Abstract
Purpose
Proteins are the principal biomolecules in bacteria that are affected by the oxidants produced by the phagocytic cells. Most of the protein damage is irreparable though few unfolded proteins and covalently modified amino acids can be repaired by chaperones and repair enzymes respectively. This study reviews the three protein repair enzymes, protein l-isoaspartyl O-methyl transferase (PIMT), peptidyl proline cis-trans isomerase (PPIase), and methionine sulfoxide reductase (MSR).
Methods
Published articles regarding protein repair enzymes were collected from Google Scholar and PubMed. The information obtained from the research articles was analyzed and categorized into general information about the enzyme, mechanism of action, and role played by the enzymes in bacteria. Special emphasis was given to the importance of these enzymes in Salmonella Typhimurium.
Results
Protein repair is the direct and energetically preferred way of replenishing the cellular protein pool without translational synthesis. Under the oxidative stress mounted by the host during the infection, protein repair becomes very crucial for the survival of the bacterial pathogens. Only a few covalent modifications of amino acids are reversible by the protein repair enzymes, and they are highly specific in activity. Deletion mutants of these enzymes in different bacteria revealed their importance in the virulence and oxidative stress survival.
Conclusion
PIMT repairs isoaspartate residues, PPiase catalyzes the conversion of cis-trans forms of proline residues, while MSR repairs oxidized methionine (Met) residues in the proteins. These repair enzymes maintain the activities of the target protein(s), thus aid in bacterial survival and virulence. The interventions which can interfere with this mechanism could be used for the development of novel therapeutics.
Similar content being viewed by others
Introduction
Phagocytes constitute a very important part of host innate immunity. After sensing microbial ligands, phagocytes produce various reactive oxygen species (ROS), reactive nitrogen species (RNS), antimicrobial peptides, chemokines, and cytokines with an overall goal to contain or kill the invaders and help other immune cells to generate adaptive immunity. Upon activation, NADPH oxidase (NOX) gets assembled on the phagosomal membrane and pumps the electrons from NADPH to oxygen by generating superoxide anions (O2−). O2− is then metabolized into a variety of other toxic ROS, like hydrogen peroxide (H2O2) and hydroxyl radicals (•OH) (Miller and Britigan, 1997). Further, myeloperoxidase catalyzes the production of highly toxic reactive chlorine species (RCS), hypochlorous acid (HOCl) from H2O2, and chloride ions (Fig. 1). It has been demonstrated that phagocyte-generated NADPH oxidase plays a very vital role in controlling bacterial pathogens, including Salmonella enterica serovar Typhimurium infection. Simultaneously, inducible nitric oxide synthase (iNOS) gets activated and catalyzes the oxidation of one of the guanidine nitrogens of l-arginine to generate nitric oxide (NO). NO gets autooxidized to produce other reactive nitrogen species like NO2•, N2O3, and S-nitrosothiols which are more reactive and have enhanced cytotoxic abilities (Fang, 1997). Further, the combination of NO and O2− forms peroxynitrite (ONOO−), which is one of the most potent RNS. Phagosomal oxidase and iNOS knockout mice were found to be more susceptible to Salmonella infection (Felmy et al. 2013). Almost all macromolecules, including DNA, RNA, lipids, and proteins, are susceptible to ROS- and RNS-mediated damage (Fig. 1).
Antioxidant defenses of Salmonella: Phagocytes produce an array of ROS/RNS which exert microbicidal effect by damaging macromolecules. Salmonella handles ROS/RNS in three ways. First, by injecting SPI2 effectors into the host phagosome, Salmonella inhibits NADPH oxidase assembly thus interfere with the O2− production. Second, various primary antioxidants of Salmonella directly inactivate ROS/RNS. Third, DNA and protein repair enzymes restore the functions of damaged DNA, and proteins
The genus Salmonella includes two species, Salmonella enterica and Salmonella bongori. Salmonella enterica is further divided into six subspecies I (enterica), II (salamae), IIIa (arizonae), IIIb (diarizonae), IV (houtenae), and VI (indica) (Le Minor L & Popoff MY 1987; Popoff et al. 1998). These subspecies are further classified into various serovars. Based on host preference and adaptability, the Salmonella enterica subspecies enterica are categorized into two serovars, i.e., typhoidal (includes S. Typhi and S. Paratyphi) and non-typhoidal (NTS, includes S. Enteritidis and S. Typhimurium) (Hohmann, 2001; Parry et al. 2002; Gal-Mor et al. 2012). Typhoidal Salmonellae are the causative agents of “enteric fever,” which is characterized by prolonged fever, fatigue, and severe gastroenteritis. Most pathogenic species of Salmonella which are the leading cause of foodborne gastroenteritis across the globe causing illness in humans belong to the species Salmonella enterica. Poultry serves as reservoir of Salmonellae and acts as major source of human infection (Bailey et al. 2002, Velge et al. 2005). Poultry meat and eggs have been implicated in the large outbreaks of foodborne Salmonellosis (Leach et al. 1999; Humphery, 2000). In 2015, WHO in its 10 year report of global burden of foodborne diseases identified Salmonellosis as the most common foodborne illness and estimated approximately 230,000 deaths in the USA alone due to non-typhoidal Salmonella enterica (NTS) (WHO, 2015). In the same year, the eggs contaminated with Salmonella spp. were associated with the highest number of foodborne outbreaks reported and were among the top five foodborne pathogen in terms of overall illness (EFSA and ECDC, 2016). Normally, NTS causes self-limiting, mild to moderate gastroenteritis in healthy adults. However, young, old, and immuno-compromised individuals are at a higher risk, wherein NTS causes severe gastroenteritis to invasive, extra-intestinal disease culminating in bacteremia and infections in multiple organs (Mandal and Brennand, 1988; Lê-Bury and Niedergang, 2018).
