Abstract
Background
d-Cycloserine (DCS) enhances extinction learning across species, but it has proven challenging to identify consistent benefit of DCS when added to therapeutic interventions. We conducted a placebo-controlled trial of DCS to potentiate social skills training in autism spectrum disorder (ASD) but found substantial improvement in both the DCS and placebo groups at the conclusion of active treatment. Here, we assess the impact of DCS 11 weeks following active treatment to evaluate the impact of DCS on treatment response durability.
Methods
Study participants included 60 outpatient youth with ASD, ages 5–11 years, all with IQ above 70, and significantly impaired social functioning who completed a 10-week active treatment phase during which they received weekly single doses of 50 mg of DCS or placebo administered 30 min prior to group social skills training. Following the 10-week active treatment phase, blinded follow-up assessments occurred at week 11 and week 22. The primary outcome measure for our durability of treatment evaluation was the parent-rated social responsiveness scale (SRS) total raw score at week 22.
Results
Analysis of the SRS total raw score demonstrated significant decrease for the DCS group compared to the placebo group (p = 0.042) indicating greater maintenance of treatment effect in the DCS group. DCS was well tolerated, with irritability being the most frequently reported adverse effect in both groups.
Conclusions
The findings of this study suggest that DCS may help youth with ASD to maintain skills gained during sort-term social skills training. Larger-scale studies with longer follow-up will be necessary to further understand the long-term impact of DCS paired with structured social skills training.
Trial registration
ClinicalTrials.gov, NCT01086475
Similar content being viewed by others
Background
Social impairment is a defining feature and key predictor of long-term outcome in autism spectrum disorder (ASD) [1, 2]. Social skills training explicitly targets this core deficit and is widely implemented in ASD treatment [2]. Adolescent social skills groups consistently demonstrate immediate improvements in social and communication skills in participants during treatment; however, participants tend to show limited sustained treatment response over longer term follow-up [2–4].
One possible way to improve long-term outcomes of social skills training would be to potentiate the effect of the intervention with adjunctive pharmacotherapy. Recent studies have shown that d-cycloserine (DCS), a partial agonist at the glycine site of the N-methyl-d-aspartate (NMDA) receptor, may potentiate response to behavioral therapy [5–9]. DCS appears to potentiate extinction learning, with most significant benefit from intermittent dosing immediately prior to behavioral conditioning in animals or psychotherapy session in humans [10]. The ability of DCS to augment learning appears to be related to NMDA receptor-dependent neural plasticity within the basolateral amygdala [8]. Multiple studies have suggested a role of the glutamate system in the pathophysiology of ASD [11], including rare, disruptive mutations in an NMDA receptor subunit gene [12]. In mouse models of ASD, glutamatergic modulators have been associated with improvement in sociability [12–14], but human studies have been less promising [15–17]. DCS studies in ASD have been primarily short-term monotherapeutic interventions which have not demonstrated consistent, convincing improvements in core features of ASD [18–20]. To date, no studies in ASD have evaluated the combined impact of DCS and therapeutic interventions.
With these facts in mind, our group designed a randomized, double-blind, placebo-controlled trial of low-dose DCS given 30 min prior to weekly peer-mediated group social skills training in youth with ASD. As described by Minshawi et al., at the conclusion of active treatment (week 11), participants in the DCS and placebo groups both demonstrated notable improvement in social functioning, but there was no statistically significant difference between the groups on primary or secondary outcome measures at the end of treatment (primary outcome measure social responsiveness scale (SRS) change score t test p value = 0.45) [21]. In the current manuscript, we review results from the post-treatment observation phase of the study (week 11 through week 22), during which participants had no further medication or study interventions, but returned for assessment at week 22 with the goal of measuring potential sustained treatment effects of DCS plus social skills training.
