Abstract
Background
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that severely affects cognitive functions and social behaviors, leading to a significant decline in an individual’s quality of life. Auditory processing deficits often precede the clinical symptoms of AD, prompting interest in auditory-based interventions as potential treatments. This scoping review aimed to compile the existing evidence on active and passive auditory-based interventions for individuals with AD and its prodromal stages.
Method and results
This scoping review followed Arksey and O’Malley's five-step framework to identify the existing evidence on auditory-based interventions for AD. Four databases (PubMed, Web of Science, CINAHL, and Embase) were used to search for studies on auditory stimulation techniques to treat cognitive decline in AD patients. In total, 14 studies were included in the analysis. Seven studies explored active auditory stimulation techniques, such as the Brain Fitness Program (BrainHQ), aiming to improve cognitive function in individuals with Mild Cognitive Impairment (MCI). The other seven studies focused on passive auditory stimulation, often combined with other sensory stimuli such as light or tactile inputs. Passive stimulation studies have focused mainly on Gamma Entrainment Using Sensory Stimulation (GENUS). The intervention frequency and duration varied across studies, ranging from one session lasting 8 h to a year. Both active and passive auditory stimulation showed potential for enhancing cognitive function in individuals with AD.
Conclusion
The literature suggests that auditory stimulation may positively influence cortical wiring and enhance cognitive abilities. Multimodal interventions that combine auditory stimulation with other sensory or behavioural approaches could yield more substantial effects on global cognition. However, the study design, intervention characteristics and outcome measures varied across studies, underscoring the necessity for standardised reporting. Well-designed studies using standard cognitive assessment protocols are recommended.
Similar content being viewed by others
Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder that gradually affects an individual's thinking and sociobehavioural skills, significantly reducing their quality of life [1]. Neural degeneration in AD occurs in three stages, with the prodromal stage, i.e., mild cognitive impairment (MCI), primarily impacting the basal regions of the frontal and temporal lobes. Deeper brain areas become increasingly affected in the later stages as the disease progresses [2, 3]. The temporal lobe plays a significant role in auditory signal processing and is implicated in this degenerative process. Previous studies have suggested that auditory processing (AP) deficits precede the clinical symptoms of AD by at least 10 years [4,5,6,7].
Behavioural interventions such as cognitive training, physical exercise, lifestyle modifications, meditation, mindfulness, and psychomotor stimulation are commonly used in managing AD, especially in its prodromal stages [8,9,10]. Processing complex auditory signals often requires greater cognitive skills; hence, auditory stimulation is also suggested as a rehabilitation method for improving cognition [11]. Studies on healthy older adults suggest that auditory training can improve cognitive function, particularly attention and working memory [12, 13]. However, the potential benefits of auditory training have not been much explored in individuals with prodromal AD.
In a conventional auditory training regimen, active participation is typically required of individuals. Recent studies have revealed that passive auditory and visual stimulation at a specific frequency (such as 40 Hz) may induce changes in cortical wiring and enhance cognitive abilities [14]. Monteiro et al. (2021) reviewed studies investigating the motor and cognitive effects of multimodal sensory stimulation in people with cognitive decline or AD [15]. It specifically focused on passive auditory stimulation, and at the time, there were only three human studies on passive auditory stimulation in people with AD [16,17,18].
Literature suggests that even active auditory stimulation is effective in enhancing cognitive function for individuals with prodromal AD [4]. The addition of recent research and growing interest in this area led us to undertake a comprehensive literature review that included both active and passive auditory training. This review summarised the current state of knowledge on auditory-based interventions, both active and passive, in individuals with AD or its prodromal stages. The objectives were to identify the types of auditory stimulation used, the outcome measures used, and the effects of such stimulation on cognitive function.
Methodology
To map the different types of auditory stimulation, this scoping review used the five-step framework of Arksey and O’Malley [19]. The five steps include a) formulating the research question, b) searching for literature, c) selecting eligible studies, d) data charting, and e) collating, summarising, and analysing the data. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews Checklist (PRISMA-ScR) guidelines [20] were followed to perform the scoping review. The review protocol was developed and registered on the Open Science Framework (OSF), which can be accessed at https://doi.org/https://doi.org/10.17605/OSF.IO/89YZ2.