Salmonella can replicate in a variety of host cells. One important feature of this bacterium is its ability to infect and replicate inside the phagocytic cells. Recent studies suggest that Salmonella can survive and replicate in neutrophils, which are considered a dead-end for most bacterial pathogens (Geddes et al. 2007). Virulence of S. Typhimurium has been correlated with its survival in the phagocytic cells, whereas mutants that are unable to survive inside the macrophages are considered avirulent (Fields et al. 1986).
Salmonella handles oxidants in three ways. First, it injects Salmonella pathogenicity island 2 (SPI2) effectors into the host cell that interfere with the assembly of phagosomal oxidase, thus modulating the production of O2− (Vázquez-Torres et al. 2000; Gallois et al. 2001) and consequently other ROS and RNS. Second, the primary antioxidants of Salmonella directly detoxify the oxidant species. Third, macromolecular (DNA and protein) repair enzymes mend damaged DNA and proteins and restore their functions without de novo synthesis.
Among primary antioxidants, Salmonella encodes four superoxide dismutases (SodA, SodB, SodCI, SodCII), three catalases (KatE, KatG, KatN), and three peroxiredoxins (AhpC, TsaA, Tpx). SODs neutralize O2−; Kats scavenge H2O2; peroxiredoxins reduce organic hydroperoxides and H2O2 (Fang, 2011; Slauch, 2011; Aussel et al. 2011). AhpC and KatG degrade ONOO– (McLean et al. 2010; Henard and Vázquez-Torres, 2011) (Fig. 1). During the respiratory burst, the quantity of phagocyte-generated oxidants can be much higher than the scavenging capacity of primary antioxidants (SOD, catalases, peroxiredoxins, etc.) of Salmonella. Furthermore, microbial enzymes that can degrade host-generated toxic oxidants like .OH and HOCl which results in macromolecular damage are not known yet. Therefore, DNA and protein repair enzymes help Salmonella to cope with oxidative insult and ensure its propagation in the host.
Due to their abundance and reactivity, proteins are highly prone to oxidative damage. Two types of protein damage are known including covalent modifications to amino acids and changes in the secondary structure (Mahawar et al. 2011). Degradation of damaged proteins to amino acids followed by ribosomal synthesis is an obvious and well-studied way to replenish damaged proteins. On the other hand, protein repair is a rapid and energy-efficient approach to reactivate damaged proteins without de novo synthesis (Brot et al. 1981; Zhang and Weissbach 2008). Under stress conditions, when limited resources are available to the cell, the repair of vital protein(s) becomes indispensable for cellular survival (either the cell repairs them or it dies). Chaperones can refold unfolded proteins. However, even though various covalent modifications have been described (Hawkins et al. 2003), only three types of repair enzymes are known: (1) protein l-isoaspartyl O-methyltransferase (PIMT), (2) peptidyl prolyl cis-trans isomerase (PPIase), and (3) methionine sulfoxide reductase (MSR) which repair damaged aspartate or asparagine (isoaspartate), isomerized proline, and oxidized methionine residues respectively (Li and Clarke, 1992; Boschi-Muller et al. 2008; Ünal and Steinert, 2014).
Protein l-isoaspartyl O-methyl transferase
Aspartyl (Asp)/asparaginyl (Asn) residues in proteins spontaneously get converted into iso-aspartate (iso-Asp) as a part of normal post-translational modification which decides the half-life of the protein (Güttler et al. 2013). But under the condition of certain stresses, their rate of formation has been shown to accelerate. Iso-Asp formation leads to distortion of protein structure resulting in unfolding and aggregation of the proteins. Thus iso-Asp formation has been linked to compromised protein function (Kern et al. 2005; Shimizu et al. 2005; Dimitrijevic et al. 2014) which consequently affects cellular survival. Protein l-isoaspartyl O-methyl transferase (PIMT) (EC 2.1.1.77), a product of pcm gene in bacteria, methylates the α-carboxyl group on iso-Asp residues by using the methyl group of S-adenosyl-l-methionine (AdoMet), thus producing methyl esters. By repairing iso-Asp to Asp, PIMT restores the protein function(s) partially and thereby enhances cellular survival under stress conditions (Dimitrijevic et al. 2014).
Mechanism of PIMT-mediated repair
Asp/Asn residues in proteins, spontaneously or under stress, get converted into succinimide. The hydrolysis of succinimide yields isoAsp and Asp in a ratio of 3:1 (Vigneswara et al. 2006). The PIMT transfers methyl group from S-adenosyl methionine to isoAsp residues resulting in the formation of iso-aspartyl methyl esters which are unstable and rapidly hydrolyzed to form succinimide (Fig. 2). With multiple cycles of such reactions, the aspartyl residues can be salvaged and thereafter proteins regain their functions (DeVry and Clarke, 1999; Dimitrijevic et al. 2014).
Conversion of Asp to iso-Asp and their repair by PIMT: Under stress Asp or Asn residues in the proteins convert into succinimide. Succinimide spontaneously convert into normal Asp or abnormal iso-Asp residues in a ratio of 1:3. PIMT catalyzes conversion of iso-Asp residues into succinimide. Few cycles of PIMT-mediated repair converts all iso-Asp to Asp
Effect of pcm gene deletion on the survival of various organisms
The pcm gene knockout strain of E. coli (Li and Clarke, 1992; Visick et al. 1998; Hicks et al. 2005) showed hypersensitivity to oxidative, temperature, and other stresses. On the other hand, the PIMT overexpressing E. coli cells showed enhanced tolerance to oxidative and temperature stresses (Kindrachuk et al. 2003;Verma et al. 2010). The enhanced survival capabilities of PIMT overexpressing cells under temperature stress were shown to be due to the methyltransferase independent activities of PIMT (Kindrachuk et al. 2003). The structure crystallographic study of PIMT in E. coli has revealed the presence of 2 highly conserved Glu81 (E81) and Glu104 (E104) in the binding site of PIMT for AdoMet (Kindrachuk et al. 2003). The same study undertook the in situ mutagenesis of glutamine residues to alanine in PIMT of E.coli cells (E81A and E104A mutants) to analyze the specific effects of PIMT on cellular survival. It was observed that upon exposure to temperature stress, overexpressed wild and inactive PIMT (E81A) led to increased but comparable survival rates, while the E104A inactive mutant showed the highest cellular survival. Since, E104A inactive mutant had no methyltransferase activity, the study postulated a different role of PIMT in enhancing cellular survival other than its stereotypical role of enzymatic repair. Further, the western blot study revealed the overexpression of DnaK chaperone protein in E104A inactive mutant under temperature stress. Therefore, the study highlights the importance of a methyltransferase independent role of PIMT in increasing cellular survival through the induction of heat shock proteins. Thus, this study suggested methyl-transferase dependent as well as the independent role of PIMT in the survival of E. coli against temperature stress (Kindrachuk et al. 2003).