Methods
Trial design and participants
This study evaluated blinded week 22 durability of treatment data collected following a 10-week randomized, double-blind, placebo-controlled DCS plus peer-mediated social skills group intervention in high functioning youth with ASD completed between August 4, 2009 and January 23, 2014 at Indiana University School of Medicine (IUSM) and Cincinnati Children’s Hospital Medical Center (CCHMC). Please see manuscript by Minshawi et al. for full details describing study design, participants, and statistical analysis of the data collected during and immediately following the 10-week intervention [21]. Both the 10-week intervention and week 22 treatment durability analysis were approved by the institutional review board (IRB) at each participating site (Indiana University Institutional Review Board and Cincinnati Children’s Hospital Medical Center Institutional Review Board). Guardians of all participants provided written informed consent prior to study enrollment. Assent was obtained from enrolled youth when possible.
Study participants were youth ages 5–11 years recruited from outpatient psychiatric clinics at both sites and via IRB approved advertising. Participants were diagnosed with autistic disorder, Asperger’s disorder, or pervasive developmental disorder, not otherwise specified (PDD-NOS) by clinical assessment based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text revision (DSM-IV-TR) diagnostic criteria [22], and corroborated by administration of the Autism Diagnostic Observation Schedule (ADOS) [23] and the Autism Diagnostic Interview-Revised (ADI-R) [24]. Due to the cognitive and verbal demands of the social skills intervention, subjects were required to have an Intellectual Quotient (IQ) above 70 as measured by the Stanford-Binet Fifth Edition (SB-V) [25] and a Vineland Adaptive Behavior Scale Second Edition (VABS-II) [26] communication standard score above 70. At baseline, all subjects demonstrated significant social impairment with T-scores of 60 or greater on the SRS [27] and scores of 70% or less on both the parent questionnaire and child assessment portions of the Triad Social Skills Assessment (TSSA) [28].
Concurrent psychotropic medication use was permitted, though all subjects were required to remain on stable doses throughout the treatment intervention and through week 22 durability of treatment effect analysis. Participants were excluded if treated with more than two psychotropic medications or known glutamatergic modulators such as riluzole, memantine, acamprosate, topiramate, or amantadine among others. Participants enrolled in psychosocial interventions independent from the study protocol were required to have stable regimens throughout the study. Participants were excluded from the study if they were participating in concurrent group social skills training programs.
For the 10-week intervention phase of the study, participants were enrolled in a series of 17 social skills groups (13 at IUSM and 4 at CCHMC), each containing four children with ASD and two typically developing, age-matched peer models. Peer models were determined to be free of psychiatric symptoms or developmental disability via the Child Symptom Inventory-4 [29] and a medical and psychiatric interview completed by a study physician. Subjects with ASD were randomized to either DCS or placebo in a 1:1 ratio by computer-generated randomization list accessible only by the investigational pharmacist. A dose of 50 mg DCS or placebo was administered 30 min prior to 10 weekly, 2-h sessions of manualized social skills training. Social skills intervention followed a curriculum utilizing ABA-based techniques designed to teach skills including greetings, understanding emotions, creative play, and social conversations [30]. Social skills groups were divided by age (5–7 years or 8–11 years), and minor modifications were made to the group curriculum to accommodate the different age ranges. Groups were instructed by masters or doctorate level clinicians with specific expertise and experience working with youth with ASD.
Following the 10-week intervention phase, participants received no ongoing study-related therapeutic intervention or treatment with study drug. Participants were asked to return at week 11 and week 22 for blinded assessment. Participants, caregivers, and investigators remained blind to study group-assignment until after all week 22 follow-up visits were completed, all data was recorded in a RedCap database, quality checks were completed, and the data set was locked.
Finally, we incorporated a pilot eye-tracking paradigm of gaze preference employing a Tobii T120 Infrared Eye Tracker integrated with a 17-inch thin film transistor monitor controlled with Tobii Studio software (Version 3.0). Eye-tracking assessment was completed at week 11 and week 22 in a subset of participants beginning in year 3 of the project. In this pilot assessment, participants viewed 60 colored photographs of adult human faces from the NimStim Face Stimuli Set [31] and percent time looking at the eye, nose, mouth, or whole face region was calculated (Fig. 1). Time spent viewing the eye region and face as a whole implied greater social interest in comparison to time spent looking at other facial regions [32–34].