Step 1: identifying the research question
The review team used the Population, Intervention, and Outcome (PIO) framework to construct the research question (Table 1): What are the existing auditory stimulation techniques used to treat cognition in individuals with Alzheimer's disease and its prodromal stages?
Step 2: searching for literature
A comprehensive search strategy was developed after discussion with subject experts (HP, KG) and consideration of relevant recent reviews. The possible literature sources were identified by searching the following databases prior to the search date of July 10, 2023: PubMed (NCBI), Web of Science (Clarivate), CINAHL (EBSCO), and Embase (Elsevier). The search was conducted by one of the authors (DSP) using the search terms "Alzheimer’s Disease", "Cognitive Dysfunction", "Cognitive Impairment", "Auditory Rehabilitation", "Auditory Training", "Sound Stimulation", "Gamma Entrainment", "Cognition", "Working Memory", and "Attention", integrating MESH terms where applicable. These keywords were combined using Boolean operators to develop the search strategy (see Appendix 1).
Step 3: selecting eligible studies
All the identified citations were collected and imported to Covidence systematic review software (accessible at www.covidence.org), with duplicates subsequently removed. Two reviewers conducted a two-stage article review process to mitigate bias or errors. Initially, reviewers (LT, KG) independently screened titles and abstracts, excluding articles that did not meet the criteria. Any disagreements were resolved by a third reviewer (HP). In the second stage, both reviewers examined the full texts, with conflicts resolved by a third reviewer.
Step 4: charting the data
A predetermined data charting format was used for data extraction by one of the reviewers (LT). Relevant data on country/region, study design, sample characteristics, recruitment site, auditory stimulation techniques, intervention characteristics (duration and frequency), and outcome measures used were extracted. The extracted data were cross-verified by the review team (KG and HP).
Step 5: collecting, summarizing, and reporting results
The findings are summarized in a narrative way aided by tables where appropriate. The results include details on the study characteristics (study setting and design), participant characteristics (study sample, age range), intervention characteristics (type, equipment used, duration and frequency) and outcome measures.
Results
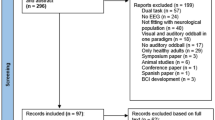
The search yielded a total of 3,879 articles. After removing duplicates (n = 882), conducting title and abstract screening (n = 2,984), and performing full-text screening (n = 43), fourteen articles were ultimately included in the analysis. The PRISMA flow diagram below reports the reasons for excluding articles (see Fig. 1).
Characteristics of the included studies
Study settings
Six of the fourteen studies were conducted in the United States, three in Greece, two each in Canada and China, and one in Brazil.
Study designs
The included studies were published between 2009 and 2022. The research designs of these studies included eight randomized controlled trials [16, 18, 21,22,23,24,25,26], four quasi-experimental studies [17, 27,28,29], a case series [30] and a case report [31].
Participant characteristics
Participants were predominantly recruited from clinic or hospital settings and/or through services for community-dwelling older people. Participants in the study were aged between 59 and 90 years, with diagnoses of either AD or MCI across both sexes and no specific sex ratio requirement. Several different diagnostic criteria have been used to identify cognitive impairment in participants. To recruit individuals with MCI, most studies followed the diagnostic criteria of Peterson et al. published in 1999 [32] [18, 23, 26,27,28,29]. Barnes et al. (2009) [21] followed the recommendations of an international consensus committee [33], and Lee et al. (2017) [25] used the Montreal Cognitive Assessment (MoCA) [34]. A few studies recruited individuals who were already diagnosed with AD/MCI and were receiving medication [17, 22, 24, 30, 31]. Clements-Cortes et al. (2016) [16] followed the clinical standards published by the National Institute on Aging and Alzheimer’s Association (NIA-AA) [35].
Intervention characteristics
Two categories of auditory stimulation interventions existed in the included studies: active auditory stimulation (7 studies) and passive auditory stimulation (7 studies).