PIMT in S. Typhimurium
PIMT contributes to the resistance of S. Typhimurium against hydrogen peroxide, hypochlorous acid, and temperature stresses in vitro (Kumawat et al. 2016; Pesingi et al. 2017). The survival of Δpcm mutant strain inside interferon-γ (IFN-γ) stimulated macrophages was found to be 10-folds less as compared to the parent WT strain (p < 0.001), and it also showed attenuated virulence to mice (Kumawat et al. 2016). Further, the pcm gene is required for colonization in poultry cecum and dissemination to the spleen and liver (Pesingi et al. 2017). The expression of PIMT protein was about three-fold higher following exposure of S. Typhimurium at 42 °C (Pesingi et al. 2017). On the other hand, HOCl exposure induced PIMT by 1.5-fold (Kumawat et al. 2016), suggesting a greater role of this protein under thermal than oxidative stress.
Peptidyl proline cis-trans isomerase
Among the various known post-translational modifications of proteins, the dynamics of polypeptide chains can also be affected by catalytic activity of foldases. PPIases are a type of foldases that catalyze the isomerization between the cis and trans forms of peptide bonds, by the 180° rotation about the prolyl bond (Fig. 3) (Lin et al. 2019) and expedite the folding of nascent polypeptides as well as the refolding of unfolded and misfolded proteins (Compton, et al. 1992; Schmid et al. 1993). Due to steric hindrance exerted by side chains of amino acids, almost all peptide bonds exist as trans-conformers. However, in the case of proline, due to the formation of the pyrrolidine ring, the peptide bond can be present either in normal trans- or in cis-configuration. The presence of the abnormal proline-cis bond spontaneously affects folding, refolding, and protease-mediated degradation of the polypeptide chain and consequently influences protein function(s) (Brandts et al. 1975; Cook et al. 1979). PPIases are mostly found to be localized in the bacterial periplasm, inner membrane, and cytoplasm and are sometimes present in the supernatant (secreted) (Hayano et al. 1991; Kim et al. 2002; Söderberg and Cianciotto. 2008; Delpino et al. 2009). Localization in different compartments suggests a variety of differentially distributed targets for PPIases.
The PPIases can be grouped under one superfamily comprising three families of proteins, namely cyclophilins (Cyps)—PpiA, FK506-binding proteins (FKBPs)—FkpA, SylD, and parvulins like SurA (Ünal and Steinert, 2014). PpiA is localized in the periplasm. FKBP-type peptidyl-prolyl cis-trans isomerase (FkpA), a product of the fkpA gene, and cyclophilin PpiA catalyze the same isomerization reaction. Chaperone SurA encoded by the surA gene helps in the correct folding and assembly of outer membrane proteins. Structurally, SurA has an N-terminal region, two parvulin-like domains, and a C-terminal tail. The PPIase activity resides in one of the parvulin domain. The N-terminal region and the C-terminal tail are necessary and sufficient for the chaperone activity of SurA. This was demonstrated by a study in which a variant of SurA composed of only N-terminal region and the C-terminal tail (lacking the parvulin domains) exhibited chaperone activity in spite of lacking the PPIase parvulin domain (Behrens et al. 2001).
PPIase in bacterial virulence
PPIases are shown to be induced during the accumulation of misfolded proteins, heat and cold stresses, (Kandror and Goldberg, 1997; Söderberg and Cianciotto, 2008; Fasseas et al. 2012) and infection processes (Port and Freitag, 2007). The role of PPIase in bacterial virulence is mostly explained by its ability to facilitate proper folding of secreted proteins, adhesins, and other virulence factors (Hermans et al. 2006; Purdy et al. 2007; Alonzo and Freitag, 2010; Behrens-Kneip, 2010; Forster et al. 2011). In Streptococcus suis, Listeria monocytogenes, and Clostridioides difficile, PPIases are required for resistance against several stresses including thermal, oxidative, and acid stresses, thereby contributing to virulence in mice (Bigot et al. 2006; Wu et al. 2011; Ünal et al. 2018). PPIase gene deletion strains of E. coli and Yersinia pseudotuberculosis showed defective adherence to and invasion of host cells (Justice et al. 2006; Obi and Francis, 2013) and virulence in mice (Hermans et al. 2006; Cron et al. 2009). A recent study demonstrated the role of Legionella pneumophila PPIase in the infection of both Acanthamoeba castellanii and human macrophages (Rasch et al. 2018).
PPIase in Salmonella
The fkpA and surA genes are required for Salmonella survival during long-term carbon starvation and the cross-resistance of carbon-starved cells to acidic pH, high temperature, and antimicrobials (Kenyon et al. 2010). Deletion mutant strains of fkpA and surA genes were found to be defective in survival in epithelial cells and macrophages and showed attenuated virulence in mice (Horne et al. 1997; Sydenham et al. 2000; Humphreys et al. 2003). Cyclophilin A (CypA) is a eukaryotic protein belonging to the PPIase family. A recent study suggested the role of CypA in the membrane ruffling and internalization of S. Typhimurium into HeLa cells (Dhanda et al. 2018).
