Abstract
Background
Angioedema with eosinophilia (AE) is a rare allergic disease classified as episodic or nonepisodic. AE is characterized by angioedema, urticaria, fever, weight gain, and eosinophilia, but its etiology and pathogenesis have not yet been clarified.
Case presentations
We present a 70-year-old woman presented with generalized edema and urticaria after Moderna COVID-19 vaccination. Peripheral blood eosinophil count was marked elevated and echocardiography and Doppler ultrasonography of both the upper and lower extremities were unremarkable. Her symptoms and peripheral blood eosinophil count were improved after systemic steroid therapy, but she failed to respond to steroid tapering. Reslizumab (anti-interluekin-5) was administered intravenously, and she remained symptom free with a normal eosinophil count during 8 months of reslizumab treatment without steroids.
Conclusions
We report a case of nonepisodic AE after COVID-19 vaccination that was successfully treated with reslizumab.
Similar content being viewed by others
Introduction
As of 24 November 2022, 13 billion doses of coronavirus disease 2019 (COVID-19) vaccine have been administered globally, and 68.5% of the world’s population has received at least one dose of vaccine in the era of the pandemic COVID-19 [1]. Various adverse reactions to vaccines, such as systemic, neurologic, cardiovascular, hematologic, and cutaneous reactions, have been reported [2]. Cutaneous reactions to COVID-19 vaccines are relatively mild and self-limited [3].
Angioedema with eosinophilia (AE) is a rare allergic disease but its etiology and pathogenesis have been unclear. We describe a case of AE after COVID-19 vaccination with steroid-dependency that was successfully treated with reslizumab, an anti-interluekin-5 (IL-5) antibody. Written informed consent was obtained from the patient.
Case
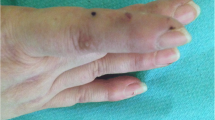
A 70-year-old woman presented with generalized edema and urticaria 5 days after the first Moderna vaccination (the 3rd dose of COVID-19 vaccine). She had a medical history of diabetes mellitus. There were no adverse reactions after the previous 1st and 2nd doses of Oxford-AstraZeneca vaccination. Edema firstly developed in the left upper arm at the injection site and then spread to both extremities and trunk, especially on the left side (Fig. 1A and B), and she gained 10 kg body weight. Urticaria was also observed in both the arms and legs.
Laboratory tests showed an elevated peripheral blood eosinophil count (3,312/µL), which increased to 18,125/µL. Serum total immunoglobulin (Ig) E (293 IU/mL) was slightly elevated, and IgM (122.4 mg/dL) was within the normal range. C1 esterase inhibitor, C1 inactivator activity, C4, anti-nuclear antibody, and stool analysis results were normal or negative. There were no genetic abnormalities, including JAK2, CALR, MPL, FIP1L1-PDGFR A, and BCR-ABL1. Echocardiography, chest computed tomography, abdominal ultrasonography, and Doppler ultrasonography of both the upper and lower extremities were unremarkable. Intravenous steroids (methylprednisolone 1 mg/kg/day) were administered to the patient for 7 days. Urticaria resolved rapidly, whereas angioedema improved gradually and disappeared completely (Fig. 1 C and D). Oral prednisolone was tapered off over 5 weeks. The blood eosinophil counts became normal. Seven days after steroid discontinuation, the blood eosinophil count increased (2,528/µL) and angioedema affecting the face and upper limbs recurred. Oral prednisolone was restarted and tapered from 30 to 5 mg/day over 4 weeks. On the 5th day of maintaining 5 mg/day of prednisolone, laryngeal edema developed, and the blood eosinophil count increased to 7.248/µL. Reslizumab 200 mg (3 mg/kg) was administered every 4 weeks along with prednisolone (30 mg/day for 7 days, 20 mg/day for the next 7 days) for the first 14 days. At the 3rd injection, reslizumab was tapered to 100 mg and maintained at the same dose for 4 weeks, and the 6th infusion was done at 8-week intervals. (Fig. 2). She remained symptom free with a normal eosinophil count during 8 months of reslizumab treatment without steroids.
Discussion
AE is classified as episodic (EAE) or nonepisodic (NEAE). EAE first described in 1976 [4] is characterized by recurrent episodes of angioedema, urticaria, fever, weight gain, elevated peripheral blood eosinophil counts, and elevated serum IgM levels [5]. NAEA has been mainly reported in Korea and Japan [6, 7], has a less severe clinical course than EAE, and is suggested to be a milder form of EAE [7,8,9]. NEAE is characterized by a single episode of edema of the extremities, arthralgia, eosinophilia, and normal IgM levels [8]. The pathogenesis of AE has not yet been clarified. Activated T-cell-derived cytokines, mainly IL-5, may be involved in the activation of blood and tissue eosinophils that drive AE [5, 10]. Serum IL-5 and total IgE concentrations were parallel with peripheral blood eosinophilia and clinical symptoms, and related with disease activity [11]. Eosinophilic degranulation releases proinflammatory cytokines, including IL-5, chemokines, and eosinophil-specific granules, such as major basic protein, eosinophil cationic protein, and eosinophil-derived neurotoxin, which are associated with inflammatory reactions and edema [12]. Another study suggested that multiple lineages other than eosinophils, including lymphocytes, neutrophils and mast cells, are involved in AE pathogenesis [13]. However, the triggers of these immunologic reactions, and whether these cells act or promote eosinophil activation, have not been elucidated. Systemic corticosteroids have been widely administered for the management AE owing to their suppressive effects on eosinophil and Th2 cytokines.
