Abstract
Abstract
Health systems worldwide are implementing lung cancer screening programmes to identify early-stage lung cancer and maximise patient survival. Volumetry is recommended for follow-up of pulmonary nodules and outperforms other measurement methods. However, volumetry is known to be influenced by multiple factors. The objectives of this systematic review (PROSPERO CRD42022370233) are to summarise the current knowledge regarding factors that influence volumetry tools used in the analysis of pulmonary nodules, assess for significant clinical impact, identify gaps in current knowledge and suggest future research. Five databases (Medline, Scopus, Journals@Ovid, Embase and Emcare) were searched on the 21st of September, 2022, and 137 original research studies were included, explicitly testing the potential impact of influencing factors on the outcome of volumetry tools. The summary of these studies is tabulated, and a narrative review is provided. A subset of studies (n = 16) reporting clinical significance were selected, and their results were combined, if appropriate, using meta-analysis. Factors with clinical significance include the segmentation algorithm, quality of the segmentation, slice thickness, the level of inspiration for solid nodules, and the reconstruction algorithm and kernel in subsolid nodules. Although there is a large body of evidence in this field, it is unclear how to apply the results from these studies in clinical practice as most studies do not test for clinical relevance. The meta-analysis did not improve our understanding due to the small number and heterogeneity of studies testing for clinical significance.
Critical relevance statement
Many studies have investigated the influencing factors of pulmonary nodule volumetry, but only 11% of these questioned their clinical relevance in their management. The heterogeneity among these studies presents a challenge in consolidating results and clinical application of the evidence.
Key points
• Factors influencing the volumetry of pulmonary nodules have been extensively investigated.
• Just 11% of studies test clinical significance (wrongly diagnosing growth).
• Nodule size interacts with most other influencing factors (especially for smaller nodules).
• Heterogeneity among studies makes comparison and consolidation of results challenging.
• Future research should focus on clinical applicability, screening, and updated technology.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Health systems worldwide are implementing Lung Cancer Screening programmes (LCS) to identify early-stage lung cancer and maximise patient survival. However, false positive findings presenting as mostly benign, small, non-calcified pulmonary nodules are present in 22–51% of participants, which may cause morbidity and undermines the cost-effectiveness of LCS [1, 2].
Before the Dutch-Belgian randomised lung cancer screening (NELSON) trial, any pulmonary nodule was considered potentially malignant until proven stable for two years. This trial linked the risk of malignancy to the nodule's size, with small nodules (≤ 100 mm3 in volume or ≤ 5 mm in diameter) having a low risk of cancer (0.4%), while large nodules (> 300 mm3 or > 10 mm) see this risk raise to 16.9%. The risk of malignancy for medium-sized nodules depends on their growth rate, increasing from 0.8% for nodules with a volume doubling time (VDT) ≥ 600 days to 9.9% for nodules with a VDT < 400 days [1].
Volumetry has consistently outperformed other methods of measuring pulmonary nodules and has been recommended by several international scientific societies for their follow-up [1, 3, 4]. However, the growth curves based on volumetry are highly variable and influenced by multiple known factors [5, 6]. These influencing factors can be related to the scanner, acquisition (e.g., radiation dose exposure, slice thickness) and reconstruction parameters (e.g., kernel), software package, nodule (e.g., size, shape, location), patient (e.g., breathing, comorbidities) or even to the observer (e.g., experience and training). The consistent use of the same scanner, protocol, and software during the follow-up of a pulmonary nodule reduces measurement variability. Still, it is often impractical, such as in cases of equipment failure, critical software upgrades, or the patient moving house.
The primary objective of this systematic review is to summarise the current knowledge regarding the factors that influence the outcome of volumetry tools dedicated to pulmonary nodules. The secondary objectives are to assess the clinical significance of the evidence, identify gaps in current knowledge and suggest future research.
Methods
The protocol and search strategy were registered with PROSPERO with the registration number CRD42022370233.
The authors defined the primary and secondary research questions as “What factors influence the outcome of volumetry tools dedicated to pulmonary nodules?” and “What is the clinical significance of their effect?” respectively.
The authors searched the following databases on the 21st of September 2022: MEDLINE, SCOPUS, Journals@Ovid, Embase, and Ovid Emcare, using the query: (((Volume OR Volumetry OR Volumetric) AND (lung OR pulmonary) AND (nodule OR nodules)).
Eligibility criteria
The inclusion criteria were defined as follows:
-
Original research studies using dedicated volumetry tools in solid or part-solid pulmonary nodules.
-
Study design explicitly tests the potential impact of influencing factors on these tools' outcomes (i.e., volume, segmentation quality).
The exclusion criteria were defined as follows:
-
Case reports reviews, or opinion articles.
-
Study design exclusively investigating ground-glass opacities (GGOs), using a dedicated (i.e., less generalisable) segmentation algorithm.
The authors excluded duplicate records using the Rayyan online tool (Perdue University).
Assessment of methodological quality
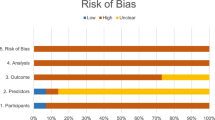
The quality of the included studies was assessed independently by two authors (chest radiologists with over five years of experience in LCS) based on the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2), and all disagreement was resolved through discussion with a third chest radiologist. The risk of bias was rated as high, low, or unclear.
Data extraction
Both authors agreed on the final list of reports and retrieved the respective full articles.
Non-English articles (i.e., Chinese, German) were translated using an online service (www.translated.com).
The authors then screened the complete reference lists of all included articles for additional pertinent entries. Grey literature reports were used to identify potential candidate studies.
The variables collected included: population, nodule features, statistical methodology, influencing factor(s), outcome variable, observed effect(s), interactions between different influencing factors, and the statistical significance of relevant tests.
Statistical analysis and data presentation
To assess the evidence for clinical significance, we selected all in vivo studies reporting interscan variability using relative Bland–Altman analysis. The variables collected at this stage included: influencing factor(s), systematic bias, Limits of Agreement (LOA), and sub-group analysis. The LOA were deduced from the standard deviation and systematic bias if needed. When appropriate, the authors synthesised LOA and systematic bias from groups of studies using the inverse-variance method with a random-effects model (SPSS v26 [IBM, Armonk, NY, USA]).
The heterogeneity between the primary studies was assessed using the heterogeneity variance (τ2) and Forest plots. The Deeks’ funnel plot was planned to determine study asymmetry and potential publication bias if comparing more than ten studies.
Missing values were excluded after an unsuccessful attempt to contact the corresponding author of the primary study.
Results
The search returned 1259 (MEDLINE), 1697 (SCOPUS), 53 (Journals@Ovid), 223 (Embase), and 126 (Emcare) results from 1960 to 2022. The PRISMA flow diagram is presented in Fig. 1.
After the study selection and critical appraisal, the first stage of the systematic review included a cohort of 137 studies. A consolidated summary of results is presented in Table 1, and the complete list of the summarised results is provided as Additional file 1: Table S1.
The second stage of the review identified a cohort of 16 studies, summarising their results in Table 2. Meta-analysis was attempted in two study groups, with results presented as Additional file 1 (Table S3 and Figures S1 and S2). Funnel plots were not performed since the minimum of 10 studies was unmet.
Influencing factors related to the scanner
Acquisition parameters
Radiation dose exposure, tube current, and tube potential Minimising radiation dose exposure is essential to LCS and can be done by manipulating tube current and potential, often interchangeably. The interaction between dose exposure and tube current–time product (mAs) is linear and well understood. However, the interaction with tube potential is not, with a reduction of tube voltage from 100 to 80kVp resulting in a reduction of dose exposure in the order of 1.5 [23].
Several studies investigated the impact of radiation dose exposure, tube voltage, or tube current on the outcome of volumetry tools. Less than half of the studies showed a statistically significant difference in accuracy, and the vast majority concluded this difference to be clinically insignificant [13, 15, 24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Some studies reported worsening segmentation quality with lower dose exposure [30] and reduced precision with lower dose exposure, tube current–time product, or tube voltage, limited to small 5 mm and non-solid nodules [28, 39,40,41,42,43,44,45,46].
The impact of radiation dose exposure on volumetry showed clinically significant differences between standard-dose (SDCT) vs low-dose (LDCT) [18] CT protocols and SDCT vs ultra-low-dose CT protocols (ULDCT) [15, 17], contradicting the consensus that reducing the radiation dose does not affect the outcome of volumetry. Studies comparing LDCT vs ULDCT did not confirm this result, thus supporting their use in LCS [10, 13]. Despite the acceptance of SDCT, LDCT and ULDCT protocols, their definition varies among authors, and the effective radiation dose depends on the patient’s body weight. The estimated effective dose acceptable for LCS is 2 mSv [47].
The signal-to-noise ratio (SNR) is not an independent influencing factor [32, 48].
Collimation
The effect of collimation is statistically significant between thin (≤ 0.75 mm) and thick (≥ 1.5 mm) settings, with some authors recommending thinner [37, 38] while others recommend thicker [49, 50] settings for volumetry. However, the consensus considers collimation as not clinically significant.
High-resolution scan mode
The development of garnet detectors in CT scanners enabled the high-resolution scan mode, increasing the sampling per gantry rotation, spatial resolution, and image quality while reducing volume overestimation [51].
Field-of-view (FOV)
The scanners’ spatial resolution in the axial plane depends on the FOV and the matrix size. The scan FOV determines the amount of raw data acquired, but images can be later reconstructed with a different and smaller display FOV.
Several authors investigated the effect of changing the FOV (between 9.6 cm and 36 cm) and showed no statistically significant impact on volumetry [52,53,54,55].
Pitch
Likewise, the pitch parameter has no significant impact on volumetry within conventionally used values [36, 49, 53, 56], apart from improved repeatability with smaller pitch values (0.9 vs 1.2) [49]. However, the high pitch mode (i.e., pitch factor of 3) reduces the accuracy of volumetry in small (< 5 mm) solid nodules [56].
Contrast enhancement
Contrast enhancement overestimates the volume, possibly by increasing the attenuation of the nodules or adjacent structures [57,58,59,60,61]. Rampinelli et al. found volumetry comparable across different delay times (i.e., phases) in contrast-enhanced CT [58].