Methionine sulfoxide reductases
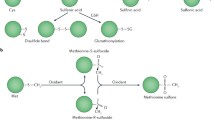
The sulfur-containing amino acids (Met and Cys) are highly prone to oxidation (Hawkins et al. 2003). Upon oxidation, Met residues convert into methionine sulfoxides (Met-SO) and further oxidation leads to the formation of methionine sulfones. MSRs reductively repair Met-SO to Met; however, sulfone repair enzymes are not yet known (Fig. 4). MSR-mediated repair of Met-SO plays two important roles in the cell. First, the MSR-mediated repair restores the functions of Met-rich oxidized proteins (Mahawar et al. 2011; Kuhns et al. 2013). Second, surface-exposed Met residues in proteins act as oxidant sinks. These surface-exposed Met residues get oxidized during the respiratory burst of host immune response and sop up excess oxidants, thus limiting damage to the cell until oxidized Met-SO gets repaired by MSRs (Abulimiti et al. 2003; Luo et al. 2009; Benoit and Maier, 2016; and Schmalstig et al. 2018).
Mechanism of MSR-mediated repair
Oxidation of sulfur in methionine forms either S or R epimers. According to localization, there are two types of MSRs, cytoplasmic and periplasmic (Boschi-Muller et al. 2008). MsrA and MsrB, which are present in cytoplasmic compartment repair S and R epimers of Met-SO, respectively. A third MSR was later discovered in the cytoplasm and named as MsrC. It is specific for free Met-R-SO and had been first described in E. coli (Lin et al. 2007) and then in S. Typhimurium (Denkel et al. 2011). MsrA reduces both free and protein-bound Met-S-SO whereas, MsrB reduces mainly protein-bound Met-R-SO with limited action on free Met-R-SO. All these MSRs repair Met-SO in thioredoxin -thioredoxin reductase manner where NADPH serves as an electron donor for the reduction process. The catalytic mechanism of MSR is a three step process involving three cysteine residues. In the first step, a nucleophilic Cys residue (CysA) attacks a Met-SO substrate, which leads to the formation of a sulfenic acid (–SOH) group on CysA and the release of reduced Met. In the second step, a nucleophilic Cys residue (CysB) attacks CysA–SOH, which leads to the formation of an intramolecular disulfide intermediate and the release of a water molecule. In the third step, the intramolecular disulfide intermediate is reduced by a Trx protein, and a catalytically active MSR enzyme is regenerated (Ezraty et al. 2017).
Periplasmic MSR (MsrP) has been discovered very recently in Gram negative bacteria. This new methionine sulfoxide reductase system, named MsrPQ, involves two proteins encoded in the same operon. MsrP, which carries out the reductase activity, is a periplasmic, soluble protein with a molybdenum atom in its active site. It was previously named YedY until its MSR activity was discovered (Loschi et al. 2004). To be functional in vivo, MsrP has to be specifically associated with MsrQ, an integral B-type heme-containing membrane-spanning protein, previously named YedZ (Drew et al. 2002). For the reduction reaction, MsrP receives electrons from MsrQ which in turn acquires electrons from quinones (Juillan-Binard et al. 2017).
Role of MSRs in bacterial virulence
The role of bacterial msr gene in combating various stresses within the host system has been well established. Further, its role in virulence also has been shown in many studies. An msrA gene deletion mutant of Mycoplasma genitalium exhibited hypersusceptibility to H2O2 in comparison to wild-type strain and showed decreased ability to colonize in hamster lungs (Dhandayuthapani et al. 2001). In certain bacteria, MsrA and MsrB activities are carried out by a single fused protein. In Neisseria gonorrhoeae, one such protein called PilB, which was earlier supposed to have a role in pilin gene expression, was found homologous to both MsrA and MsrB of E. coli (Skaar et al. 2002). PilB is found in both secreted and cytoplasmic form, but only the secreted form of PilB was involved in the oxidative stress survival of the bacteria which was evident from the increased sensitivity of ΔpilB null mutant and mutant overexpressing truncated form of PilB to hydrogen peroxide and superoxide compared to the wild-type strain (Skaar et al. 2002). ΔmsrAB double deletion mutant strain of Helicobacter pylori was shown to be defective in host colonization in vivo suggesting the role of MSR in pathogenesis and virulence of H. pylori (Alamuri and Maier, 2004). In Campylobacter jejuni, MsrA and MsrB proteins were found to protect against oxidative and nitrosative stress. Moreover, the ΔmsrAB double mutant strain of this bacteria showed a severe growth defect in in vitro media due to accumulation of Met-SO in proteins (Atack and Kelly, 2008). msrA and msrB genes were very essential for the protection of Enterococcus faecalis against H2O2 stress, and the deletion of these genes lead to attenuated virulence in mice model studies (Zhao et al. 2010). The msrA and B gene deletion mutant strain of E. coli was hypersusceptible to HOCl-mediated killing (Rosen et al. 2009). Mycobacterium tuberculosis lacking both MsrA and B were shown to be hypersusceptible to nitrite and HOCl stress (Lee et al. 2009). The msrA mutant strain of Staphylococcus aureus were sensitive to H2O2 stress in vitro and phagocytic cells. Further, this mutant was less adherent to human lung epithelial cells and showed reduced survival in mouse model (Singh et al. 2015, Singh et al. 2018). In Francisella tularensis, the msrB deletion mutant was shown to be defective in growth significantly in comparison to wild type in in vitro media. Further, this strain was hypersusceptible to H2O2 stress and showed decreased growth in macrophages and defective in colonization in mice (Saha et al. 2017) indicating that MsrB contributes to virulence.