A wide range of adverse reactions to COVID-19 vaccines have been reported. Inflammatory cytokines, autoimmune involvement, angiotensin-converting enzyme 2 downregulation, and eosinophil association have been suggested to be related to post-vaccine adverse reactions [14]. Eosinophilic diseases after COVID-19 vaccination included eosinophilic myocarditis [15, 16], eosinophilic pneumonia [16,17,18], eosinophilic granulomatosis with polyangiitis [19], eosinophilic cellulitis [20, 21], eosinophilic panniculitis [22], eosinophilic gastroenteritis [23] and NEAE [24]. Eosinophil responses are observed not only during COVID-19 infection but also during coronavirus vaccination [25]. Whether eosinophils have a protective or exacerbating role during COVID-19 remains unclear. Eosinopenia was noted in patients with COVID-19, and it may be a prognostic factor for COVID-19 severity. A Th2-skewed immunopathology was observed, and isolated coronavirus spike protein caused eosinophilia and eosinophil infiltration in the lungs associated with a Th2 response in severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) murine vaccine studies [26,27,28]. The SARS-CoV-2 spike protein of COVID-19 can also lead to eosinophil activation owing to the sharing of high identity between SARS-CoV-1 and SARS-CoV-2 [25].
To date, only one case of AE after COVID-19 vaccination has been reported [24]. The patient developed NEAE after the 2nd dose of Pfizer-BioNTech vaccination and showed a good response to systemic steroids. The patient in this case developed NEAE after Moderna vaccination (previous 1st and 2nd Oxford-AstraZeneca vaccines). Many conditions can cause eosinophilia, and a comprehensive approach is needed for an accurate diagnosis. Moreover, the diagnostic criteria for AE is not yet well defined, it remains one of exclusion. Although it was not perfect, a thorough evaluation was conducted for this patient. She failed to respond to steroid tapering, but her symptoms and blood eosinophilia were well-controlled after reslizumab administration. Anti-IL-5 therapy is indicated for eosinophilic diseases, such as asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis, and blood eosinophil count is the best-established biomarker for the prediction of its efficacy [29]. Besides theses indications, bullous pemphigoid with eosinophilia [30] and NEAE [31] also showed a good response to reslizumab.
Conclusions
AE appears to have occurred after COVID-19 vaccination. Reslizumab would be a treatment option for patients with AE who are refractory to systemic steroid therapy.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Our World In Data. Coronavirus (COVID-19) vaccinations [Internet], 2022. https://ourworldindata.org/covid-vaccinations. Accessed 02 Dec 2022.
Beatty AL, Peyser ND, Butcher XE, Cocohoba JM, Lin F, Olgin JE, Pletcher MJ, Marcus GM. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4(12): e2140364.
Seirafianpour F, Pourriyahi H, Gholizadeh Mesgarha M, Pour Mohammad A, Shaka Z, Goodarzi A. A systematic review on mucocutaneous presentations after COVID-19 vaccination and expert recommendations about vaccination of important immune-mediated dermatologic disorders. Dermatol Ther. 2022;35(6): e15461.
Cooper BJ, Patterson R. Elevated IgM Levels, Edema, and Fatigue Syndrome. Arch Intern Med. 1976;136(12):1366–9.
Gleich GJ, Schroeter AL, Marcoux JP, Sachs MI, O’Connell EJ, Kohler PF. Episodic angioedema associated with eosinophilia. N Engl J Med. 1984;310(25):1621–6.
Nakachi S, Inokuma S. Eleven cases of angioedema with eosinophilia treated in a single hospital in Japan. Allergol Int. 2012;61(2):259–63.
Cho HJ, Yoo HS, Kim MA, Shin YS, Ye YM, Nahm DH, et al. Clinical characteristics of angioedema with eosinophilia. Allergy Asthma Immunol Res. 2014;6(4):362–5.
Chikama R, Hosokawa M, Miyazawa T, Miura R, Suzuki T, Tagami H. Nonepisodic angioedema associated with eosinophilia: report of 4 cases and review of 33 young female patients reported in Japan. Dermatology. 1998;197(4):321–5.
Matsuda M, Fushimi T, Nakamura A, Ikeda S. Nonepisodic angioedema with eosinophilia: a report of two cases and a review of the literature. Clin Rheumatol. 2006;25(3):422–5.
Butterfield JH, Leiferman KM, Abrams J, Silver JE, Bower J, Gonchoroff N, et al. Elevated serum levels of interleukin-5 in patients with the syndrome of episodic angioedema and eosinophilia. Blood. 1992;79(3):688–92.