Outcome assessments and statistical analysis
Demographic data were collected at screen, prior to randomization to study group. Monitoring for adverse events was completed by a study physician at all visits via discussion of new symptoms, recent doctor visits, and current medication review. The primary outcome measure at week 22 (and at week 11) was the parent-rated SRS total raw score. Exploratory outcome measures at week 22 were the SRS subscale component scores. The SRS total raw score ranges from 0 to 195, with higher scores indicating more significant social impairment [27].
Demographic data describing the week 22 completers in the DCS and placebo groups (age, gender, ASD subtype, and concomitant medications), as well as reported adverse events/frequencies were compared using Student’s t tests for continuous variables and Fisher’s exact tests for categorical variables. Wilcoxon rank sum tests were completed to validate results of the Student’s t tests.
To assess potential impact of participants lost to follow-up, we compared using Wilcoxon rank sum tests the week 22 completers versus those who dropped out before week 11 on the SRS total raw score at baseline. We also compared these completers to those who dropped out before week 22 on the change in SRS total raw score from baseline to week 11.
To examine the differences between the groups on SRS total raw scores from week 11 to week 22, we employed a robust linear model based on M-estimation, specifically the bisquare weight function (or biweight), which is the default method in the SAS ® statistical software package (SAS ® version 9.3, SAS Institute Inc., Cary, NC). A robust linear model was used because preliminary analyses indicated that the data contained some outliers. Rather than omitting these points (i.e., setting their weight to zero) and fitting parameters using least squares, we preferred to downweight the outliers based on their distance from the bulk of the data. The biweight was used because it provides smoothly changing weights to all of the observations, including the outliers [35]. In our model, the response was the SRS total raw score at week 22 and the independent variable was treatment group (DCS or placebo). The weightings account for the SRS total raw score at week 11 as well as the covariates gender, age group (5–7 versus 8–11 years), and ASD diagnosis (autistic disorder versus Asperger’s disorder and PDD-NOS combined). Since this model is predicting week 22 SRS total raw scores while controlling for week 11 SRS total raw scores with the weightings, this model predicts relative change in this measure from week 11 to week 22. The weighted estimates are similar to means, but are more resistant to the adverse effects of outliers, and refer to the effect plus the value of the biweight estimator at week 22. The biweight estimator is a weighted average of the week 22 results; adding this value to the treatment effects allows the estimates to be comparable to raw values.
The effect sizes resulting from the robust linear model described above were calculated by dividing the magnitude of treatment effect by the robust measure of scale produced by the model above. In the spirit of Cohen’s d, which is based on means and the standard deviation, we will refer to this effect size as d biw, to indicate that this effect size is based on robust biweight estimators. Similar versions of the effect size based on different robust models have been discussed by Wilcox and Tian [36]. To correct for multiple exploratory post hoc comparisons, false discovery rate (FDR) procedures were utilized for the secondary SRS subscale analyses.
For the pilot eye-tracking data, a repeated measures linear mixed model was conducted where the response was the percent fixation time at a particular area of interest (SAS ® version 9.3). The continuous covariate was the baseline percent fixation time and the categorical independent variables were treatment group and week 11 or week 22 as well as their interaction term. The “week” term was the repeated measure within subjects (which is the random effect). Other covariates included the face identifier, expression, mouth (open or closed), age group, gender, and ASD subtype. The least squares means were derived for each treatment by week combination. The contrast comparing the treatment change between the two weeks of interest was then derived.
Results
Review of week 11 findings [21]
In brief, the 10-week intervention phase of the study, described in detail by Minshawi et al., enrolled 68 children with ASD with no statistically significant treatment group differences in demographics, clinical factors, concomitant medications, therapeutic interventions, or SRS total raw score at baseline [21]. Mean SRS total raw score decreased during the intervention phase in both groups. At week 11, the SRS change scores from baseline demonstrated no statistically significant difference attributable to DCS treatment. DCS was well tolerated, with irritability being the most frequently reported adverse effect in both groups. There was no statistically significant difference in number of reported adverse events between groups. A supplementary figure providing a comprehensive overview of the mean SRS total raw scores across all study phases, including both the initial study treatment phase (week 11 endpoint) and the long-term follow-up (week 22 endpoint) was included as a supplementary file for reference (please see Additional File 1).