MSRs in Salmonella
In S. Typhimurium, three cytoplasmic MSRs namely, MsrA, MsrB, and MsrC, have been reported. All the three are present in cytoplasmic compartment. MsrA repairs protein-bound as well as free Met-SO and is essential for the survival of this bacterium under oxidative stress (Denkel et al. 2011; Trivedi et al. 2015). MsrB repairs protein-bound Met-SO though it is dispensable for stress survival of this bacterium (Denkel et al. 2011). The msrA and msrC gene deletion strains are hypersensitive to phagocytic cells and showed attenuated virulence in mice and poultry (Denkel et al. 2011; Sarkhel et al. 2017). Salmonella also harbors MsrP, though the role of this protein in stress survival of most bacterial pathogens including Salmonella is not known.
Conclusions
Different stresses induce unfolding and covalent modifications in bacterial proteins that result in protein inactivation and consequently affecting cellular survival. Chaperones can refold unfolded proteins. Covalently modified amino acids are repaired by special enzymatic systems (PIMT, MSR, and PPIase). The overall goal of protein repair is to reactivate the damaged proteins without their de novo synthesis, and this process helps bacterial pathogens to overcome stress conditions, as those encountered during the infection (Fig. 5). The ways that can inhibit this process can pave the way to develop novel prophylactic and therapeutic agents against bacterial diseases.
Model: Different stresses cause unfolding and covalent modifications in polypeptide chain resulting in protein inactivation and consequently affect cellular survival. Chaperones can refold unfolded proteins. Protein repair enzymes (PIMT, MSR, and PPIase) can repair covalently modified amino acids. The repair of damaged proteins not only restores their functions but also enhance cellular survival under stress conditions
References
Abulimiti A, Qiu X, Chen J, Liu Y, Chang Z (2003) Reversible methionine sulfoxidation of Mycobacterium tuberculosis small heat shock protein Hsp16.3 and its possible role in scavenging oxidants. Biochem Biophys Res Commun 305:87–93
Alamuri P, Maier RJ (2004) Methionine sulphoxide reductase is an important antioxidant enzyme in the gastric pathogen Helicobacter pylori. Mol Microbiol 53:1397–1406
Alonzo F, Freitag NE (2010) Listeria monocytogenes PrsA2 is required for virulence factor secretion and bacterial viability within the host cell cytosol. Infect Immun 78:4944–4957
Atack JM, Kelly DJ (2008) Contribution of the stereospecific methionine sulphoxide reductases MsrA and MsrB to oxidative and nitrosative stress resistance in the food-borne pathogen Campylobacter jejuni. Microbiol 154:2219–2230
Aussel L, Zhao W, Hébrard M, Guilhon AA, Viala JPM, Henri S, Chasson L, Gorvel JP, Barras F, Méresse S (2011) Salmonella detoxifying enzymes are sufficient to cope with the host oxidative burst. Mol Microbiol 80:628–640
Bailey JS, Cox NA, Craven SE, Cosby DE (2002) Serotype tracking of Salmonella through integrated broiler chicken operations. J Fd Prot 65:742–745
Behrens S, Maier R, de Cock H, Schmid FX, Gross CA (2001) The SurA periplasmic PPIase lacking its parvulin domains functions in vivo and has chaperone activity. EMBO J 20(1-2):285–294
Behrens-Kneip S (2010) The role of SurA factor in outer membrane protein transport and virulence. Int J Med Microbiol 300:421–428
Benoit SL, Maier RJ (2016) Helicobacter catalase devoid of catalytic activity protects the bacterium against oxidative stress. J Biol Chem 291:23366–23373
Bigot A, Botton E, Dubail I, Charbit A (2006) A homolog of Bacillus subtilis trigger factor in Listeria monocytogenes is involved in stress tolerance and bacterial virulence. Appl Environ Microbiol 72:6623–6631
Boschi-Muller S, Gand A, Branlant G (2008) The methionine sulfoxide reductases: catalysis and substrate specificities. Arch Biochem Biophys 474:266–273
Brandts JF, Halvorson HR, Brennan M (1975) Consideration of the possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochem 14:4953–4963
Brot N, Weissbach L, Werth J, Weissbach H (1981) Enzymatic reduction of protein-bound methionine sulfoxide. Proc Natl Acad Sci 78(4):2155–2158
Compton LA, Davis JM, Macdonald JR, Bächinger HP (1992) Structural and functional characterization of Escherichia coli peptidyl-prolyl cis-trans isomerases. Eur J Biochem 206:927–934
Cook KH, Schmid FX, Baldwin RL (1979) Role of proline isomerization in folding of ribonuclease a at low temperatures. Proc Natl Acad Sci U S A 76:6157–6161
Cron LE, Bootsma HJ, Noske N, Burghout P, Hammerschmidt S, Hermans PWM (2009) Surface-associated lipoprotein PpmA of Streptococcus pneumoniae is involved in colonization in a strain-specific manner. Microbiol 155:2401–2410
Delpino MV, Comerci DJ, Wagner MA, Eschenbrenner M, Mujer CV, Ugalde RA, Fossati CA, Baldi PC, DelVecchio VG (2009) Differential composition of culture supernatants from wild-type Brucella abortus and its isogenic virB mutants. Arch Microbiol 191:571–581
Denkel LA, Horst SA, Rouf SF, Kitowski V, Böhm OM, Rhen M, Jäger T, Bange FC (2011) Methionine sulfoxide reductases are essential for virulence of Salmonella Typhimurium. PLoS One 6(11):e26974
DeVry CG, Clarke S (1999) Polymorphic forms of the protein L-isoaspartate (D-aspartate) O- methyltransferase involved in the repair of age-damaged proteins. J Hum Genet 44:275–288
Dhanda AS, Warren KE, Chiu RH, Guttman JA (2018) Cyclophilin a controls Salmonella internalization levels and is present at E. coli actin-rich pedestals. Anat Rec 2094:2086–2094
Dhandayuthapani S, Blaylock MW, Bebear CM, Rasmussen WG, Baseman JB (2001) Peptide methionine sulfoxide reductase (MsrA) is a virulence determinant in Mycoplasma genitalium. J Bacteriol 183:5645–5650
Dimitrijevic A, Qin Z, Aswad DW (2014) Isoaspartyl formation in creatine kinase B is associated with loss of enzymatic activity; implications for the linkage of isoaspartate accumulation and neurological dysfunction in the PIMT knockout mouse. PLoS One 9:1–8
Drew D, Sjöstrand D, Nilsson J, Urbig T, Chin C, de Gier J-W, von Heijne G (2002) Rapid topology mapping of Escherichia coli inner-membrane proteins by prediction and PhoA/GFP fusion analysis. Proc Natl Acad Sci 99:2690–2695
EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) (2016) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J 14: 4634–4865.