Murakami T, Kato J, Kogawa K, Watanabe N, Sakamaki S, Kohgo Y, et al. Increased serum level of interleukin-5 in a patient with episodic angioedema and eosinophilia syndrome. Intern Med. 1993;2(4):343–5.
Fettrelet T, Gigon L, Karaulov A, Yousefi S, Simon HU. The Enigma of eosinophil degranulation. Int J Mol Sci. 2021;22(13):7091.
Khoury P, Herold J, Alpaugh A, Dinerman E, Holland-Thomas N, Stoddard J, et al. Episodic angioedema with eosinophilia (Gleich syndrome) is a multilineage cell cycling disorder. Haematologica. 2015;100(3):300–7.
Awaya T, Moroi M, Enomoto Y, Kunimasa T, Nakamura M. What Should We Do after the COVID-19 Vaccination? Vaccine-associated diseases and precautionary measures against adverse reactions. Vaccines (Basel). 2022;10(6):866.
Kounis NG, Mplani V, Koniari I, Velissaris D. Hypersensitivity myocarditis and COVID-19 vaccines. Kardiol Pol. 2022;80(1):109–10.
Takeda M, Ishio N, Shoji T, Mori N, Matsumoto M, Shikama N. Eosinophilic myocarditis following coronavirus disease 2019 (COVID-19) vaccination. Circ J. 2022;86(6):1020.
Barrio Piqueras M, Ezponda A, Felgueroso C, Urtasun C, Di Frisco IM, Larrache JC, et al. Acute Eosinophilic Pneumonia following mRNA COVID-19 vaccination: a case report. Arch Bronconeumol. 2021;S0300–2896(21):00379–83.
Miqdadi A, Herrag M. Acute Eosinophilic Pneumonia Associated With the Anti-COVID-19 Vaccine. Cureus. 2021;13(10):e1895.
Chan-Chung C, Ong CS, Chan LL, Tan EK. Eosinophilic granulomatosis with polyangiitis after COVID-19 vaccination. QJM. 2022;114(11):807–9.
Ikediobi O, Eichenfield DZ, Barrio VR. Eosinophilic cellulitis in response to BNT162b2 COVID-19 vaccination. Pediatr Dermatol. 2022. https://doi.org/10.1111/pde.15019.
de Montjoye L, Marot L, Baeck M. Eosinophilic cellulitis after BNT162b2 mRNA Covid-19 vaccine. J Eur Acad Dermatol Venereol. 2022;6(1):e26–8.
Kaikati J, Ghanem A, El Bahtimi R, Helou J, Tomb R. Eosinophilic panniculitis: a new side effect of Sinopharm COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2022;36(5):e337–9.
Lee JY, Lee JH. mRNA COVID-19 vaccine-associated subserosal Eosinophilic Gastroenteritis: a case report. J Korean Med Sci. 2022;37(30): e233.
Ishizuka K, Katayama K, Kaji Y, Tawara J, Ohira Y. Non-episodic angioedema with eosinophilia after BNT162b2 mRNA COVID-19 vaccination Non-episodic angioedema with eosinophilia after BNT162b2 mRNA COVID-19 vaccination. QJM. 2021;114(10):745–6.
Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146(1):1–7.
Tseng CT, Sbrana E, Iwata-Yoshikawa N, Newman PC, Garron T, Atmar RL, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE. 2012;7(4): e35421.
Iwata-Yoshikawa N, Uda A, Suzuki T, Tsunetsugu-Yokota Y, Sato Y, Morikawa S, et al. Effects of Toll-like receptor stimulation on eosinophilic infiltration in lungs of BALB/c mice immunized with UV-inactivated severe acute respiratory syndrome-related coronavirus vaccine. J Virol. 2014;88(15):8597–614.
Honda-Okubo Y, Barnard D, Ong CH, Peng BH, Tseng CT, Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol. 2015;89(6):2995–3007.
Nagase H, Ueki S, Fujieda S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol Int. 2020;69(2):178–86.
Rhyou HI, Han SH, Nam YH. Successful induction treatment of bullous pemphigoid using reslizumab: a case report. Allergy Asthma Clin Immunol. 2021;17(1):117.
Chu HA, Youn DA, Park HA, Ye YA, Park YA, Ban GA. Non-episodic Angioedema With Eosinophilia Successfully Treated With Reslizumab. Allergy Asthma Immunol Res. 2020;12(2):371–4.
Acknowledgements
Not applicable.
Funding
This work was supported by Dong-A University Research Fund.
Author information
Authors and Affiliations
Contributions
YN interview with patient, obtained consent, conceptualized and designed the study. YN wrote and reviewed the manuscript for intellectual content. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The work was written obeying the principles ethics committee.
Consent for publication
Patient consented to the publication of this case report. All authors consented to the publication.
Competing interests
None of the authors report potential conflicts of the interest with this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nam, YH. Nonepisodic angioedema with eosinophilia after COVID-19 vaccination: a case successfully treated with reslizumab. Allergy Asthma Clin Immunol 19, 11 (2023). https://doi.org/10.1186/s13223-023-00765-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13223-023-00765-8