Active auditory stimulation
This review identified seven studies that used active auditory stimulation intending to improve cognitive function in individuals with MCI. Active auditory stimulation included the use of computerized software called the Brain Fitness Program (BrainHQ by Posit Science) (as shown in Table 2). Four studies used the BrainHQ for auditory stimulation [21, 23, 25, 26]. This brain fitness program comprises various exercises targeted to enhance the speed and accuracy of AP. The exercises included time order judgment, syllable discrimination, and adaptive recognition of verbal instructions. The other three studies in this category used the BrainHQ along with physical exercise for research conducted in Greece, which was named long-lasting memories (LLM) [27,28,29]. LLM involves a computerized game-based physical exercise designed particularly for older adults, along with BrainHQ.
Passive auditory stimulation
Seven studies utilized passive auditory stimulation. However, only one of them employed solely acoustic stimulation, while the remaining six studies used acoustic stimulation in combination with other sensory stimuli, including light, tactile, and transcranial alternating current stimulation (tACS) (Table 3). Papalambros et al. (2019) [18] employed a phase-locked loop (PLL), a method used in neuroscience to deliver acoustic stimulation in synchrony with brain waves [36]. This involved using EEG to monitor the natural slow-wave oscillations of individuals with MCI during nonrapid eye movement sleep (NREM) stages. Next, brief pink noise pulses were generated in synchrony with specific phases of the recorded brain waves. The pink noise pulses were transmitted through headphones at the most comfortable level (MCL) so as not to disturb the participants' sleep. This acoustic stimulation delivered over one night enhanced slow-wave activity and, in turn, memory recall in individuals with aMCI.
Six articles employed passive auditory stimulation along with other sensory or noninvasive stimulation. Among them, three studies used light stimulation in combination with auditory stimulation. Chan et al. (2022) [22] utilized the Gamma Entrainment Using Sensory Stimulation (GENUS) device from the Picower Institute (https://picower.mit.edu/innovations-inventions/genus), and He et al. (2021) [24] employed the Gamma Sense Stimulation system from Cognito Therapeutics (https://cognitotx.com/). Calomeni et al. (2017) [17] investigated the synergistic effects of light and binaural beats using the Brain Wave Synthesizer named SIRIUS by Mind Place Center, Canada. The study employed a multimodal approach, sequentially combining visual and auditory stimulation with binaural beats and working memory training. However, specific details regarding stimulation parameters and duration were not provided.
Additionally, two studies combined tactile stimulation with auditory stimulation. In the study by Clements-Cortes et al. (2016) [16], participants with AD were randomized into two groups using a crossover design, with a wash-out period between sessions. The sessions comprised either 30 min of 40 Hz rhythmic sensory stimulation (RSS) or visual stimulation. The NextWave chair delivered the RSS via 40 Hz sinusoidal sound waves, providing vibrotactile stimulation across the body. Following a washout period, participants underwent visual stimulation while seated on the NextWave chair. The chair remained inactive, prompting them to engage with visual stimuli such as ocean waves and nature images on a television screen. Another study by Clements-Cortes and Bartel (2022) [30] detailed the experiences of three participants (two with MCI and one with AD) and their caregivers following multisensory gamma stimulation. The intervention involved auditory stimulation with isochronous sound at 40 Hz and tactile stimulation at 40 Hz, delivered through the Sound Oasis VTS 1000.
Finally, Liu et al. (2022) [31] combined tACS at gamma frequency (40 Hz) and sound stimulation simultaneously. The sound stimulation at 40 Hz was delivered through two sponge earbuds placed in the patient’s ears and synchronized with tACS. The tACS was administered using two electrodes positioned at the left dorsolateral prefrontal cortex (F3) and the contralateral supraorbital area (F4).
Intervention frequency and duration
The duration and frequency of stimulation varied across studies, ranging from a minimum of one session lasting 8 h to 30-min sessions conducted over a year (3 to 5 days per week). The most prevalent approach for active and passive stimulation involved one-hour sessions held 3 to 5 days per week for 8 weeks.
Outcome measures
Objective measures, such as EEG and/or functional magnetic resonance imaging (fMRI), were utilised in three studies [22, 24], one study employed auditory event-related potentials (ERPs) [18]. Other behavioural outcome measures included MMSE, MoCA, the Dementia Rating Scale (DRS), the Trail Making Test (TMT) A & B, the Digit Span Test (DST), the National Institutes of Health (NIH) Toolbox Cognition Battery, Saint Louis University Mental Status (SLUMS) and several behavioural and neuropsychological tests.