Ezraty B, Gennaris A, Barras F, Collet J-F (2017) Oxidative stress, protein damage and repair in bacteria. Nat Rev Microbiol 15:385–396
Fang FC (1997) Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest 99(12):2818–2825
Fang FC (2011) Antimicrobial actions of reactive oxygen species. MBio. 2(5)
Fasseas MK, Dimou M, Katinakis P (2012) The Caenorhabditis elegans parvulin gene subfamily and their expression under cold or heat stress along with the fkb subfamily. Biochem Biophys Res Commun 423:520–525
Felmy B, Songhet P, Slack EMC, Müller AJ, Kremer M, Van Maele L, Cayet D, Heikenwalder M, Sirard JC, Hardt WD (2013) NADPH oxidase deficient mice develop colitis and bacteremia upon infection with normally avirulent, TTSS-1- and TTSS-2-deficient Salmonella Typhimurium. PLoS One 8:1–10
Fields PI, Swanson R, Haidaris CG, Heffron F (1986) Mutants of Salmonella Typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci 83:5189–5193
Forster BM, Zemansky J, Portnoy DA, Marquis H (2011) Post-translocation chaperone PrsA2 regulates the maturation and secretion of Listeria monocytogenes proprotein virulence factors. J Bacteriol 193:5961–5970
Gallois A, Klein JR, Allen LAH, Jones BD, Nauseef WM (2001) Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J Immunol 166:5741–5748
Gal-Mor O, Suez J, Elhadad D, Porwollik S, Leshem E, Valinsky L, McClelland M, Schwartz E, Rahav G (2012) Molecular and cellular characterization of a Salmonella enterica serovar paratyphi a outbreak strain and the human immune response to infection. Clin Vaccine Immunol 19:146–156
Geddes K, Cruz F, Heffron (2007) Analysis of cells targeted by Salmonella type III secretion in vivo. PLoS Pathog 3: 2017-2028.
Güttler BHO, Cynis H, Seifert F, Ludwig HH, Porzel A, Schilling S (2013) A quantitative analysis of spontaneous isoaspartate formation from N-terminal asparaginyl and aspartyl residues. Amino Acids 44(4):1205–1214
Hawkins CL, Pattison DI, Davies MJ (2003) Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids 25:259–274
Hayano T, Takahashi N, Kato S, Maki N, Suzuki M (1991) Two distinct forms of peptidylprolyl-cis-trans-isomerase are expressed separately in periplasmic and cytoplasmic compartments of Escherichia coli cells. Biochem 30:3041–3048
Henard CA, Vázquez-Torres A (2011) Nitric oxide and Salmonella pathogenesis. Front Microbiol 2:1–11
Hermans PWM, Adrian PV, Albert C, Estevão S, Hoogenboezem T, Luijendijk IHT, Kamphausen T, Hammerschmidt S (2006) The streptococcal lipoprotein rotamase a (SlrA) is a functional peptidyl-prolyl isomerase involved in pneumococcal colonization. J BiolChem 281:968–976
Hicks WM, Kotlajich MV, Visick JE (2005) Recovery from long-term stationary phase and stress survival in Escherichia coli require the L-isoaspartyl protein carboxyl methyltransferase at alkaline pH. Microbiol 151:2151–2158
Hohmann EL (2001) Nontyphoidal salmonellosis. Clin Infect Dis 32(2):263–269
Horne SM, Kottom TJ, Nolan LK, Young KD (1997) Decreased intracellular survival of an fkpA mutant of Salmonella Typhimurium Copenhagen. Infect Immun 65:806–810
Humphrey T (2000) Public health aspects of Salmonella infection. In: Wray, C. and Wray, A. (eds.) Salmonella in domestic animals. CABI Publishing, U.K. pp. 245–264.