Reconstruction parameters
Slice thickness
Slice thickness has been investigated as an influencing factor of volumetry between 0.625 and 5 mm. Thinner slices resulted in statistically significant improvement in accuracy and precision in all but one study [19, 21, 31, 34, 36, 39, 44, 48,49,50, 52,53,54,55, 62,63,64,65,66,67]. In comparison, thicker slices are related to lower measurement agreement and reduced segmentation quality [52, 54, 66].
The slice thickness determines the scan's longitudinal (z-axis) spatial resolution. The difference between the higher axial and lower longitudinal spatial resolution explains why the FOV is insignificant while the slice thickness is, especially for thicker slices.
Increasing the slice thickness increases the volume of voxels along the z-axis. Larger voxels may increase the volume measurement, but surface voxels will also suffer more partial volume effects, increasing measurement variability [54]. Since smaller nodules have a higher ratio of surface to inner voxels, the volumetry of smaller nodules is more affected by slice thickness [21, 36, 49, 52].
The slice thickness should be thin enough to make any nodule visible in at least three consecutive slices [52]. Likewise, a thickness ≥ 2.5 mm is inadequate to detect 1 mm changes in diameter [63, 65].
Reconstruction interval
When the reconstruction interval is smaller than the slice thickness (i.e., overlap), the longitudinal spatial resolution improves independently of slice thickness [36, 55].
In a study by Gavrielides et al., the accuracy and precision of volumetry tools improved with 50% overlap, with significant cross-effects between reconstruction interval, slice thickness, nodule size, and radiation dose exposure [36]. Honda et al. reported that non-overlapping reconstructions were associated with volume overestimation in scans obtained with 2.5 mm and 3.75 mm slice thicknesses [54]. Eberhard et al. found no significant benefit of overlapping protocols when using a 1 mm slice thickness, arguing in favour of skipping them to improve LCS cost-effectiveness [68].
Raw-data reconstruction algorithm and kernel
CT image reconstruction involves converting the raw data to a sinogram (representing the number and angulation of photons as they hit the detectors) and then to a matrix of attenuation values, known as the image model. This process is called direct back-projection and results in significant blurring. In filtered back projection (FBP), filters (or kernels) are applied to the image model to reduce the blurring effect, provide smoothing or edge enhancement, and highlight certain features and anatomical components.
Most studies investigating the impact of kernels on volumetry have considered them statistically significant (10 out of 13 studies). High-spatial frequency (sharp) kernels, like lung or bone, improved accuracy, precision, and repeatability in most studies [36, 45, 49, 63]. In contrast, a single study reported increased repeatability with a low-spatial frequency (smooth) kernel [64]. Larici et al. investigated the interaction between kernel and slice thickness to conclude that a sharp kernel provides the best performance for volumetry in 1.25 mm slice thickness. A smooth kernel outperforms the sharp kernel in 2.5 mm slice thickness [66].
Several studies reported an overestimation of volume associated with the sharp kernel [54, 59, 64], especially in non-overlapping acquisition and solid nodules (or solid components of part-solid nodules) [54]. Conversely, volumetry of GGOs (or ground-glass components of part-solid nodules) results in higher estimates when using a smooth kernel [9].
In iterative reconstruction (IR), the scanner converts the image model into an artificial sinogram (forward projection). It then compares it to the original sinogram with each iteration, correcting random fluctuations in photon measurement. This process minimises noise and improves image quality at significantly lower radiation exposure [69]. However, this noise reduction is less significant at the edges of the pulmonary nodules, resulting in IR-specific measurement error for small nodules and lower doses or higher noise levels [25, 34].
Multiple studies investigated the influence of raw data reconstruction algorithms on volumetry tools [13, 24, 25, 28, 30, 32,33,34, 39,40,41,42, 51, 70,71,72], with the consensus being that IR outperforms FBP for small, part-solid nodules or at lower tube currents [28, 39,40,41], allowing IR-based protocols to replace FBP safely.
Recently, Kim et al. [73] investigated two deep learning (DL)-based raw-data reconstruction algorithms (Truefidelity and ClariCT.AI), showing improved accuracy against the adaptive statistical iterative reconstruction (ASiR) algorithm using LDCT and ULDCT.
The scientific literature often refers to raw-data reconstruction algorithms and kernels as just reconstruction algorithms, which could be confusing since the former is generally considered not to influence volumetry measurements. At the same time, the latter is known to do so [42].
Post-processing
Despite the earlier warning by Ko et al. regarding image compression [74], Santos et al. found no significant deterioration in the performance of volumetry tools within the limits proposed in the European Society of Radiology (ESR) position paper [75, 76].
The influence of vessel suppression on volumetry was investigated by Milanese et al. using commercially available software (ClearRead, Riverain, Miamisburg, OH, USA). The authors reported high measurement agreement with and without vessel suppression, although the rate of manual correction was unusually high (49/77, 75.4%) [77].
CT scanner equipment
CT scanner vendor
Comparing the performance of volumetry tools using different scanners showed good accuracy regardless of the scanner vendor [37]. Two later studies found a statistically significant difference between scanner vendors, but only for small nodules, which would not require follow-up according to current guidelines [26, 78].
CT scanner technology
Several studies have compared the performance of volumetry between different scanner technologies (e.g., single or multiple detectors, flat-panel, and dual-energy CT scanners) [19, 37, 55, 79, 80]. Das et al. reported increasing accuracy in volumetry with more detector rows [38], although Xie et al. did not confirm this [81].
Flat-panel scanners outperform multi-detector scanners in pulmonary nodule volumetry, especially in small nodules (< 5 mm) [82,83,84,85].
Mono-energetic reconstructions at 70 keV using dual-energy spectral CT are considered equivalent to conventional CT images acquired using 120 kVp, and several authors found no significant difference in volumetry accuracy between them [86,87,88]. In addition, mono-energetic reconstructions improved the repeatability of volumetry at the same radiation dose [87].
Influencing factors related to the software
Software package and segmentation algorithm
Several studies compared different software packages and different segmentation algorithms for pulmonary nodule volumetry, reporting statistically significant differences in all but one study [45, 83, 89,90,91,92] and even between different versions of the same software [93]. Adjusting the attenuation threshold, as some segmentation algorithms allow, also influences the volume measurement outcome [45, 55, 92].
Several international societies firmly recommend consistently using the same software package, version, and segmentation algorithm during follow-up [94].
Influencing factors related to the nodule
Nodule size
Volumetry is less performant for small nodules [10, 13, 16, 18, 21, 25, 28, 29, 31, 36,37,38, 40, 41, 45, 48,49,50,51, 53, 55, 56, 60, 63, 70, 72, 74, 78, 79, 81, 82, 89, 92, 93, 95,96,97,98,99,100,101], explained mainly by partial volume effects, and is considered unreliable for nodules < 5 mm in diameter [60, 102].
Multiple interactions between nodule size and other influencing factors are known, including collimation [31, 49], tube current [29, 41], reconstruction algorithm [29, 41, 51, 70, 72], kernel [36], reconstruction interval [36], slice thickness [20, 21, 31, 36, 48, 49, 53], scanner technology [41, 52, 82], software [16, 45, 89, 93], compression level [74], density [28, 41], and level of inspiration [16, 20].
Hwang et al. suggested that raising the threshold to 9 mm for starting follow-up would lead to a significant increase in specificity (i.e., from 91.7% to 96.7%) at the cost of only a modest decrease in sensitivity (i.e., from 96.2% to 94.2%). The impact of such a change to current recommendations would result in a 60% reduction of follow-up scans at the cost of delaying the diagnosis of 1.9% of lung cancer patients [103]. Volumetry tools should be robust to influencing factors for solid nodules ≥ 9 mm when using current LDCT protocols in LCS programmes.
Density
Published studies in the literature describe the density of a nodule as either a qualitative (e.g., solid, part-solid, ground-glass, calcified) or quantitative feature (i.e., in Hounsfield Units).
Non-solid nodules are more challenging to segment manually and using volumetry tools and present lower accuracy and higher variability than solid nodules [11, 25, 26, 28, 36, 41, 45, 92].
Interactions between density and other influencing factors have been described, including nodule size [28, 36, 92], reconstruction algorithms [28, 41, 70], slice thickness [36], tube current [41], level of inspiration [104], and image compression [74]. Higher nodule density is correlated to larger volume [88].
Shape and margin
The shape of a pulmonary nodule can be round, elongated, smooth, lobulated, spiculated, or irregular.
An irregular or spiculated shape is associated with lower accuracy [62, 64] and precision [20] of volumetry tools. It is also associated with a lower volume measurement [78], lower segmentation quality [97, 105], and increased variability [21, 96, 97, 100, 106].
The ratio of surface to inner voxels increases in nodules with an irregular or spiculated shape (i.e., larger surface area), deteriorating the performance of volumetry tools due to partial volume effects [78, 100]. Therefore, volumetry of small (≤ 6 mm) pulmonary nodules with irregular or spiculated shapes (i.e., high-risk features for malignancy) may be unreliable and can justify an optional follow-up period [107].
The shape of a nodule also interacts with other influencing factors, such as the nodule's density [28], location, slice thickness, and kernel [64].
Several authors describe spiculation as a feature of the nodule's margin, which can be a source of confusion. We defined the margin as either well or poorly defined. In a study by Iwano et al., volume measurements of nodules with poorly defined margins had a significantly higher variability [108].
Location
Most authors categorise a nodule’s location as either intra-parenchymal, juxta-pleural, juxta-fissural, or juxta-vascular [37, 38, 51, 64, 66, 96, 109, 110], with intra-parenchymal nodules further classified as either central or peripheric [74, 111, 112].
Attachments to adjacent structures (e.g., vessels, bronchial wall, and pleura) may result in the latter's inclusion, overestimating the volume and increasing the measurement variability [111, 112].