Week 22 findings
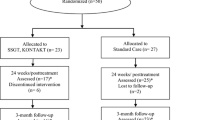
Sixty participants completed week 22 (four subjects were lost during active treatment phase and four lost during follow-up period, consort diagram, Fig. 2). At week 22, there were no statistically significant differences between treatment groups in age, gender, diagnostic subtype, or concomitant medications (Table 1). In our examination of the impact of participants lost to follow-up, we found no statistically significant difference between the week 22 completers and those who were lost to follow-up prior to week 11 on the SRS total raw score at baseline (Wilcoxon Rank Sum p value = 0.90). Furthermore, we found no statistically significant difference when we compared these completers to those who dropped out before week 22 on the change in SRS total raw score from baseline to week 11 (Wilcoxon rank sum p value = 0.91).
The robust linear model employed in this analysis to examine the difference between DCS and placebo groups on SRS total raw scores from week 11 to week 22 demonstrates that the DCS group decreased significantly compared to the placebo group (DCS mean estimate = 85.1 (SE 7.3), placebo mean estimate = 91.5 (SE 7.6), DCS-placebo estimate (treatment effect) = −6.4 (SE 3.1), p = 0.042, d biw = 0.69). This suggests that DCS increased durability of social skills training gains at week 22 in those children treated with active drug. These differences account for week 11 SRS scores as well as gender, age, and diagnosis because of the robust linear model used in analysis. Effect size is in the moderate to large range, indicating that the observed change was meaningful [37]. The size of the effect is apparent in that the treatment effect (the difference between the DCS estimated mean and the placebo estimated mean) is only slightly smaller than the SEs of each of the estimated means.
In the secondary analysis of the SRS subscales, the social cognition subscale showed the greatest between groups difference (p = 0.003, d biw = 0.82). When we corrected for multiple exploratory comparisons utilizing FDR procedures, this effect remained significant. There were no statistically significant differences between groups on the social awareness, social communication, social motivation, and autistic mannerism subscales.
Finally, the pilot eye-tracking measure was completed in 38 subjects, 21 in the DCS group and 17 in the placebo group. There was no statistically significant difference identified between these groups in age, sex, diagnostic subtype, or concomitant medications. Results demonstrated that the DCS treatment group had decreased percent time looking at the nose, but increased percent time looking at the face as a whole (p < 0.0001) when compared to the placebo group. There was no difference in percent time spent viewing the eye or mouth regions between groups. As the social skills curriculum employed in this study directly taught and reinforced eye gaze to the face and eyes region, this data suggests that the DCS treated group was potentially more successful at gaining this skill and was therefore more socially interested in viewing faces in the eye-tracking paradigm at week 22 as a result of receiving active drug.
Discussion
To our knowledge, this study combing social skills therapeutic intervention with medication is the first of its kind to be completed in ASD research. The week 11 results reported by Minshawi et al. demonstrating decreased SRS total scores in both treatment groups but not between group treatment effects align with previous reports of substantial immediate impact of social skills training in youth with ASD [2]. In this follow-up study, we show enhanced durability of treatment response in those subjects who received weekly DCS, a novel finding considering previous studies indicating lack of treatment durability following social skills intervention in ASD [4]. In this analysis, DCS appears to support maintenance of social skills gains made during short-term group therapy compared to placebo. This improved maintenance of effect was measured both by parent-reported increased social cognition and by our pilot eye-tracking paradigm. Furthermore, this medication’s long track record of safe use in children, limited adverse effects in this study, and demonstrated impact with intermittent dosing in other disorders suggests significant potential for future treatment development of DCS in ASD.
