Beuque M, et al (2021) Radiomics for precision medicine: Current challenges, future prospects, and the proposal of a new framework. Methods 188:20–29. https://doi.org/10.1016/j.ymeth.2020.05.022
Galavis PE, Hollensen C, Jallow N,et al (2010) Variability of textural features in FDG PET images due to different acquisition modes and reconstruction parameters. Acta Oncol 49:1012–6. https://doi.org/10.3109/0284186X.2010.498437
Balagurunathan Y, Kumar V, Gu Y, et al (2014) Test–retest reproducibility analysis of lung CT image features. J Digit Imaging 27:805–23. https://doi.org/10.1007/s10278-014-9716-x
Zhou SK, Greenspan H, Davatzikos C et al (2021) A review of deep learning in medical imaging: imaging traits, technology trends, case studies with progress highlights and future promises. Proc IEEE Inst Electr Electron Eng 109:820–838. https://doi.org/10.1109/JPROC.2021.3054390
Sun Q, Lin X, Zhao Y, et al (2020) Deep learning vs. radiomics for predicting axillary lymph node metastasis of breast cancer using ultrasound images: don’t forget the peritumoral region. Front Oncol 10:53. https://doi.org/10.3389/fonc.2020.00053
Lee HS, Lee HE, Yang HK, et al (2013) Perigastric tumor deposits in primary gastric cancer: Implications for patient prognosis and staging. Ann Surg Oncol 20:1604–13. https://doi.org/10.1245/s10434-012-2692-9
Lugassy C, Zadran S, Bentolila LA, et al (2014) Angiotropism, pericytic mimicry and extravascular migratory metastasis in melanoma: an alternative to intravascular cancer dissemination. Cancer Microenviron 7:139–52. https://doi.org/10.1007/s12307-014-0156-4
Acknowledgements
Thanks to the technical staff of the Radiology department for their assistance in copying the imaging images.
Funding
This study received funding from the Hospital-level Scientific Research Foundation of Qujing First People’s Hospital (Grant No. YJKTZ04) and the Scientific Research Fund of the Education Department of Yunnan Province (Grant No. 2023Y0700).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: Y Jin, B Song. (II) Administrative support: B Song. (III) Provision of study materials or patients: Y Wang, S Liu, L Yang. (IV) Collection and assembly of data: Y Jin, H Yin. (V) Data analysis and interpretation: H Yin, H Zhang. (VI) Manuscript writing: Y Jin, H Yin. (VII) Final approval of manuscript: all authors. (VII) All figures/tables in our manuscripts are original and are not adapted from any published ones.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Biomedical Research Ethics Committee of West China Hospital of Sichuan University (20210101) and individual consent for this retrospective analysis was waived.
Consent for publication
All authors agreed to submit and further publicize this paper.
Competing interests
B Song is a Deputy Editor for Insights into Imaging. He has not taken part in the review or selection process of this article.
H Yin and H Zhang are employees of Beijing Infervision Technology Co., Ltd.
The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure 1.
Example of the manual three-dimensional segmentation results. Supplementary Figure 2. Workflow chart and schematic of the single-DL model (A) and the multi-DL model (B).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jin, Y., Yin, H., Zhang, H. et al. Predicting tumor deposits in rectal cancer: a combined deep learning model using T2-MR imaging and clinical features. Insights Imaging 14, 221 (2023). https://doi.org/10.1186/s13244-023-01564-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-023-01564-w