In a recent study by Guedes Pinto et al., the authors reported the location in both the axial (anterior, middle, or posterior) and coronal (upper, middle, lower) planes, additionally measuring the vascular distance along the pulmonary arteries, from the main pulmonary artery (MPA) to the nodule using multiplanar reformatting, which proved to be statistically significant [113]. Conversely, the location within a lobe [18] or segment [98] was not proven to be statistically significant.
Interactions have been reported between the location and software [111], shape [64], slice thickness [64, 66], kernel [64], tube current [66], and compression [74].
Influencing factors related to the patient
Parenchymal changes
Both global and regional parenchymal changes in emphysema patients (i.e., reduced parenchymal attenuation) have been investigated and found not significantly to affect pulmonary nodule volumetry (108. However, in diseases with increased parenchyma attenuation, like interstitial lung disease (ILD), the reduced contrast between nodule and surrounding parenchyma could deteriorate the performance of volumetry tools. In two phantom studies by Gavrielides et al., the difference in attenuation between a synthetic nodule and the background was statistically significant [39, 67]. Recently, Penha et al. reported that the quality of pulmonary nodule segmentation by volumetry tools decreases with increasing attenuation of the surrounding parenchyma [114].
Breathing
Breathing artefacts are related to overestimating volume and increased measurement variability of volumetry tools [12, 16, 20, 43, 99, 104, 115, 116]. This effect is most significant at the end of expiration and for smaller nodules but is considered unlikely to be clinically relevant [12, 16, 20, 115]. However, Goo et al. reported a volume overestimation of 23.1% from inspiration to expiration, interpreted as clinically significant [116].
The level of inspiration interacts with other influencing factors like the nodule size [16], density [104], and software package [16].
Cardiopulmonary haemodynamic factors
Studies designed with coronary CT angiography (CCTA) can compare the performance of volumetry tools at different cardiac phases in a single acquisition.
Boll et al. reported changes in volume measurement related to a complex interaction between the cardiac phase, location (i.e., pulmonary segments), and nodule size [98].
Guedes Pinto et al. investigated the impact of cardiopulmonary haemodynamic factors on volumetry tools, including the cardiac phase, calibre change of the MPA between systole and diastole, the vascular distance between the MPA and the nodule, and nodule's location along the axial (related to hydrostatic pressure) and coronal plane (related to vascular section area), all statistically significant except the cardiac phase. The authors proposed a theoretical model where the volume of a given nodule is affected by the dynamic vascular pressure as blood travels from the heart to the nodule [113]. In another study by the same authors, the variability of volumetry vastly exceeded the criterion for clinical significance when comparing measurements in opposing cardiac phases (systole vs diastole [− 47%, 52.3%]), with the lower variability seen when comparing two measurements in diastole ([− 18.9%, 19.7%]) [8].
CCTA is not appropriate for LCS. However, there is considerable overlap in risk factors between coronary artery disease and lung cancer. Patients enrolled in LCS are also at risk of cardiovascular events, with some authors advocating a role for dual screening [113].
Influencing factors related to the observer
Manual correction, observer experience and training
The promise of (semi)automated tools is to reduce interobserver variability by limiting the observer's influence in the measurement [102, 117]. Counter-intuitively, allowing manual correction of the segmentation improves the tool’s performance [60, 102, 118]. This is explained because inadequately segmented nodules tend to be outliers (i.e., either including adjacent structures [113] or incompletely segmenting the nodule [20]), resulting in higher variability and lower observer agreement.
The outcome of volumetry tools is independent of observer experience (i.e., radiologists vs non-radiologists), even when manually correcting the segmentation result. However, in the un-experienced group of observers, training with the tool was statistically significant for volume measurements [119].
Regarding concerns of bias and excluded studies
The most common concern of bias in the included studies (Table 3) is the use of experimental algorithms [9, 28, 45, 53, 74, 89, 92, 117, 120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152], followed by the assumption of zero-change datasets over more extended periods, relying on the perceived stability of the nodules [80, 95, 152]. Two studies use non-consecutive or convenience sample techniques, possibly introducing selection bias [60, 71]. Still, others present an incomplete description of their methods, poorly defining their population or the statistical analysis [26, 72, 77, 93, 148, 152].
Several promising candidate studies were excluded after full-text analysis based on their choice of outcome (Additional file 1: Table S2). These outcomes include the risk of malignancy [1, 100, 103, 153,154,155,156,157,158,159,160,161], prognosis [162,163,164,165,166,167], growth [5, 67, 101, 112, 168,169,170,171,172,173,174], or comparison to other methods of measurement like diameter [100, 175], area [175], the diameter of an equivalent volume sphere [3] or manual segmentations (e.g., most of the recent research using DL-based segmentation).
Although these outcomes are clinically interesting, they are unrelated to our research questions.
Discussion
The influencing factors of volumetry tools have been investigated extensively. However, the possibility of wrongly diagnosing a nodule as stable or growing between follow-up scans has only been tested in a little over 10% of studies. Consolidating the results from different studies is difficult due to the heterogeneity, but an impact on clinical decision-making seems more likely in smaller nodules.
The contrast between nodule and surrounding lung parenchyma and the surface-to-inner voxel ratio are two key concepts in understanding how volumetry tools can be influenced.
Pulmonary nodule volumetry benefits from the contrast between the nodule and the surrounding well-aerated lung parenchyma. This contrast is decreased in sub-solid nodules when the surrounding parenchyma has increased attenuation (e.g., ILD, expiratory phase, contrast enhancement) or when the nodule contacts adjacent structures. Image reconstruction with different kernels and raw-data reconstruction algorithms may also expand or contract the segmentation by changing the attenuation value of the voxels.
Surface voxels contain both nodule tissue and surrounding parenchyma and suffer partial volume effects leading to measurement error and variability.
The surface-to-inner voxel ratio depends primarily on the size difference between the nodule and the voxel (i.e., how many voxels fit in the nodule). Still, it can also be increased by an irregular shape or ill-defined nodule margin (i.e., increased surface area).
Reducing the slice thickness and measuring nodules of increasing size rapidly decreases the ratio of surface to inner voxels, improving the performance of volumetry tools.
Apart from these two key concepts, implementation details involved in the segmentation algorithms account for most of the remaining observed influence in volumetry tools.
Despite the large number of included studies in this review, comparing study results is problematic given a large number of influencing factors and heterogeneity in study design, outcomes, statistical analysis, nodule features and demographics. Additionally, multiple authors report statistically significant results while openly questioning their clinical relevance. Changing a factor that influences a volumetry tool may not be enough to change our assessment of nodule growth and clinical management. Therefore, using this evidence to support clinical decisions is challenging. We consider this a limitation of the evidence and a strong motivator for this review.
A clarification of clinical significance seems needed. The optimal waiting period for a follow-up scan is based on the inherent in vivo interscan measurement variability of volumetry tools, accepted as ≤ 25% of total volume [6]. Higher measurement variability implies a longer time to distinguish real growth from measurement error. Therefore, we defined clinical significance as interscan variability > 25% of volume change since false-positive growth estimation would become more likely in this setting. We used this criterion to select a subset of all studies reporting interscan variability using Bland–Altman analysis (n = 16). Influencing factors investigated regarding their clinical relevance include radiation dose exposure, slice thickness, raw-data reconstruction algorithms, kernels, size, cardiac cycle phase, software package, segmentation algorithm, and level of inspiration.
We combined the results of two studies comparing SDCT vs ULDCT protocols [15, 17], and the synthesised result confirmed the primary studies’ conclusions. We also combined the results in a second group (three studies) by disregarding sub-group analysis concerning size [7, 22] and quality of segmentation [20], with a synthesised result within the clinically acceptable a priori LOA, but losing the influence of the factors (i.e., size and quality of segmentation) under study. Due to significant population, outcome, and design heterogeneity, we could not combine other studies. Therefore, our attempted meta-analysis failed to advance the current knowledge meaningfully (Additional file 1: Table S3 and Figures S1 and S2).
Several other factors have been statistically shown to influence the outcome of volumetry tools. However, the clinical relevance of these findings still needs to be investigated (Table 1) and represents gaps in current knowledge and opportunities for future research.
Implications of the results for practice, policy, and future research
Findings from this review confirm the clinically significant impact of some known influencing factors on pulmonary nodule volumetry, including the segmentation algorithm, quality of the segmentation, slice thickness, the level of inspiration for solid nodules, and the reconstruction algorithm and kernel in subsolid nodules (Table 3).
Much of the evidence collected has yet to be tested for potential clinical significance and is thus open for future research.
A concern related to this systematic review is the long period of the included studies in a rapidly changing field, suggesting that this review may not reflect current performance. A comparison of recent (i.e., last five years) and older studies show an improving performance trend likely related to software and scanner technology innovations. In a recent study by Bartlett et al., the reported interscan variability was not clinically relevant (95CI [− 16.8%; 16%]) even for very small (30–80 mm3) solid, non-metastatic and non-calcified pulmonary nodules (n = 58), suggesting that a shorter optimal waiting time may already be appropriate [7].
We propose a standard for future studies around the Bland–Altman analysis and restricted to nodules between 5 and 10 mm where growth estimation is useful. Such studies should investigate the persisting gaps in current knowledge, focusing on clinical applicability and currently available technology. Future research should also explore the cost and benefits of potential changes to current practices, like raising the threshold for follow-up or shortening the optimal waiting period in the follow-up schedule.
Availability of data and materials
All data extracted or synthesised and the analytic code used for the meta-analysis can be obtained from the corresponding author on reasonable request.