Humphreys S, Rowley G, Stevenson A, Kenyon WJ, Spector MP, Roberts M (2003) Role of periplasmic peptidylprolyl isomerases in Salmonella enterica serovar Typhimurium virulence. Infect Immun 71:5386–5388
Juillan-Binard C, Picciocchi A, Andrieu JP, Dupuy J, Petit-Hartlein I, Caux-Thang C, Vivès C, Nivière V, Fieschi F (2017) A two-component NADPH oxidase (NOX)-like system in bacteria is involved in the electron transfer chain to the methionine sulfoxide reductase MsrP. J Biol Chem 292:2485–2494
Justice SS, Lauer SR, Hultgren S, Hunstad DA (2006) Maturation of intracellular Escherichia coli communities requires SurA. Infect Immun 74:4793–4800
Kandror O, Goldberg AL (1997) Trigger factor is induced upon cold shock and enhances viability of Escherichia coli at low temperatures. Proc Natl AcadSci 94:4978–4981
Kenyon WJ, Humphreys S, Roberts M, Spector MP (2010) Periplasmic peptidyl-prolyl isomerases SurA and FkpA play an important role in the starvation-stress response (SSR) of Salmonella enterica serovar Typhimurium. Antonie Van Leeuwenhoek 98(1):51–63
Kern R, Malki A, Abdallah J, Liebart JC, Dubucs C, Yu MH, Richarme G (2005) Protein isoaspartate methyltransferase is a multicopy suppressor of protein aggregation in Escherichia coli. J Bacteriol 187:1377–1383
Kim N, Weeks DL, Shin JM, Scott DR, Young MK, Sachs G (2002) Proteins released by helicobacter pylori in vitro. J Bacteriol 184:6155–6162
Kindrachuk J, Parent J, Davies GF, Dinsmore M, Attah-Poku S, Napper S (2003) Overexpression of L-Isoaspartate O-methyltransferase in Escherichia coli increases heat shock survival by a mechanism independent of methyltransferase activity. J Biol Chem 278:50880–50886
Kuhns LG, Mahawar M, Sharp JS, Benoit S, Maier RJ (2013) Role of Helicobacter pylori methionine sulfoxide reductase in urease maturation. Biochem J 450:141–148
Kumawat M, Pesingi PK, Agarwal RK, Goswami TK, Mahawar M (2016) Contribution of protein isoaspartate methyl transferase (PIMT) in the survival of Salmonella Typhimurium under oxidative stress and virulence. Int J Med Microbiol 306:222–230
Le Minor L, Popoff MY (1987) Designation of salmonella enterica sp. nov. as the type and only species of the genus Salmonella. Int J Syst Bacteriol 37:465–468
Leach SA, Williams A, Davies AC, Wilson J, Marsh PD, Humphrey TJ (1999) Aerosol route enhances the contamination of intact eggs and muscle of experimentally infected laying hens by Salmonella Typhimurium DT104. FEMS Microbiol Lett 171:203–207
Lê-Bury G, Niedergang F (2018) Defective phagocytic properties of HIV-infected macrophages: how might they be implicated in the development of invasive Salmonella Typhimurium. Front Immunol 9:1–12
Lee WL, Gold B, Darby C, Brot N, Jiang X, De Carvalho LPS, Wellner D, St. John G, Jacobs WR, Nathan C (2009) Mycobacterium tuberculosis expresses methionine sulphoxide reductases a and B that protect from killing by nitrite and hypochlorite. Mol Microbiol 71:583–593
Li C, Clarke S (1992) A protein methyltransferase specific for altered aspartyl residues is important in Escherichia coli stationary-phase survival and heat-shock resistance. Proc Natl Acad Sci 89:9885–9889
Lin W, Bonin M, Boden A, Wieduwild R, Murawala P, Wermke M, Andrade H, Bornhäuser M, Zhang Y (2019) Peptidyl prolyl cis/trans isomerase activity on the cell surface correlates with extracellular matrix development. Commun biol 2(1):1–11
Lin Z, Johnson LC, Weissbach H, Brot N, Lively MO, Lowther WT (2007) Free methionine-(R)-sulfoxide reductase from Escherichia coli reveals a new GAF domain function. Proc Natl Acad Sci 104(23):9597–9602
Loschi L, Brokx SJ, Hills TL, Zhang G, Bertero MG, Lovering AL, Weiner JH, Strynadka NCJ (2004) Structural and biochemical identification of a novel bacterial oxidoreductase. J Biol Chem 279:50391–50400
Luo S, Levine RL (2009) Methionine in proteins defends against oxidative stress. FASEB J 23:464–472
Mahawar M, Tran V, Sharp JS, Maier RJ (2011) Synergistic roles of Helicobacter pylori methionine sulfoxide reductase and GroEL in repairing oxidant-damaged catalase. J Biol Chem 286:19159–19169
Mandal BK, Brennand J (1988) Bacteraemia in salmonellosis: a 15 year retrospective study from regional infectious diseases unit. Br Med J 297:1242–1243
McLean S, Bowman LAH, Poole RK (2010) KatG from Salmonella Typhimurium is a peroxynitritase. FEBS Lett. 584 1628–1632.
Miller RA, Britigan BE (1997) Role of oxidants in microbial pathophysiology. Clin Microbiol Rev 10:1–18
Obi IR, Francis MS (2013) Demarcating SurA activities required for outer membrane targeting of Yersinia pseudotuberculosis adhesins. Infect Immun 81:2296–2308
Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ (2002) Typhoid fever is a systemic infection with the bacterium. N Engl J Med 347:1770–1782
Pesingi PK, Kumawat M, Behera P, Dixit SK, Agarwal RK, Goswami TK, Mahawar M (2017) Protein-L-isoaspartyl methyltransferase (PIMT) is required for survival of Salmonella Typhimurium at 42 °C and contributes to the virulence in poultry. Front Microbiol 8:1–9
Popoff MY, Bockemühl J, Brenner FW (1998) Supplement 1997 (no. 41) to the Kauffmann-White scheme. Res Microbiol 149(8):601–604
Port GC, Freitag NE (2007) Identification of novel Listeria monocytogenes secreted virulence factors following mutational activation of the central virulence regulator, PrfA. Infect Immun 75:5886–5897
Purdy GE, Fisher CR, Payne SM (2007) IcsA surface presentation in Shigella flexneri requires the periplasmic chaperones DegP, Skp, and SurA. J Bacteriol 189:5566–5573
Rasch J, Ünal CM, Klages A, Karsli Ü, Heinsohn N, Brouwer RMHJ, Richter M, Dellmann A, Steinert M (2018) Peptidyl-prolyl- cis/trans-isomerases Mip and PpiB of Legionella pneumophila contribute to surface translocation, growth at suboptimal temperature, and infection. Infect Immun 87(1)