Effect on cognitive function
Overall, the findings indicate that active auditory training had a positive impact on cognitive function. Several studies have reported improvements in overall cognition [26, 27], as well as in specific cognitive domains such as delayed memory [21], spatial span test [25, 26], CVLT-II [27], TMT [27], and DST [17, 25, 27]. However, Chandler et al. (2017) [23] reported no improvements in any cognitive measures among participants in a brain fitness program.
Passive auditory stimulations, on the other hand, resulted in improvements in cognitive measures such as ADAS-Cog, MMSE, MoCA, AVLT [37], DST [17], face-name association task [22] and SLUMS score [16, 30]. A study by Papalambros et al. (2019) did not show significant improvement in cognitive tests used, such as the verbal paired association test and NIHTB [18].
Studies by Klados et al. (2016) [28] and He et al. (2021) [24] did not directly assess cognition using neuropsychological or behavioural tests; instead, they employed electrophysiological measures such as ERPs, resting-state EEG, and/or fMRI. Results of active intervention using resting-state EEG indicated heightened EEG band activity, particularly in the beta band [28] and the theta band [29]. In addition, fMRI revealed enhanced functional connectivity in the default mode network (DMN) following passive stimulation of light and sound [24].
Discussion
This scoping review aimed to synthesize the existing evidence on auditory-based interventions for individuals with AD and its prodromal stages. This review identified two primary categories of auditory interventions: active auditory stimulation and passive auditory stimulation. A significant proportion of the included studies adopted a combined modality approach, integrating auditory stimulation with other sensory or behavioural interventions.
Auditory stimulation
There are various ways to modulate the neurons, one of which is through auditory stimulation [38]. Studies have shown that passive auditory stimulation can significantly change brain function [39]. This is because auditory stimulation can potentially alter neuronal plasticity by increasing the levels of certain neurotransmitters [40]. These improvements could be due to increased phase locking of cortical neurons (even outside the auditory cortex) in response to external stimuli [41]. Furthermore, a study demonstrated the effectiveness of targeted auditory stimulation in modulating slow-wave activity (SWA), a phenomenon crucial for memory consolidation during the nonrapid eye movement (NREM) stage of sleep [18]. Reduced SWA is associated with age-related memory decline [42]. By presenting the SWA frequency through a transducer during this NREM stage of sleep, it is believed that the SWA can be increased, and memory can be improved.
Both passive and active auditory stimulation can result in neuroplastic changes [38]. In the hearing field, traditional auditory training methods focus on active auditory stimulation, requiring active participation from individuals [43]. BrainHQ software encompasses several sets of exercises with different elements to improve cognition, one of which is an auditory module designed to improve speed and accuracy in AP. Auditory processing deficits commonly precede the clinical symptoms of AD [7], and training using the BrainHQ has demonstrated efficacy in enhancing various cognitive skills, including attention, working memory, and language abilities [44]. Generally, active auditory training has resulted in medium to large cognitive enhancement effects in individuals with MCI.
Multimodal stimulation
Cognitive processes are closely interconnected with various physiological and neural systems. Interventions addressing multiple components and mechanisms through a multimodal approach may yield more substantial effects on global cognition [45]. This review identified studies combining physical exercise with active auditory stimulation, i.e., using BrainHQ software (Auditory component). Exercise promotes synaptic plasticity and neurogenesis by increasing the levels of growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1) [46]. Additionally, physical exercise can increase hippocampal size and decrease amyloid deposition, contributing to improved cognitive functions [46, 47]. This review also identified studies that have explored the application of passive auditory stimulation in conjunction with light or other sensory inputs. These studies target sensory entrainment processes, aiming to synchronize neural network rhythms with external stimuli, potentially modulating brain oscillations and altering memory functions [48, 49]. Studies have focused on 40 Hz stimulation, as the results from preclinical studies demonstrated the ability of stimulation at this frequency to reduce the accumulation of β-amyloid plaques, a hallmark feature of AD, in animal models [50, 51]. Recent research aimed to understand the neurobiological mechanisms of sensory entrainment and optimize its therapeutic effectiveness for Alzheimer's disease and related neurodegenerative conditions [52, 53].