Abbreviations
- CCTA:
-
Coronary CT angiography
- DL:
-
Deep-learning
- FBP:
-
Filtered back-projection
- FOV:
-
Field of view
- GGO:
-
Ground-glass opacity
- IR:
-
Iterative reconstruction
- LCS:
-
Lung cancer screening
- LDCT:
-
Low-dose CT
- LOA:
-
Limits of agreement
- QUADAS-2:
-
Quality Assessment of Diagnostic Accuracy Studies tool
- SDCT:
-
Standard dose CT
- SNR:
-
Signal-to-noise ratio
- ULDCT:
-
Ultra-low-dose CT
- VDT:
-
Volume doubling time
References
Horeweg N, van Rosmalen J, Heuvelmans MA et al (2014) Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 15(12):1332–1341. https://doi.org/10.1016/S1470-2045(14)70389-4
Walter JE, Heuvelmans MA, Dorrius M, Oudkerk M (2019) Low-dose lung cancer screening: nodule measurement and management. Precis Cancer Med 2:24–24. https://doi.org/10.21037/pcm.2019.07.03
Dicken V, Bornemann L, Moltz JH, Peitgen HO, Zaim S, Scheuring U (2015) Comparison of volumetric and linear serial ct assessments of lung metastases in renal cell carcinoma patients in a clinical phase IIB study. Acad Radiol 22(5):619–625. https://doi.org/10.1016/j.acra.2014.12.018
Han D, Heuvelmans MA, Oudkerk M (2017) Volume versus diameter assessment of small pulmonary nodules in CT lung cancer screening. Transl Lung Cancer Res 6(1):52–61. https://doi.org/10.21037/tlcr.2017.01.05
Korst RJ, Lee BE, Krinsky GA, Rutledge JR (2011) The utility of automated volumetric growth analysis in a dedicated pulmonary nodule clinic. J Thorac Cardiovasc Surg 142(2):372–377. https://doi.org/10.1016/j.jtcvs.2011.04.015
Devaraj A, van Ginneken B, Nair A, Baldwin D (2017) use of volumetry for lung nodule management: theory and practice. Radiology 284(3):630–644. https://doi.org/10.1148/radiol.2017151022
Bartlett EC, Kemp S, Rawal B, Devaraj A (2022) Defining growth in small pulmonary nodules using volumetry: results from a “coffee-break” CT study and implications for current nodule management guidelines. Eur Radiol 32(3):1912–1920. https://doi.org/10.1007/s00330-021-08302-0
Pinto EG, Penha D, Hochhegger B et al (2022) Variability of pulmonary nodule volumetry on coronary CT angiograms. Medicine (Baltimore) 101(35):e30332. https://doi.org/10.1097/MD.0000000000030332
Werner S, Gast R, Grimmer R, Wimmer A, Horger M (2022) Accuracy and reproducibility of a software prototype for semi-automated computer-aided volumetry of the solid and subsolid components of part-solid pulmonary nodules. Rofo 194(3):296–305. https://doi.org/10.1055/a-1656-9834
Paks M, Leong P, Einsiedel P, Irving LB, Steinfort DP, Pascoe DM (2018) Ultralow dose CT for follow-up of solid pulmonary nodules. Medicine (Baltimore) 97(34):e12019. https://doi.org/10.1097/MD.0000000000012019
Cohen JG, Kim H, Park S et al (2017) Comparison of the effects of model-based iterative reconstruction and filtered back projection algorithms on software measurements in pulmonary subsolid nodules. Eur Radiol 27(8):3266–3274. https://doi.org/10.1007/s00330-016-4716-5
Moser JB, Mak SM, McNulty WH et al (2017) The influence of inspiratory effort and emphysema on pulmonary nodule volumetry reproducibility. Clin Radiol 72(11):925–929. https://doi.org/10.1016/j.crad.2017.06.117
Sui X, Meinel FG, Song W et al (2016) Detection and size measurements of pulmonary nodules in ultra-low-dose CT with iterative reconstruction compared to low dose CT. Eur J Radiol 85(3):564–570. https://doi.org/10.1016/j.ejrad.2015.12.013
Kim H, Park CM, Woo S et al (2013) Pure and part-solid pulmonary ground-glass nodules: measurement variability of volume and mass in nodules with a solid portion less than or equal to 5 mm. Radiology 269(2):585–593. https://doi.org/10.1148/radiology.13121849
Hein PA, Romano VC, Rogalla P et al (2010) Variability of semiautomated lung nodule volumetry on ultralow-dose CT: comparison with nodule volumetry on standard-dose CT. J Digit Imaging 23(1):8–17. https://doi.org/10.1007/s10278-008-9157-5
Hoop B, Gietema H, Ginneken B et al (2009) A comparison of six software packages for evaluation of solid lung nodules using semi-automated volumetry: What is the minimum increase in size to detect growth in repeated CT examinations. Eur Radiol 19(4):800–808. https://doi.org/10.1007/s00330-008-1229-x
Hein P, Romano V, Rogalla P et al (2009) Linear and volume measurements of pulmonary nodules at different CT dose levels – intrascan and interscan analysis. Rofo 181(1):24–31. https://doi.org/10.1055/s-2008-1027874
Rampinelli C, de Fiori E, Raimondi S, Veronesi G, Bellomi M (2009) In vivo repeatability of automated volume calculations of small pulmonary nodules with CT. AJR Am J Roentgenol 192(6):1657–1661. https://doi.org/10.2214/AJR.08.1825
Vogel MN, Vonthein R, Schmücker S et al (2008) Automated pulmonary nodule volumetry with an optimized algorithm. Accuracy at different slice thicknesses compared to unidimensional and bidimensional measurements. Rofo 180(9):791–797. https://doi.org/10.1055/s-2008-1027562
Gietema HA, Schaefer-Prokop CM, Mali WPTM, Groenewegen G, Prokop M (2007) Pulmonary nodules: interscan variability of semiautomated volume measurements with multisection CT—influence of inspiration level, nodule size, and segmentation performance. Radiology 245(3):888–894. https://doi.org/10.1148/radiol.2452061054
Petrou M, Quint LE, Nan B, Baker LH (2007) Pulmonary nodule volumetric measurement variability as a function of CT slice thickness and nodule morphology. AJR Am J Roentgenol 188(2):306–312. https://doi.org/10.2214/AJR.05.1063
Wormanns D, Kohl G, Klotz E et al (2004) Volumetric measurements of pulmonary nodules at multi-row detector CT: in vivo reproducibility. Eur Radiol 14(1):86–92. https://doi.org/10.1007/s00330-003-2132-0
Lira D, Padole A, Kalra MK, Singh S (2015) Tube potential and CT radiation dose optimization. AJR Am J Roentgenol 204(1):W4-10. https://doi.org/10.2214/AJR.14.13281
Lee HN, Kim JI, Shin SY (2020) Measurement accuracy of lung nodule volumetry in a phantom study. Medicine (Baltimore) 99(23):e20543. https://doi.org/10.1097/MD.0000000000020543
Eberhard M, Stocker D, Milanese G et al (2019) Volumetric assessment of solid pulmonary nodules on ultralow-dose CT: a phantom study. J Thorac Dis 11(8):3515–3524. https://doi.org/10.21037/jtd.2019.08.12
Liu J, Qing H, Luo H et al (2019) Accuracy of pulmonary nodule volumetry at different exposure parameters in low-dose computed tomography. J Comput Assist Tomogr 43(6):926–930. https://doi.org/10.1097/RCT.0000000000000908
Jin S, Zhang B, Zhang L, Li S, Li S, Li P (2018) Lung nodules assessment in ultra-low-dose CT with iterative reconstruction compared to conventional dose CT. Quant Imaging Med Surg 8(5):480–490. https://doi.org/10.21037/qims.2018.06.05
Gavrielides MA, Berman BP, Supanich M et al (2017) Quantitative assessment of nonsolid pulmonary nodule volume with computed tomography in a phantom study. Quant Imaging Med Surg 7(6):623–635. https://doi.org/10.21037/qims.2017.12.07
Su D, Feng L, Jiang Y, Wang Y (2017) Effect of scanning and reconstruction parameters on three dimensional volume and CT value measurement of pulmonary nodules: a phantom study. Zhongguo Fei Ai Za Zhi 20(8):562–567. https://doi.org/10.3779/j.issn.1009-3419.2017.08.11
den Harder AM, Willemink MJ, van Hamersvelt RW et al (2016) Pulmonary nodule volumetry at different low computed tomography radiation dose levels with hybrid and model-based iterative reconstruction. J Comput Assist Tomogr 40(4):578–583. https://doi.org/10.1097/RCT.0000000000000408
Gavrielides MA, Li Q, Zeng R, Myers KJ, Sahiner B, Petrick N (2016) Volume estimation of multidensity nodules with thoracic computed tomography. J Med Imaging (Bellingham) 3(1):013504. https://doi.org/10.1117/1.JMI.3.1.013504
Young S, Kim HJG, Ko MM, Ko WW, Flores C, McNitt-Gray MF (2015) Variability in CT lung-nodule volumetry: effects of dose reduction and reconstruction methods. Med Phys 42(5):2679–2689. https://doi.org/10.1118/1.4918919
Kim H, Park CM, Song YS, Lee SM, Goo JM (2014) Influence of radiation dose and iterative reconstruction algorithms for measurement accuracy and reproducibility of pulmonary nodule volumetry: A phantom study. Eur J Radiol 83(5):848–857. https://doi.org/10.1016/j.ejrad.2014.01.025
Chen B, Barnhart H, Richard S, Robins M, Colsher J, Samei E (2013) Volumetric quantification of lung nodules in CT with iterative reconstruction (ASiR and MBIR). Med Phys 40(11):111902. https://doi.org/10.1118/1.4823463
Christe A, Szucs-Farkas Z, Huber A et al (2013) Optimal dose levels in screening chest ct for unimpaired detection and volumetry of lung nodules, with and without computer assisted detection at minimal patient radiation. PLoS One 8(12):e82919. https://doi.org/10.1371/journal.pone.0082919
Gavrielides MA, Zeng R, Myers KJ, Sahiner B, Petrick N (2013) Benefit of overlapping reconstruction for improving the quantitative assessment of CT lung nodule volume. Acad Radiol 20(2):173–180. https://doi.org/10.1016/j.acra.2012.08.014
Das M, Ley-Zaporozhan J, Gietema HA et al (2007) Accuracy of automated volumetry of pulmonary nodules across different multislice CT scanners. Eur Radiol 17(8):1979–1984. https://doi.org/10.1007/s00330-006-0562-1
Das M, Muhlenbruch G, Katoh M et al (2007) Automated volumetry of solid pulmonary nodules in a phantom. Invest Radiol 42(5):297–302. https://doi.org/10.1097/01.rli.0000258683.20123.c4