Rosen H, Klebanoff SJ, Wang Y, Brot N, Heinecke JW, Fu X (2009) Methionine oxidation contributes to bacterial killing by the myeloperoxidase system of neutrophils. Proc Natl Acad Sci 106:18686–18691
Saha SS, Hashino M, Suzuki J, Uda A, Watanabe K, Shimizu T, Watarai M (2017) Contribution of methionine sulfoxide reductase B (MsrB) to Francisella tularensis infection in mice. FEMS Microbiol Lett 364:1–7
Sarkhel R, Rajan P, Gupta AK, Kumawat M, Agarwal P, Shome A, Puii L, Mahawar M (2017) Methionine sulfoxide reductase a of Salmonella Typhimurium interacts with several proteins and abets in its colonization in the chicken. Biochem Biophys Acta - Gen Subj 1861:3238–3245
Schmalstig AA, Benoit SL, Misra SK, Sharp JS, Maier RJ (2018) Non catalytic antioxidant role for Helicobacter pylori urease. J Bacteriol 200(17):e00124–e00118
Schmid FX, Mayr LM, Mucke M, Schonbrunner ER (1993) Prolyl isomerases: role in protein folding. Adv Protein Chem 44:25–66
Shimizu T, Matsuoka Y, Shirasawa T (2005) Biological significance of isoaspartate and its repair system. Biol Pharm Bull 28:1590–1596
Singh V, Singh K, Baum K (2018) The role of methionine sulfoxide reductases in oxidative stress tolerance and virulence of Staphylococcus aureus and other bacteria. Antioxidants 7:128
Singh VK, Vaish M, Johansson TR, Baum KR, Ring RP, Singh S, Shukla SK, Moskovitz J (2015) Significance of four methionine sulfoxide reductases in Staphylococcus aureus. PLoS One 10:1–20
Skaar EP, Tobiason DM, Quick J, Judd RC, Weissbach H, Etienne F, Brot N, Seifert HS (2002) The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc Natl Acad Sci 99:10108–10113
Slauch JM (2011) How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol Microbiol 80:580–583
Söderberg MA, Cianciotto NP (2008) A Legionella pneumophila peptidyl-prolyl cis-trans isomerase present in culture supernatants is necessary for optimal growth at low temperatures. Appl Environ Microbiol 74:1634–1638
Sydenham M, Douce G, Bowe F, Ahmed S, Chatfield S, Dougan G (2000) Salmonella enterica serovar Typhimurium surA mutants are attenuated and effective live oral vaccines. Infect Immun 68:1109–1115
Trivedi RN, Agarwal P, Kumawat M, Pesingi PK, Gupta VK, Goswami TK, Mahawar M (2015) Methionine sulfoxide reductase a (MsrA) contributes to Salmonella Typhimurium survival against oxidative attack of neutrophils. Immunobiol 220:1322–1327
Ünal CM, Berges M, Smit N, Schiene-Fischer C, Priebe C, Strowig T, Jahn D, Steinert M (2018) PrsA2 (CD630_35000) of Clostridioides difficile is an active parvulin-type PPIase and a virulence modulator. Front Microbiol 9:2913
Unal CM, Steinert M (2014) Microbial peptidyl-prolyl cis/trans isomerases (PPIases): virulence factors and potential alternative drug targets. Microbiol Mol Biol Rev 78:544–571
Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM, Dinauer MC, Mastroeni P, Fang FC (2000) Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Sci 287:1655–1658
Velge P, Cloeckaert A, Barrow P (2005) Emergence of Salmonella epidemics: the problems related to Salmonella enterica serotype Enteritidis and multiple antibiotic resistance in other major serotypes. Vet Res 36:267–288
Verma P, Singh A, Kaur H, Majee M (2010) Protein l-isoaspartyl methyltransferase1 (CaPIMT1) from chickpea mitigates oxidative stress-induced growth inhibition of Escherichia coli. Planta 231:329–336
Vigneswara V, Lowenson JD, Powell CD, Thakur M, Bailey K, Clarke S, Ray DE, Carter WG (2006) Proteomic identification of novel substrates of a protein isoaspartyl methyltransferase repair enzyme. J Biol Chem 281:32619–32629
Visick JE, Cai HUI, Clarke S (1998) The L-isoaspartyl protein repair methyltransferase enhances survival of aging Escherichia coli subjected to secondary environmental stresses. J Bacteriol 180:2623–2629
WHO (2015) WHO estimates of the global burden of foodborne diseases. Foodborne Dis Burden Epidemiol Reference group:2007
Wu T, Zhao Z, Zhang L, Ma H, Lu K, Ren W, Liu Z, Chang H, Bei W, Qiu Y, Chen H (2011) Trigger factor of Streptococcus suis is involved in stress tolerance and virulence. Microb Pathog 51(1-2):69–76
Zhang XH, Weissbach H (2008) Origin and evolution of the protein-repairing enzymes methionine sulphoxide reductases. Biol Rev 83(3):249–257
Zhao C, Hartke A, La Sorda M, Posteraro B, Laplace J, Auffray Y, Sanguinetti M (2010) Role of methionine sulfoxide reductases a and B of Enterococcus faecalis in oxidative stress and virulence. Infect Immun 78:3889–3897
Acknowledgements
The authors are thankful to Indian Council of Agricultural Research, India, Department of Biotechnology, Govt. of India, and the Director, ICAR- Indian Veterinary Research Institute, Izatnagar, India.
Funding
National Agricultural Sustainable Fund, Indian Council of Agricultural Research, India and Department of Biotechnology, Govt. of India.
Author information
Authors and Affiliations
Contributions
AS and MM both conceptualized the idea and wrote the review article along with the valuable inputs from all the other authors. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research does not contain any studies with human participants or animals.
Consent for publication
Informed consent is not applicable in this work.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shome, A., Sarkhel, R., Apoorva, S. et al. Role of protein repair enzymes in oxidative stress survival and virulence of Salmonella. Ann Microbiol 70, 55 (2020). https://doi.org/10.1186/s13213-020-01597-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13213-020-01597-2