Study design
Studies utilising active auditory stimulation predominantly adopted stronger study designs, with four RCTs [21, 23, 25, 26] and three pre- and post-experimental designs [27,28,29]. Passive auditory stimulation studies included case reports [31], case series [30], a few RCTs [16, 18, 22, 24] and a pre-post experimental design [17]. It is noticed that research methodologies employed for passive auditory stimulation with two studies being case reports and case series. To strengthen support for the effectiveness of passive auditory stimulation, further research, with a strong study design and adequate sample, is needed.
Outcome measures
The review revealed a range of outcome measures employed in the identified studies, including objective measures such as EEG and fMRI, along with various behavioural assessments. The lack of consistency in these measures, coupled with variations in intervention duration, poses a challenge in determining the most effective protocols and poses a challenge for future meta-analyses. Standardisation of outcome measures and intervention protocols would facilitate more robust comparisons and meta-analyses, ultimately advancing our understanding of the potential benefits of auditory interventions in individuals with AD. Additionally, considering the baseline/premorbid abilities of participants, such as their variability in AP abilities, could enhance the precision and efficacy of interventions.
Strengths and limitations
The strengths of this review are that we conducted a comprehensive literature search encompassing different databases over a wide period of time and were able to identify studies utilising a range of auditory stimulation techniques. However, a limitation is the inclusion of only English language studies. Additionally, the authors did not appraise the level of evidence or examine bias in the included studies, as the aim was to identify and synthesise the current evidence to gain an overview of the topic as a basis for future studies.
Conclusion
Active interventions show potential for improving cognitive function, while passive interventions, especially when combined with other sensory inputs, have the potential to modulate brain oscillations and impact memory functions. To ensure reliable results, it is important to have strong study designs coupled with standardised intervention protocols and outcome measures.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Kumar A, Sidhu J, Goyal A, Tsao JW, Doerr C. Alzheimer Disease (Nursing). StatPearls [Internet]. 2024 Jan.
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189–a006189. https://doi.org/10.1101/cshperspect.a006189.
Deture MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegen. 2019;14:1–18. https://doi.org/10.1186/S13024-019-0333-5.
Swords GM, Nguyen LT, Mudar RA, Llano DA. Auditory system dysfunction in Alzheimer disease and its prodromal states: a review. Ageing Res Rev. 2018;44:49. https://doi.org/10.1016/J.ARR.2018.04.001.
Tarawneh HY, Menegola HK, Peou A, Tarawneh H, Jayakody DMP. Central auditory functions of Alzheimer’s disease and its preclinical stages: a systematic review and meta-analysis. Cells. 2022;11:1007. https://doi.org/10.3390/CELLS11061007.
Haggstrom J, Hederstierna C, Rosenhall U, Ostberg P, Idrizbegovic E. Prognostic value of a test of central auditory function in conversion from mild cognitive impairment to dementia. Audiol Neurotol. 2020;25:276–82. https://doi.org/10.1159/000506621.
Gates GA, Beiser A, Rees TS, D’Agostino RB, Wolf PA. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J Am Geriatr Soc. 2002;50:482–8. https://doi.org/10.1046/J.1532-5415.2002.50114.X.
Fischer CE, Churchill N, Leggieri M, Vuong V, Tau M, Fornazzari LR, et al. Long-known music exposure effects on brain imaging and cognition in early-stage cognitive decline: a pilot study. J Alzheimers Dis. 2021;84:819–33. https://doi.org/10.3233/JAD-210610.
Levy SA, Smith G, De Wit L, DeFeis B, Ying G, Amofa P, et al. Behavioral interventions in Mild Cognitive Impairment (MCI): lessons from a multicomponent program. Neurotherapeutics. 2022;19:117–31. https://doi.org/10.1007/s13311-022-01225-8.
van der Steen JT, Smaling HJA, van der Wouden JC, Bruinsma MS, Scholten RJPM, Vink AC. Music-based therapeutic interventions for people with dementia. Cochrane Database Syst Rev. 2018;7:CD003477. https://doi.org/10.1002/14651858.CD003477.pub4.
Ferguson M, Henshaw H. How does auditory training work? Joined-Up Thinking Listening Semin Hear. 2015;36:237–49. https://doi.org/10.1055/S-0035-1564456.