Gavrielides MA, Li Q, Zeng R et al (2019) Discrimination of pulmonary nodule volume change for low- and high-contrast tasks in a phantom CT study with low-dose protocols. Acad Radiol 26(7):937–948. https://doi.org/10.1016/j.acra.2018.09.006
Ohno Y, Yaguchi A, Okazaki T et al (2016) Comparative evaluation of newly developed model-based and commercially available hybrid-type iterative reconstruction methods and filter back projection method in terms of accuracy of computer-aided volumetry (CADv) for low-dose CT protocols in phantom study. Eur J Radiol 85(8):1375–1382. https://doi.org/10.1016/j.ejrad.2016.05.001
Doo KW, Kang EY, Yong HS, Woo OH, Lee KY, Oh YW (2014) Accuracy of lung nodule volumetry in low-dose CT with iterative reconstruction: an anthropomorphic thoracic phantom study. Br J Radiol 87(1041):20130644. https://doi.org/10.1259/bjr.20130644
Wielpütz MO, Lederlin M, Wroblewski J et al (2013) CT volumetry of artificial pulmonary nodules using an ex vivo lung phantom: Influence of exposure parameters and iterative reconstruction on reproducibility. Eur J Radiol 82(9):1577–1583. https://doi.org/10.1016/j.ejrad.2013.04.035
Tateishi U, Tsukagoshi S, Inokawa H, Okumura M, Moriyama N (2008) Fluctuation in measurements of pulmonary nodule under tidal volume ventilation on four-dimensional computed tomography: preliminary results. Eur Radiol 18(10):2132–2139. https://doi.org/10.1007/s00330-008-1002-1
Kuhnigk JM, Dicken V, Bornemann L et al (2006) Morphological segmentation and partial volume analysis for volumetry of solid pulmonary lesions in thoracic CT scans. IEEE Trans Med Imaging 25(4):417–434. https://doi.org/10.1109/TMI.2006.871547
Ko JP, Rusinek H, Jacobs EL et al (2003) Small pulmonary nodules: volume measurement at chest CT—phantom study. Radiology 228(3):864–870. https://doi.org/10.1148/radiol.2283020059
Kinnard LM, Gavrielides MA, Myers KJ, et al (2008) Volume error analysis for lung nodules attached to pulmonary vessels in an anthropomorphic thoracic phantom. In: Proceedings, Medical imaging: computer-aided diagnosis, vol 6915 (2008). https://doi.org/10.1117/12.773039
Larke FJ, Kruger RL, Cagnon CH et al (2011) Estimated radiation dose associated with low-dose chest CT of average-size participants in the National Lung Screening Trial. AJR Am J Roentgenol 197:1165–1169. https://doi.org/10.2214/AJR.11.6533
Diwakar M, Kumar M (2018) A review on CT image noise and its denoising. Biomed Signal Process Control 42:73–88. https://doi.org/10.1016/j.bspc.2018.01.010
Li Q, Gavrielides MA, Sahiner B, Myers KJ, Zeng R, Petrick N (2015) Statistical analysis of lung nodule volume measurements with CT in a large-scale phantom study. Med Phys 42(7):3932–3947. https://doi.org/10.1118/1.4921734
Bolte H, Riedel C, Knöß N et al (2007) Computed tomography-based lung nodule volumetry—do optimized reconstructions of routine protocols achieve similar accuracy, reproducibility and interobserver variability to that of special volumetry protocols? Rofo 179(3):276–281. https://doi.org/10.1055/s-2007-962929
Coenen A, Honda O, van der Jagt EJ, Tomiyama N (2013) Computer-assisted solid lung nodule 3D volumetry on CT: influence of scan mode and iterative reconstruction: a CT phantom study. Jpn J Radiol 31(10):677–684. https://doi.org/10.1007/s11604-013-0235-3
Ravenel JG, Leue WM, Nietert PJ, Miller J, Taylor KK, Silvestri GA (2008) Pulmonary nodule volume: effects of reconstruction parameters on automated measurements—a phantom study. Radiology 247(2):400–408. https://doi.org/10.1148/radiol.2472070868
Way TW, Chan HPP, Goodsitt MM et al (2008) Effect of CT scanning parameters on volumetric measurements of pulmonary nodules by 3D active contour segmentation: a phantom study. Phys Med Biol 53(5):1295–1312. https://doi.org/10.1088/0031-9155/53/5/009
Honda O, Sumikawa H, Johkoh T et al (2007) Computer-assisted lung nodule volumetry from multi-detector row CT: influence of image reconstruction parameters. Eur J Radiol 62(1):106–113. https://doi.org/10.1016/j.ejrad.2006.11.017
Goo JM, Tongdee T, Tongdee R, Yeo K, Hildebolt CF, Bae KT (2005) Volumetric measurement of synthetic lung nodules with multi-detector row CT: effect of various image reconstruction parameters and segmentation thresholds on measurement accuracy. Radiology 235(3):850–856. https://doi.org/10.1148/radiol.2353040737
Hwang SH, Oh YW, Ham SY, Kang EY, Lee KY (2015) Effect of the high-pitch mode in dual-source computed tomography on the accuracy of three-dimensional volumetry of solid pulmonary nodules: a phantom study. Korean J Radiol 16(3):641–647. https://doi.org/10.3348/kjr.2015.16.3.641
de Jong PA, Leiner T, Lammers JWJ, Gietema HA (2012) Can low-dose unenhanced chest ct be used for follow-up of lung nodules? AJR Am J Roentgenol 199(4):777–780. https://doi.org/10.2214/AJR.11.7577
Rampinelli C, Raimondi S, Padrenostro M et al (2010) Pulmonary nodules: contrast-enhanced volumetric variation at different CT scan delays. AJR Am J Roentgenol 195(1):149–154. https://doi.org/10.2214/AJR.09.3212
Honda O, Johkoh T, Sumikawa H et al (2007) Pulmonary nodules: 3D volumetric measurement with multidetector CT–effect of intravenous contrast medium. Radiology 245(3):881–887. https://doi.org/10.1148/radiol.2453062116
Goodman LR, Gulsun M, Washington L, Nagy PG, Piacsek KL (2006) Inherent variability of CT lung nodule measurements in vivo using semiautomated volumetric measurements. AJR Am J Roentgenol 186(4):989–994. https://doi.org/10.2214/AJR.04.1821
Mohamed HFA, Bülbül M, de Jong PA (2016) Pulmonary nodule follow-up: be careful with volumetry between contrast enhanced and unenhanced CT. Ann Transl Med 4(18):346. https://doi.org/10.21037/atm.2016.08.43
Petrick N, Kim HJG, Clunie D et al (2014) Comparison of 1D, 2D, and 3D nodule sizing methods by radiologists for spherical and complex nodules on thoracic CT phantom images. Acad Radiol 21(1):30–40. https://doi.org/10.1016/j.acra.2013.09.020
Yang R, Yu T, Wang Y, Wang Q (2012) Effects of different reconstruction parameters on CT volumetric measurement of pulmonary nodules. Zhongguo Fei Ai Za Zhi 15(2):72–77. https://doi.org/10.3779/j.issn.1009-3419.2012.02.02
Wang Y, de Bock GH, van Klaveren RJ et al (2010) Volumetric measurement of pulmonary nodules at low-dose chest CT: effect of reconstruction setting on measurement variability. Eur Radiol 20(5):1180–1187. https://doi.org/10.1007/s00330-009-1634-9
Nietert PJ, Ravenel JG, Leue WM et al (2009) Imprecision in automated volume measurements of pulmonary nodules and its effect on the level of uncertainty in volume doubling time estimation. Chest 135(6):1580–1587. https://doi.org/10.1378/chest.08-2040
Larici AR, Storto ML, Torge M et al (2008) Automated volumetry of pulmonary nodules on multidetector CT: influence of slice thickness, reconstruction algorithm and tube current. Preliminary results. Radiol Med 113(1):29–42. https://doi.org/10.1007/s11547-008-0231-3
Gavrielides MA, Li Q, Zeng R, et al. (2016) Detectable change of lung nodule volume with CT in a phantom study with high and low signal to background contrast. In: Proceedings, medical imaging 2016: physics of medical imaging, vol 9783; 978329 (2016). https://doi.org/10.1117/12.2217887
Eberhard M, Martini K, Euler A, Frauenfelder T (2022) Overlapping reconstructions in thin-section computed tomography. J Thorac Imaging 37(4):W56–W57. https://doi.org/10.1097/RTI.0000000000000631
Arndt C, Güttler F, Heinrich A, Bürckenmeyer F, Diamantis I, Teichgräber U (2021) Deep learning CT image reconstruction in clinical practice. Rofo 193(3):252–261. https://doi.org/10.1055/a-1248-2556
Kim SK, Kim C, Lee KY et al (2019) Accuracy of model-based iterative reconstruction for CT volumetry of part-solid nodules and solid nodules in comparison with filtered back projection and hybrid iterative reconstruction at various dose settings: an anthropomorphic chest phantom study. Korean J Radiol 20(7):1195–1206. https://doi.org/10.3348/kjr.2018.0893
Willemink MJ, Borstlap J, Takx RAP et al (2013) The effects of computed tomography with iterative reconstruction on solid pulmonary nodule volume quantification. PLoS One 8(2):e58053. https://doi.org/10.1371/journal.pone.0058053
Willemink MJ, Leiner T, Budde RPJJ et al (2012) Systematic error in lung nodule volumetry: effect of iterative reconstruction versus filtered back projection at different CT parameters. AJR Am J Roentgenol 199(6):1241–1246. https://doi.org/10.2214/AJR.12.8727
Kim C, Kwack T, Kim W, Cha J, Yang Z, Yong HS (2022) Accuracy of two deep learning-based reconstruction methods compared with an adaptive statistical iterative reconstruction method for solid and ground-glass nodule volumetry on low-dose and ultra-low-dose chest computed tomography: a phantom study. PLoS One 17(6):e0270122. https://doi.org/10.1371/journal.pone.0270122
Ko JP, Chang J, Bomsztyk E, Babb JS, Naidich DP, Rusinek H (2005) Effect of CT image compression on computer-assisted lung nodule volume measurement. Radiology 237(1):83–88. https://doi.org/10.1148/radiol.2371041079
dos Santos DP, Friese C, Borggrefe J, Mildenberger P, Mahringer-Kunz A, Kloeckner R (2020) The impact of irreversible image data compression on post-processing algorithms in computed tomography. Diagn Interv Radiol 26(1):22–27. https://doi.org/10.5152/dir.2019.18245120
(2011) Usability of irreversible image compression in radiological imaging. A position paper by the European Society of Radiology (ESR). Insights Imaging 2(2):103–115. https://doi.org/10.1007/s13244-011-0071-x.