There are several limitations of our trial design that must be considered when interpreting the results of this study. First, we utilized a novel, peer-mediated manualized social skills training curriculum for this study [30]. Although the curriculum is based on validated ABA-based techniques, the curriculum itself has not been validated outside of this study. Second, the social skills training design limited this intervention to a selective group of children with relatively high communication and adaptive functioning, limiting the inferences that can be made regarding intermittent DCS dosing to support learning in youth with ASD more generally. Third, the SRS was designed as an assessment tool rather than as an outcome measure, the subscales were derived via expert consensus and may not reflect a true underlying construct, and the large range of potential scores present a challenge in interpreting the exact meaning of a score change [27, 38]. However, we made the choice to employ this measure as our primary outcome as this measure was most in line with the core social impairments we hoped to address via our study intervention, and has also been used in other social skills training studies in youth with ASD [39]. Fourth, our eye-tracking paradigm was piloted only in a subgroup of participants in this study, limiting the impact of these results. Future studies could continue to explore the use of eye tracking as a potential objective measure of social interest by including all study participants in the trial design. Last, the specific mechanisms underlying the longer term benefits of DCS, such as reduction of social anxiety during treatment or improvements in learning and memory during therapy, remain to be established. Despite these limitations, the prospect of enhancing the sustained benefit of social skill training interventions with pharmacotherapy is novel and exciting. Given that social skills training is a primary intervention for ASD but one with limited long-term benefits, the added impact of DCS could significantly improve the long-term social functioning of children with ASD. Replication of the observed effects would require a larger study that is explicitly focused on sustained benefit. Additional work is also needed to determine the mechanisms underlying the benefit of DCS added to social skills training.
Conclusions
Adjunctive DCS significantly increased the sustained benefit from short-term social skills intervention 3 months after treatment cessation. This is of importance considering the body of literature suggesting limited durability of therapeutic interventions targeting core features of ASD. Additionally, the safety and time limited nature of this drug treatment, as demonstrated by the limited adverse effects reported by study participants, indicates that DCS may be a safe and effective strategy to enhance the durability of therapy impact in youth with ASD. This finding holds significant potential in ASD where there are no approved treatments for the core social skills deficits associated with the disorder.
Abbreviations
- ADI-R:
-
Autism Diagnostic Interview-Revised
- ADOS:
-
Autism Diagnostic Observation Schedule
- ASD:
-
Autism spectrum disorder
- CCHMC:
-
Cincinnati Children’s Hospital Medical Center
- DCS:
-
d-Cycloserine
- DSM-IV-TR:
-
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text revision
- IQ:
-
Intellectual quotient
- IRB:
-
Institutional review board
- IUSM:
-
Indiana University School of Medicine
- NMDA:
-
N-methyl-d-aspartate
- PDD-NOS:
-
Pervasive developmental disorder, not otherwise specified
- SB-V:
-
Stanford-Binet Fifth Edition
- SRS:
-
Social responsiveness scale
- TSSA:
-
Triad Social Skills Assessment
- VABS-II:
-
Vineland Adaptive Behavior Scale Second Edition
References
Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–50.
Reichow B, Steiner AM, Volkmar F. Cochrane review: social skills groups for people aged 6 to 21 with autism spectrum disorders (ASD). Evid Based Child Health. 2013;8:266–315.
Laugeson EA, Frankel F, Gantman A, Dillon AR, Mogil C. Evidence-based social skills training for adolescents with autism spectrum disorders: the UCLA PEERS program. J Autism Dev Disord. 2012;42:1025–36.
Soorya LV, Siper PM, Beck T, Soffes S, Halpern D, Gorenstein M, Kolevzon A, Buxbaum J, Wang AT. Randomized comparative trial of a social cognitive skills group for children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2015;54:208–16.
Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–44.
Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–8.
Nave AM, Tolin DF, Stevens MC. Exposure therapy, D-cycloserine, and functional magnetic resonance imaging in patients with snake phobia: a randomized pilot study. J Clin Psychiatry. 2012;73:1179–86.
Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, Cannistraro P, Jenike MA, Rauch SL. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–41. quiz 409.
Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–9.
Parnas AS, Weber M, Richardson R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83:224–31.
Erickson CA, Posey DJ, Stigler KA, McDougle CJ. Glutamatergic function in autism. In: Heresco-Levy U, editor. Glutamate in Neuropsychiatric Disorders. Trivandrum: Research Signpost; 2008. p. 197–212.
Lee EJ, Choi SY, Kim E. NMDA receptor dysfunction in autism spectrum disorders. Curr Opin Pharmacol. 2015;20:8–13.
Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, Bolliger MF, Sudhof TC, Powell CM. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–29.
Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J, Ha S, Chung C, Jung ES, Cho YS, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–5.
King BH, Wright DM, Handen BL, Sikich L, Zimmerman AW, McMahon W, Cantwell E, Davanzo PA, Dourish CT, Dykens EM, et al. Double-blind, placebo-controlled study of amantadine hydrochloride in the treatment of children with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2001;40:658–65.
Erickson CA, Posey DJ, Stigler KA, Mullett J, Katschke AR, McDougle CJ. A retrospective study of memantine in children and adolescents with pervasive developmental disorders. Psychopharmacology (Berl). 2007;191:141–7.
Study of pharmacokenetics, safety, efficacy, and tolerability of memantine in children with autism
Posey DJ, Kem DL, Swiezy NB, Sweeten TL, Wiegand RE, McDougle CJ. A pilot study of D-cycloserine in subjects with autistic disorder. Am J Psychiatry. 2004;161:2115–7.
Posey D, Stigler K, Erickson CA, Azzouz F, Mullett J, Diener JT, McDougle CJ. A double-blind, placebo-controlled study of D-Cycloserine in children with autistic disorder. Chicago: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; 2008. p. 219.
Urbano M, Okwara L, Manser P, Hartmann K, Herndon A, Deutsch SI. A trial of D-cycloserine to treat stereotypies in older adolescents and young adults with autism spectrum disorder. Clin Neuropharmacol. 2014;37:69–72.
Minshawi N, Wink LK, Shaffer R, Plawecki MH, Posey D, Liu H, Hurwitz S, McDougle CJ, Swiezy NB, Erickson CA. A randomized, placebo-controlled trial of D-cycloserine for the enhancement of social skills training in autism spectrum disorders. Molecular Autism. 2016;7:1.
American_Psychiatric_Association. Diagnostic and statistical manual of mental disorders, four edition, text revision. Washington, D.C: American Psychiatric Association; 2000.
Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212.
Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, Fombonne E, Leboyer M, Minshew N. Diagnosing autism: analyses of data from the autism diagnostic interview. J Autism Dev Disord. 1997;27:501–17.
Roid G. Stanford-Binet intelligence scales for early childhood. 5th ed. 2005.
Sparrow S, Cicchetti D, Balla DA. Vineland adaptive behaviors scales. 2nd ed. 2008.
Constantino J, Gruber C. Social responsiveness scale. 2005.
Stone W, Ruble LA, Coonrod E, Hepburn S, Pennington M. TRIAD social skills assessment manual. Nashville: Treatment and Research Institute for Autism Spectrum Disorders; 2003.
Sprafkin J, Gadow KD. Child symptom inventory 4. 2002.
Pozdol SL, Sweizy NB, Stuart ML, Beach EB. Social skills training in children with ASD: a comparison of weekly groups and summer camp formats. Sandiego: 33rd Association for Behavioral Analysis International; 2007.
Tottenham N. Categorization of facial expressions in children and adults:establishing a larger stimulus set. In Cognitive Neuroscience Society Annual Meeting. . 2002.
Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59:809–16.
Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65:946–54.
Spezio ML, Adolphs R, Hurley RS, Piven J. Abnormal use of facial information in high-functioning autism. J Autism Dev Disord. 2007;37:929–39.
Hampel F, Ronchetti E, Rousseeuw P, Stahel W. Robust statistics: the approach based on influence functions. New York: John Wiley & Sons; 1986.