Ferguson MA, Henshaw H. Auditory training can improve working memory, attention, and communication in adverse conditions for adults with hearing loss. Front Psychol. 2015;6:556. https://doi.org/10.3389/fpsyg.2015.00556.
Kawata NYS, Nouchi R, Oba K, Matsuzaki Y, Kawashima R. Auditory cognitive training improves brain plasticity in healthy older adults: evidence from a randomized controlled trial. Front Aging Neurosci. 2022;14:826672. https://doi.org/10.3389/fnagi.2022.826672.
Smith BC, D’Amico M. Sensory-based interventions for adults with dementia and Alzheimer’s Disease: a scoping review. Occup Ther Health Care. 2019;34:171–201. https://doi.org/10.1080/07380577.2019.1608488.
Monteiro F, Sotiropoulos I, Carvalho Ó, Sousa N, Silva FS. Multi-mechanical waves against Alzheimer’s disease pathology: a systematic review. Transl Neurodegener. 2021;10:1–28. https://doi.org/10.1186/S40035-021-00256-Z.
Clements-Cortes A, Ahonen H, Evans M, Freedman M, Bartel L. Short-term effects of rhythmic sensory stimulation in alzheimer’s disease: an exploratory pilot study. J Alzheimer’s Dis. 2016;52:651–60. https://doi.org/10.3233/JAD-160081.
Calomeni MR, Furtado da Silva V, Velasques BB, Feijó OG, Bittencourt JM, Ribeiro de Souza e Silva AP. Modulatory Effect of Association of Brain Stimulation by Light and Binaural Beats in Specific Brain Waves. Clin Pract Epidemiol Mental Health. 2017;13:134–44. https://doi.org/10.2174/1745017901713010134.
Papalambros NA, Weintraub S, Chen T, Grimaldi D, Santostasi G, Paller KA, et al. Acoustic enhancement of sleep slow oscillations in mild cognitive impairment. Ann Clin Transl Neurol. 2019;6:1191–201. https://doi.org/10.1002/acn3.796.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. https://doi.org/10.1080/1364557032000119616.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73. https://doi.org/10.7326/M18-0850.
Barnes DE, Yaffe K, Belfor N, Jagust WJ, DeCarli C, Reed BR, et al. Computer-based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Dis Assoc Disord. 2009;23:205–10. https://doi.org/10.1097/WAD.0B013E31819C6137.
Chan D, Suk HJ, Jackson BL, Milman NP, Stark D, Klerman EB, et al. Gamma frequency sensory stimulation in mild probable Alzheimer’s dementia patients: results of feasibility and pilot studies. PLoS One. 2022;17:e0278412. https://doi.org/10.1371/journal.pone.0278412.
Chandler MJ, Locke DEC, Duncan NL, Hanna SM, Cuc AV, Fields JA, et al. Computer versus compensatory calendar training in individuals with mild cognitive impairment: functional impact in a pilot study. Brain Sci. 2017;7:112. https://doi.org/10.3390/brainsci7090112.
He Q, Colon-Motas KM, Pybus AF, Piendel L, Seppa JK, Walker ML, et al. A feasibility trial of gamma sensory flicker for patients with prodromal Alzheimer’s disease. Alzheimer’s Dementia. 2021;7:e12178. https://doi.org/10.1002/trc2.12178.
Lee T, Chan FHW, Chu LW, Kwok TCY, Lam LCW, Tam HMK, et al. Auditory-based cognitive training programme for attention and memory in older people at risk of progressive cognitive decline: a randomised controlled trial. 2017.
Rosen AC, Sugiura L, Kramer JH, Whitfield-Gabrieli S, Gabrieli JD. Cognitive training changes hippocampal function in mild cognitive impairment: a pilot study. J Alzheimer’s Dis. 2011;26:349–57. https://doi.org/10.3233/JAD-2011-0009.
Bamidis PD, Fissler P, Papageorgiou SG, Zilidou V, Konstantinidis EI, Billis AS, et al. Gains in cognition through combined cognitive and physical training: the role of training dosage and severity of neurocognitive disorder. Front Aging Neurosci. 2015;7:152. https://doi.org/10.3389/fnagi.2015.00152.