Milanese G, Eberhard M, Martini K, Vittoria De Martini I, Frauenfelder T (2018) Vessel suppressed chest computed tomography for semi-automated volumetric measurements of solid pulmonary nodules. Eur J Radiol 101:97–102. https://doi.org/10.1016/j.ejrad.2018.02.020
Xie X, Willemink MJ, de Jong PA et al (2014) Small irregular pulmonary nodules in low-dose CT: observer detection sensitivity and volumetry accuracy. AJR Am J Roentgenol 202(3):W202–W209. https://doi.org/10.2214/AJR.13.10830
Xie X, Willemink MJ, Zhao Y et al (2013) Inter- and intrascanner variability of pulmonary nodule volumetry on low-dose 64-row CT: an anthropomorphic phantom study. Br J Radiol 86(1029):20130160. https://doi.org/10.1259/bjr.20130160
Marchianò A, Calabrò E, Civelli E et al (2009) Pulmonary nodules: volume repeatability at multidetector CT lung cancer screening. Radiology 251(3):919–925. https://doi.org/10.1148/radiol.2513081313
Xie X, Zhao Y, Snijder RA et al (2013) Sensitivity and accuracy of volumetry of pulmonary nodules on low-dose 16- and 64-row multi-detector CT: an anthropomorphic phantom study. Eur Radiol 23(1):139–147. https://doi.org/10.1007/s00330-012-2570-7
Marten K, Dullin C, Machann W et al (2009) Comparison of flat-panel-detector-based CT and multidetector-row CT in automated volumetry of pulmonary nodules using an anthropomorphic chest phantom. Br J Radiol 82(981):716–723. https://doi.org/10.1259/bjr/40733553
Obenauer S, Dullin C, Heuser M (2007) Flat panel detector-based volumetric computed tomography (fpVCT). Invest Radiol 42(5):291–296. https://doi.org/10.1097/01.rli.0000258663.13199.bf
Marten K, Funke M, Engelke C (2004) Flat panel detector-based volumetric CT: prototype evaluation with volumetry of small artificial nodules in a pulmonary phantom. J Thorac Imaging 19(3):156–163. https://doi.org/10.1097/01.rti.0000131591.12777.a8
Marten K, Engelke C, Grabbe E, Rummeny EJ (2004) Flat-panel detector-based computed tomography: accuracy of experimental growth rate assessment in pulmonary nodules. Rofo 176(5):752–757. https://doi.org/10.1055/s-2004-813020
He C, Liu J, Hu S et al (2020) Accuracy of pulmonary nodule volumetry using noise-optimized virtual monoenergetic image and nonlinear blending image algorithms in dual-energy computed tomography: a phantom study. J Comput Assist Tomogr 44(6):847–851. https://doi.org/10.1097/RCT.0000000000001102
Kim J, Lee KH, Kim J, Shin YJ, Lee KW (2019) Improved repeatability of subsolid nodule measurement in low-dose lung screening with monoenergetic images: a phantom study. Quant Imaging Med Surg 9(2):171–179. https://doi.org/10.21037/qims.2018.10.06
den Harder AM, Bangert F, van Hamersvelt RW et al (2017) The effects of iodine attenuation on pulmonary nodule volumetry using novel dual-layer computed tomography reconstructions. Eur Radiol 27(12):5244–5251. https://doi.org/10.1007/s00330-017-4938-1
Balagurunathan Y, Beers A, Kalpathy-Cramer J et al (2018) Semi-automated pulmonary nodule interval segmentation using the NLST data. Med Phys 45(3):1093–1107. https://doi.org/10.1002/mp.12766
Kalpathy-Cramer J, Zhao B, Goldgof D et al (2016) A comparison of lung nodule segmentation algorithms: methods and results from a multi-institutional study. J Digit Imaging 29(4):476–487. https://doi.org/10.1007/s10278-016-9859-z
Ashraf H, de Hoop B, Shaker SB et al (2010) Lung nodule volumetry: segmentation algorithms within the same software package cannot be used interchangeably. Eur Radiol 20(8):1878–1885. https://doi.org/10.1007/s00330-010-1749-z
Mullally W, Betke M, Wang J, Ko JP (2004) Segmentation of nodules on chest computed tomography for growth assessment. Med Phys 31(4):839–848. https://doi.org/10.1118/1.1656593
Rinaldi MF, Bartalena T, Braccaioli L et al (2010) Three-dimensional analysis of pulmonary nodules: variability of semiautomated volume measurements between different versions of the same software. Radiol Med 115(3):403–412. https://doi.org/10.1007/s11547-010-0511-6
MacMahon M, Naidich D, Lee KS et al (2017) Incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 284(1):228–243. https://doi.org/10.1148/radiol.2017161659
Liang M, Yip R, Tang W et al (2017) Variation in screening CT–detected nodule volumetry as a function of size. AJR Am J Roentgenol 209(2):304–308. https://doi.org/10.2214/AJR.16.17159
Wang Y, van Klaveren RJ, van der Zaag-Loonen HJ et al (2008) Effect of nodule characteristics on variability of semiautomated volume measurements in pulmonary nodules detected in a lung cancer screening program. Radiology 248(2):625–631. https://doi.org/10.1148/radiol.2482070957
Gietema HA, Wang Y, Xu D et al (2006) Pulmonary nodules detected at lung cancer screening: interobserver variability of semiautomated volume measurements. Radiology 241(1):251–257. https://doi.org/10.1148/radiol.2411050860
Boll DT, Gilkeson RC, Fleiter TR, Blackham KA, Duerk JL, Lewin JS (2004) Volumetric assessment of pulmonary nodules with ECG-gated MDCT. AJR Am J Roentgenol 183(5):1217–1223. https://doi.org/10.2214/ajr.183.5.1831217
Kostis WJ, Yankelevitz DF, Reeves AP, Fluture SC, Henschke CI (2004) Small pulmonary nodules: reproducibility of three-dimensional volumetric measurement and estimation of time to follow-up CT. Radiology 231(2):446–452. https://doi.org/10.1148/radiol.2312030553
Hwang EJ, Goo JM, Kim J et al (2017) Development and validation of a prediction model for measurement variability of lung nodule volumetry in patients with pulmonary metastases. Eur Radiol 27(8):3257–3265. https://doi.org/10.1007/s00330-016-4713-8
Smith GT, Rahman AR, Li M et al (2015) Reproducibility of volumetric computed tomography of stable small pulmonary nodules with implications on estimated growth rate and optimal scan interval. PLoS One 10(9):e0138144. https://doi.org/10.1371/journal.pone.0138144
Bolte H, Riedel C, Müller-Hülsbeck S et al (2007) Precision of computer-aided volumetry of artificial small solid pulmonary nodules in ex vivo porcine lungs. Br J Radiol 80(954):414–421. https://doi.org/10.1259/bjr/23933268
Hwang EJ, Goo JM, Kim HY, Yi J, Kim Y (2021) Optimum diameter threshold for lung nodules at baseline lung cancer screening with low-dose chest CT: exploration of results from the Korean Lung Cancer Screening Project. Eur Radiol 31(9):7202–7212. https://doi.org/10.1007/s00330-021-07827-8
Petkovska I, Brown MS, Goldin JG et al (2007) The effect of lung volume on nodule size on CT. Acad Radiol 14(4):476–485. https://doi.org/10.1016/j.acra.2007.01.008
Guo XW, Wang Y, Li D et al (2014) The intra-observer variability of volumetric measurement of pulmonary nodules: Comparison of two-dimensional and three-dimensional method. Zhongguo Fei Ai Za Zhi 17(4):336–341. https://doi.org/10.3779/j.issn.1009-3419.2014.04.08
Han D, Heuvelmans MA, Vliegenthart R et al (2018) Influence of lung nodule margin on volume- and diameter-based reader variability in CT lung cancer screening. Br J Radiol 91(1090):20170405. https://doi.org/10.1259/bjr.20170405
Nair A, Devaraj A, Callister M, Bladwin D (2018) The Fleischner Society 2017 and British Thoracic Society 2015 guidelines for managing pulmonary nodules: keep calm and carry on. Thorax 73:806–812. https://doi.org/10.1136/thoraxjnl-2018-211764
Iwano S, Okada T, Koike W et al (2009) Semi-automatic volumetric measurement of lung cancer using multi-detector CT. Acad Radiol 16(10):1179–1186. https://doi.org/10.1016/j.acra.2009.04.007
Talwar A, Willaime JMY, Pickup LC et al (2018) Pulmonary nodules: assessing the imaging biomarkers of malignancy in a “coffee-break.” Eur J Radiol 101:82–86. https://doi.org/10.1016/j.ejrad.2018.02.004
Volterrani L, Mazzei MA, Scialpi M et al (2006) Three–dimensional analysis of pulmonary nodules by MSCT with advanced lung analysis (ALA1) software. Radiol Med 111(3):343–354. https://doi.org/10.1007/s11547-006-0033-4
Zhao YR, van Ooijen PMA, Dorrius MD et al (2014) Comparison of three software systems for semi-automatic volumetry of pulmonary nodules on baseline and follow-up CT examinations. Acta Radiol 55(6):691–698. https://doi.org/10.1177/0284185113508177
Ko JP, Berman EJ, Kaur M et al (2012) Pulmonary nodules: growth rate assessment in patients by using serial CT and three-dimensional volumetry. Radiology 262(2):662–671. https://doi.org/10.1148/radiol.11100878
Pinto EG, Penha D, Hochhegger B et al (2022) The impact of cardiopulmonary hemodynamic factors in volumetry for pulmonary nodule management. BMC Med Imaging 22(1):49. https://doi.org/10.1186/s12880-022-00774-w
Penha D, Pinto EG, Hochhegger B et al (2021) The impact of lung parenchyma attenuation on nodule volumetry in lung cancer screening. Insights Imaging 12(1):84. https://doi.org/10.1186/s13244-021-01027-0