Wilcox RR, Tian TS. Measuring effect size: a robust heteroscedastic approach for two or more groups. J Appl Stat. 2011;38:1359–68.
Cohen J. Statistical power analysis for the behavioral sciences. 2 th ed. Hillsdale: Lawrence Earlbaum Associates; 1988.
Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, Reich W. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427–33.
Tse J, Strulovitch J, Tagalakis V, Meng L, Fombonne E. Social skills training for adolescents with Asperger syndrome and high-functioning autism. J Autism Dev Disord. 2007;37:1960–8.
Acknowledgements
We would like to acknowledge the contributions of Sarah Hurwitz PhD (Indiana University School of Medicine) and Stacie Pozdol MA (Indiana University School of Medicine), for the study design and group execution. We would like to thank Carrie McGinnis BS (Indiana University School of Medicine), Lyndsi Moser BS (Indiana University School of Medicine), Arlene Kohn MA (Indiana University School of Medicine), Lauren Mathieu-Frasier MA (Cincinnati Children’s Hospital Medical Center), Katherine Friedmann RN (Cincinnati Children’s Hospital Medical Center), Kaela O’Brien BS (Cincinnati Children’s Hospital Medical Center), and Sarah Fitzpatrick (Cincinnati Children’s Hospital Medical Center) for the assistance with the study coordination and data collection. We would additionally like to thank Jeremy Veenstra-Vanderweele (Columbia University Medical Center) and John Sweeny (University of Cincinnati College of Medicine) for the manuscript edits and guidance.
Funding
This study was funded by the US Department of Defense.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publically available due to ongoing analysis of portions of the data, but are available from the corresponding author on reasonable request.
Authors’ contributions
LW participated in acquisition of the data, drafting and revision of the manuscript, and approval of the final draft. NM, RS, MP, EP, and TS participated in acquisition of the data, revision of the manuscript, and approval of the final draft. DP, CM, and NS participated in conception and design of the study, revision of the manuscript, and approval of the final draft. PH and RA completed statistical analysis of the data, participated in drafting and revision of the manuscript, and approval of the final draft. CE takes responsibility for the integrity of the work as a whole, from inception to publication. CE had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. CE participated in acquisition of the data, drafting and revision of the manuscript, and approval of the final draft. All authors read and approved the final manuscript.
Competing interests
LW’s current research is supported by the Simons Research Foundation, Autism Speaks, Riovant Sciences Ltd, and Cures Within Reach, and LW has served as a past consultant for Otsuka. PH served as a consultant for inVentiHealth in 2014. EP receives research support from the Cincinnati Children’s Hospital Research Foundation. TS’s research is supported by Confluence Pharmaceuticals CE is a consultant to and holds equity in Confluence Pharmaceuticals and is a consultant to Neurotrope and Fulcrum, and is a past consultant to Alcobra Pharmaceuticals, the Roche Group, and Novartis. CE also holds non-related IP held by CCHMC and Indiana University. CE receives or has received research grant support from the John Merck Fund, Indiana University School of Medicine, Cincinnati Children’s Hospital Medical Center, Autism Speaks, the US Department of Defense, the Simons Foundation, the US Centers for Disease Control, the National Fragile X Foundation, The Roche Group, Neuren Pharmaceuticals, the National Institutes of Health, and Riovant Sciences Ltd. NM, RS, MP, DP, RA, NS, and CM have no conflicts to report.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Both the 10-week intervention and week 22 treatment durability analysis presented here were approved by the Indiana University School of Medicine Institutional Review Board and the Cincinnati Children’s Hospital Medical Center Institutional Review Board. Guardians of all participants provided written informed consent prior to study enrollment. Assent was obtained from enrolled youth when possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wink, L.K., Minshawi, N.F., Shaffer, R.C. et al. d-Cycloserine enhances durability of social skills training in autism spectrum disorder. Molecular Autism 8, 2 (2017). https://doi.org/10.1186/s13229-017-0116-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13229-017-0116-1