Biederer J, Dinkel J, Bolte H et al (2007) Respiratory-gated helical computed tomography of lung: reproducibility of small volumes in an ex vivo model. Int J Radiat Oncol Biol Phys 69(5):1642–1649. https://doi.org/10.1016/j.ijrobp.2007.08.031
Goo JM, Kim KG, Gierada DS, Castro M, Bae KT (2006) Volumetric measurements of lung nodules with multi-detector row CT: effect of changes in lung volume. Korean J Radiol 7(4):243–248. https://doi.org/10.3348/kjr.2006.7.4.243
Picozzi G, Diciotti S, Falchini M et al (2006) Operator-dependent reproducibility of size measurements of small phantoms and lung nodules examined with low-dose thin-section computed tomography. Invest Radiol 41(11):831–839. https://doi.org/10.1097/01.rli.0000242837.11436.6e
Bolte H, Riedel C, Jahnke T et al (2006) Reproducibility of computer-aided volumetry of artificial small pulmonary nodules in ex vivo porcine lungs. Invest Radiol 41(1):28–35. https://doi.org/10.1097/01.rli.0000191366.05586.4d
Bolte H, Jahnke T, Schäfer FKW et al (2007) Interobserver-variability of lung nodule volumetry considering different segmentation algorithms and observer training levels. Eur J Radiol 64(2):285–295. https://doi.org/10.1016/j.ejrad.2007.02.031
Agnes SA, Anitha J (2022) Efficient multiscale fully convolutional UNet model for segmentation of 3D lung nodule from CT image. J Med Imaging (Bellingham). 9(5):052402. https://doi.org/10.1117/1.JMI.9.5.052402
Bhattacharyya D, Rao NT, Joshua ESN, Hu YC (2022) A bi-directional deep learning architecture for lung nodule semantic segmentation. Vis Comput 8:1–17. https://doi.org/10.1007/s00371-022-02657-1
Lu D, Chu J, Zhao R, Zhang Y, Tian G (2022) A novel deep learning network and its application for pulmonary nodule segmentation. Comput Intell Neurosci 17(2022):7124902. https://doi.org/10.1155/2022/7124902
Kido S, Kidera S, Hirano Y et al (2022) Segmentation of lung nodules on CT images using a nested three-dimensional fully connected convolutional network. Front Artif Intell 17(5):782225. https://doi.org/10.3389/frai.2022.782225
Song J, Huang SC, Kelly B et al (2022) Automatic lung nodule segmentation and intra-nodular heterogeneity image generation. IEEE J Biomed Health Inform 26(6):2570–2581. https://doi.org/10.1109/JBHI.2021.3135647
Lu Z, Long F, He X (2022) Classification and segmentation algorithm in benign and malignant pulmonary nodules under different CT reconstruction. Comput Math Methods Med 21(2022):3490463. https://doi.org/10.1155/2022/3490463
Bianconi F, Fravolini ML, Pizzoli S et al (2021) Comparative evaluation of conventional and deep learning methods for semi-automated segmentation of pulmonary nodules on CT. Quant Imaging Med Surg 11(7):3286–3305. https://doi.org/10.21037/qims-20-1356
Cui Y, Arimura H, Nakano R, Yoshitake T, Shioyama Y, Yabbuchi H (2021) Automated approach for segmenting gross tumor volumes for lung cancer stereotactic body radiation therapy using CT-based dense V-networks. J Radiat Res 62(2):346–355. https://doi.org/10.1093/jrr/rraa132
Dutande P, Baid U, Talbar S (2021) LNCDS: A 2D–3D cascaded CNN approach for lung nodule classification, detection and segmentation. Biomed Signal Process Control 67(6):102527. https://doi.org/10.1016/j.bspc.2021.102527
Yu H, Li J, Zhang L, Cao Y, Yu X, Sun J (2021) Design of lung nodules segmentation and recognition algorithm based on deep learning. BMC Bioinform 22(Suppl 5):314. https://doi.org/10.1186/s12859-021-04234-0
Jain S, Indora S, Atal DK (2021) Lung nodule segmentation using salp shuffled shepherd optimization algorithm-based generative adversarial network. Comput Biol Med 137:104811. https://doi.org/10.1016/j.compbiomed.2021.104811
Meng XL, Xing ZJ, Lu S (2021) A deep learning-based lung nodule density classification and segmentation method and its effectiveness under different CT reconstruction algorithms. Zhonghua Yi Xue Za Zhi 101(7):476–480. https://doi.org/10.3760/cma.j.cn112137-20201123-03171
Wang K, Zhang X, Zhang X, Huang S, Li J, HuangFu L (2021) Multi-granularity scale-aware networks for hard pixels segmentation of pulmonary nodules. Biomed Signal Process Control 69:102890. https://doi.org/10.1016/j.bspc.2021.102890
Wang Y, Zhou C, Chan H-P, Hadjiiski L, Chughtai A, Kazerooni E (2022) Hybrid U-Net-based deep learning model for volume segmentation of lung nodules in CT images. Med Phys 49(11):7287–7302. https://doi.org/10.1002/mp.15810
Zhang X, Liu X, Zhang B et al (2021) Accurate segmentation for different types of lung nodules on CT images using improved U-Net convolutional network. Medicine (Baltimore) 100(40):e27491. https://doi.org/10.1097/MD.0000000000027491
Dong X, Xu S, Liu Y et al (2020) Multi-view secondary input collaborative deep learning for lung nodule 3D segmentation. Cancer Imaging 20(1):53. https://doi.org/10.1186/s40644-020-00331-0
Sathish R, Sathish R, Sethuraman R, Sheet D (2020) Lung segmentation and nodule detection in computed tomography scan using a convolutional neural network trained adversarially using turing test loss. Annu Int Conf IEEE Eng Med Biol Soc 2020:1331–1334. https://doi.org/10.1109/EMBC44109.2020.9175649
Usman M, Lee B-D, Byon S-S, Kim S-H, Lee B-I, Shin Y-G (2020) Volumetric lung nodule segmentation using adaptive ROI with multi-view residual learning. Sci Rep 10(1):12839. https://doi.org/10.1038/s41598-020-69817-y
Wu W, Gao L, Duan H, Huang G, Ye X, Nie S (2020) Segmentation of pulmonary nodules in CT images based on 3D-UNET combined with three-dimensional conditional random field optimization. Med Phys 47(9):4054–4063. https://doi.org/10.1002/mp.14248
Aresta G, Jacobs C, Araújo T et al (2019) iW-Net: an automatic and minimalistic interactive lung nodule segmentation deep network. Sci Rep 9(1):11591. https://doi.org/10.1038/s41598-019-48004-8
Liu H, Cao H, Song E et al (2019) A cascaded dual-pathway residual network for lung nodule segmentation in CT images. Phys Med 63:112–121. https://doi.org/10.1016/j.ejmp.2019.06.003
Chung H, Ko H, Jeon SJ, Yoon K-H, Lee J (2018) Automatic lung segmentation with juxta-pleural nodule identification using active contour model and Bayesian approach. IEEE J Transl Eng Health Med 18(6):1800513. https://doi.org/10.1109/JTEHM.2018.2837901
Kidera S, Kido S, Hirano Y, Mabu S, Tanaka N (2018) (2018) Segmentation of lung nodules on MDCT images by using 3D Conv-DeconvNet. Int J CARS 13(Suppl 1):1–273. https://doi.org/10.1007/s11548-018-1766-y
Qin Y, Zheng H, Huang X, Yang J, Zhu Y-M (2019) Pulmonary nodule segmentation with CT sample synthesis using adversarial networks. Med Phys 46(3):1218–1229. https://doi.org/10.1002/mp.13349
Shakir H, Rasool Khan TM, Rasheed H (2018) 3-D segmentation of lung nodules using hybrid level sets. Comput Biol Med 1(96):214–226. https://doi.org/10.1016/j.compbiomed.2018.03.015
Feng X, Yang J, Laine AF, Angelini ED (2017) Discriminative localization in CNNs for weakly-supervised segmentation of pulmonary nodules. Med Image Comput Comput Assist Interv 10435:568–576. https://doi.org/10.1007/978-3-319-66179-7_65
Wang S, Zhou M, Gevaert O et al (2017) A multi-view deep convolutional neural networks for lung nodule segmentation. Annu Int Conf IEEE Eng Med Biol Soc 2017:1752–1755. https://doi.org/10.1109/EMBC.2017.8037182
Wang S, Zhou M, Liu Z et al (2017) Central focused convolutional neural networks: developing a data-driven model for lung nodule segmentation. Med Image Anal 40:172–183. https://doi.org/10.1016/j.media.2017.06.014
Li B, Chen Q, Peng G et al (2016) Segmentation of pulmonary nodules using adaptive local region energy with probability density function-based similarity distance and multi-features clustering. Biomed Eng Online 15(1):49. https://doi.org/10.1186/s12938-016-0164-3
Lassen BC, Jacobs C, Kuhnigk JM, van Ginneken B, van Rikxoort EM (2015) Robust semi-automatic segmentation of pulmonary subsolid nodules in chest computed tomography scans. Phys Med Biol 60(3):1307–1323. https://doi.org/10.1088/0031-9155/60/3/1307
Tan Y, Schwartz LH, Zhao B (2013) Segmentation of lung lesions on CT scans using watershed, active contours, and Markov random field. Med Phys 40(4):043502. https://doi.org/10.1118/1.4793409
Reeves AP, Chan AB, Yankelevitz DF, Henschke CI, Kressler B, Kostis WJ (2006) On measuring the change in size of pulmonary nodules. IEEE Trans Med Imaging 25(4):435–450. https://doi.org/10.1109/TMI.2006.871548
Yankelevitz DF, Reeves AP, Kostis WJ, Zhao B, Henschke CI (2000) Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology 217(1):251–256. https://doi.org/10.1148/radiology.217.1.r00oc33251
Hammer MM, Byrne SC (2022) Cancer risk in nodules detected at follow-up lung cancer screening CT. AJR Am J Roentgenol 218(4):634–641. https://doi.org/10.2214/AJR.21.26927
Silva M, Milanese G, Sestini S et al (2021) Lung cancer screening by nodule volume in Lung-RADS v1.1: negative baseline CT yields potential for increased screening interval. Eur Radiol 31(4):1956–1968. https://doi.org/10.1007/s00330-020-07275-w
Li K, Yip R, Avila R, Henschke CI, Yankelevitz DF (2017) Size and growth assessment of pulmonary nodules: consequences of the rounding. J Thorac Oncol 12(4):657–662. https://doi.org/10.1016/j.jtho.2016.12.010
Li M, Han R, Song W et al (2016) Three dimensional volumetric analysis of solid pulmonary nodules on chest CT: cancer risk assessment. Zhongguo Fei Ai Za Zhi 19(5):279–285. https://doi.org/10.3779/j.issn.1009-3419.2016.05.05
Horeweg N, Scholten ET, de Jong PA et al (2014) Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 15(12):1342–1350. https://doi.org/10.1016/S1470-2045(14)70387-0
Shin KE, Lee KS, Yi CA, Chung MJ, Shin MH, Choi YH (2014) Subcentimeter lung nodules stable for 2 years at LDCT: Long-term follow-up using volumetry. Respirology 19(6):921–928. https://doi.org/10.1111/resp.12337
Horeweg N, van der Aalst CM, Vliegenthart R et al (2013) Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. Eur Respir J 42(6):1659–1667. https://doi.org/10.1183/09031936.00197712
van Klaveren RJ, Oudkerk M, Prokop M et al (2009) Management of lung nodules detected by volume CT scanning. N Engl J Med 361(23):2221–2229. https://doi.org/10.1056/NEJMoa0906085
Revel MP, Merlin A, Peyrard S et al (2006) Software volumetric evaluation of doubling times for differentiating benign versus malignant pulmonary nodules. AJR Am J Roentgenol 187(1):135–142. https://doi.org/10.2214/AJR.05.1228
Ahn Y, Lee SM, Kim MS et al (2022) Volume doubling times of pulmonary metastases in patients with bone and soft-tissue sarcomas: associations with subsequent new metastases and survival after metastasectomy. AJR Am J Roentgenol 218(4):624–632. https://doi.org/10.2214/AJR.21.26859
Setojima Y, Shimada Y, Tanaka T et al (2020) Prognostic impact of solid-part tumour volume doubling time in patients with radiological part-solid or solid lung cancer. Eur J Cardiothorac Surg 57(4):763–770. https://doi.org/10.1093/ejcts/ezz305
Kamiya S, Iwano S, Umakoshi H et al (2018) Computer-aided volumetry of part-solid lung cancers by using CT: solid component size predicts prognosis. Radiology 287(3):1030–1040. https://doi.org/10.1148/radiol.2018172319
Li Q, Gu Y-F, Fan L, Li Q-C, Xiao Y, Liu S-Y (2018) Effect of CT window settings on size measurements of the solid component in subsolid nodules: evaluation of prediction efficacy of the degree of pathological malignancy in lung adenocarcinoma. Br J Radiol 91(1088):20180251. https://doi.org/10.1259/bjr.20180251
Yanagawa M, Tanaka Y, Leung AN et al (2014) Prognostic importance of volumetric measurements in stage i lung adenocarcinoma. Radiology 272(2):557–567. https://doi.org/10.1148/radiol.14131903
Vogel MN, Schmücker S, Maksimovic O, Hartmann J, Claussen CD, Horger M (2012) Reduction in growth threshold for pulmonary metastases: an opportunity for volumetry and its impact on treatment decisions. Br J Radiol 85(1015):959–964. https://doi.org/10.1259/bjr/87835487
Solomon J, Ebner L, Christe A et al (2021) Minimum perceivable size difference: how well can radiologists visually detect a change in lung nodule size from CT images? Eur Radiol 31(4):1947–1955. https://doi.org/10.1007/s00330-020-07326-2
Mets OM, Chung K, Zanen P et al (2018) In vivo growth of 60 non-screening detected lung cancers: a computed tomography study. Eur Respir J 51(4):1702183. https://doi.org/10.1183/13993003.02183-2017
Sun Q, Huang Y, Zhao SJ et al (2018) The volume and mass growth of persisted pulmonary nodules detected in low-dose CT screening and its influence factors. Zhonghua Zhong Liu Za Zhi 40(4):274–279. https://doi.org/10.3760/cma.j.issn.0253-3766.2018.04.007
Heuvelmans MA, Vliegenthart R, de Koning HJ et al (2017) Quantification of growth patterns of screen-detected lung cancers: the NELSON study. Lung Cancer 108:48–54. https://doi.org/10.1016/j.lungcan.2017.02.021
Henschke CI, Yankelevitz DF, Yip R et al (2012) Lung cancers diagnosed at annual CT screening: volume doubling times. Radiology 263(2):578–583. https://doi.org/10.1148/radiol.12102489
Kim EY, Lee JI, Sung YM et al (2012) Pulmonary metastases from colorectal cancer: imaging findings and growth rates at follow-up CT. Clin Imaging 36(1):14–18. https://doi.org/10.1016/j.clinimag.2011.04.018
Tanimoto D, Ito K, Tamada T et al (2012) Serial 3-dimensional volumetric computed tomography evaluation of lung cancer growth rate in patients with chronic obstructive pulmonary disease findings. J Comput Assist Tomogr 36(2):181–186. https://doi.org/10.1097/RCT.0b013e3182483c32
Pauls S, Kürschner C, Dharaiya E et al (2008) Comparison of manual and automated size measurements of lung metastases on MDCT images: Potential influence on therapeutic decisions. Eur J Radiol 66(1):19–26. https://doi.org/10.1016/j.ejrad.2007.05.022
Shi J, Ye Y, Zhu D, Su L, Huang Y, Huang J (2021) Comparative analysis of pulmonary nodules segmentation using multiscale residual U-Net and fuzzy C-means clustering. Comput Methods Programs Biomed 209:106332. https://doi.org/10.1016/j.cmpb.2021.106332
Kakinuma R, Muramatsu Y, Yamamichi J, Gomi S, Oubel E, Moriyama N (2018) Evaluation of the 95% limits of agreement of the volumes of 5-year clinically stable solid nodules for the development of a follow-up system for indeterminate solid nodules in CT lung cancer screening. J Thorac Dis 10(1):175–189. https://doi.org/10.21037/jtd.2017.11.142
Christe A, Bronnimann A, Vock P, Brönnimann A, Vock P (2014) Volumetric analysis of lung nodules in computed tomography (CT): comparison of two different segmentation algorithm softwares and two different reconstruction filters on automated volume calculation. Acta Radiol 55(1):54–61. https://doi.org/10.1177/0284185113492454
Koike W, Iwano S, Matsuo K, Kitano M, Kawakami K, Naganawa S (2014) Doubling time calculations for lung cancer by three-dimensional computer-aided volumetry: effects of inter-observer differences and nodule characteristics. J Med Imaging Radiat Oncol 58(1):82–88. https://doi.org/10.1111/1754-9485.12128
Scholten ETh, de Hoop B, Jacobs C et al (2013) Semi-automatic quantification of subsolid pulmonary nodules: comparison with manual measurements. PLoS One 8(11):e80249. https://doi.org/10.1371/journal.pone.0080249
Dehmeshki J, Amin H, Valdivieso M, Ye X (2008) Segmentation of pulmonary nodules in thoracic CT scans: a region growing approach. IEEE Trans Med Imaging 27(4):467–480. https://doi.org/10.1109/TMI.2007.907555
Revel MP, Lefort C, Bissery A et al (2004) Pulmonary nodules: preliminary experience with three-dimensional evaluation. Radiology 231(2):459–466. https://doi.org/10.1148/radiol.2312030241
Heuvelmans MA, Walter JE, Vliegenthart R et al (2018) Disagreement of diameter and volume measurements for pulmonary nodule size estimation in CT lung cancer screening. Thorax 73(8):779–781. https://doi.org/10.1136/thoraxjnl-2017-210770
Shen S, Bui AAT, Cong J, Hsu W (2015) An automated lung segmentation approach using bidirectional chain codes to improve nodule detection accuracy. Comput Biol Med 57:139–149. https://doi.org/10.1016/j.compbiomed.2014.12.008
Funding
The authors did not receive financial or non-financial support for this systematic review.
Author information
Authors and Affiliations
Contributions
All authors critically revised the manuscript and approved it prior to submission. EGP, DP, and KI conceived this study and supervised all aspects of its implementation. EGP and DP collected the data and carried out the analysis of the data. All the authors contributed to the interpretation of the results and the proofreading of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table S1.
Studies included in the first stage of the systematic review [176,177,178,179,180,181,182]. Supplementary Table S2. Studies that seemed promising for inclusion during the screening phase but were later excluded [183, 184]. Supplementary Table S3. Results of the attempted meta-analysis of two sets of similar studies. Supplementary Figures S1. Forest plot of similar studies [15, 17]. Supplementary Figures S2. Forest plot of similar studies [7, 20, 22].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guedes Pinto, E., Penha, D., Ravara, S. et al. Factors influencing the outcome of volumetry tools for pulmonary nodule analysis: a systematic review and attempted meta-analysis. Insights Imaging 14, 152 (2023). https://doi.org/10.1186/s13244-023-01480-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-023-01480-z