Klados MA, Styliadis C, Frantzidis CA, Paraskevopoulos E, Bamidis PD. Beta-band functional connectivity is reorganized in mild cognitive impairment after combined computerized physical and cognitive training. Front Neurosci. 2016;10:55. https://doi.org/10.3389/fnins.2016.00055.
Styliadis C, Kartsidis P, Paraskevopoulos E, Ioannides AA, Bamidis PD. Neuroplastic effects of combined computerized physical and cognitive training in elderly individuals at risk for dementia: an eLORETA controlled study on resting states. Neural Plast. 2015;2015:172192. https://doi.org/10.1155/2015/172192.
Clements-Cortes A, Bartel L. Long-term multi-sensory gamma stimulation of dementia patients: a case series report. Int J Environ Res Public Health. 2022;19:15553. https://doi.org/10.3390/ijerph192315553.
Liu Y, Tang C, Wei K, Liu D, Tang K, Chen M, et al. Transcranial alternating current stimulation combined with sound stimulation improves the cognitive function of patients with Alzheimer’s disease: a case report and literature review. Front Neurol. 2022;13:1068175. https://doi.org/10.3389/FNEUR.2022.962684.
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. https://doi.org/10.1001/ARCHNEUR.56.3.303.
Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment – beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J Intern Med. 2004;256:240–6. https://doi.org/10.1111/J.1365-2796.2004.01380.X.
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. https://doi.org/10.1111/J.1532-5415.2005.53221.X.
Alzheimer’s Disease Diagnostic Guidelines. NIH National Institute on Aging. 2011.
Santostasi G, Malkani R, Riedner B, Bellesi M, Tononi G, Paller KA, et al. Phase-locked loop for precisely timed acoustic stimulation during sleep. J Neurosci Methods. 2016;259:101–14. https://doi.org/10.1016/J.JNEUMETH.2015.11.007.
Liu Y, Liu S, Tang C, Tang K, Liu D, Chen M, et al. Transcranial alternating current stimulation combined with sound stimulation improves cognitive function in patients with Alzheimer’s disease: Study protocol for a randomized controlled trial. Front Aging Neurosci. 2023;14:1068175. https://doi.org/10.3389/fnagi.2022.1068175.
Willott JF. Physiological plasticity in the auditory system and its possible relevance to hearing aid use, deprivation effects, and acclimatization. Ear Hear. 1996;17:66S-77S. https://doi.org/10.1097/00003446-199617031-00007.
Horwitz A, Klemp M, Horwitz H, Thomsen MD, Rostrup E, Mortensen EL, et al. Brain responses to passive sensory stimulation correlate with intelligence. Front Aging Neurosci. 2019;10:201. https://doi.org/10.3389/FNAGI.2019.00201/FULL.
Anderson S, White-Schwoch T, Parbery-Clark A, Kraus N. Reversal of age-related neural timing delays with training. Proc Natl Acad Sci U S A. 2013;110:4357–62. https://doi.org/10.1073/PNAS.1213555110.
Tonti E, Budini M, Vingolo EM. Visuo-acoustic stimulation’s role in synaptic plasticity: a review of the literature. Int J Mol Sci. 2021;22:10783. https://doi.org/10.3390/IJMS221910783.
Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, et al. Prefrontal atrophy, disrupted NREM slow waves, and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357. https://doi.org/10.1038/NN.3324.
Bronus K, El RA, Pryce H. Auditory training and adult rehabilitation: a critical review of the evidence. Glob J Health Sci. 2011;3:p49. https://doi.org/10.5539/GJHS.V3N1P49.
Sardone R, Battista P, Donghia R, Lozupone M, Tortelli R, Guerra V, et al. Age-related central auditory processing disorder, mci, and dementia in an older population of Southern Italy. Otolaryngol - Head Neck Surg (United States). 2020;163:348–55. https://doi.org/10.1177/0194599820913635/ASSET/IMAGES/LARGE/10.1177_0194599820913635-FIG1.JPEG.
Tsolaki AC, Tsolaki M, Pandria N, Lazarou E, Gkatzima O, Zilidou V, et al. Web-based intervention effects on mild cognitive impairment based on apolipoprotein e genotype: quasi-experimental study. J Med Internet Res. 2020;22:e14617. https://doi.org/10.2196/14617.
Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. https://doi.org/10.1016/S0166-2236(02)02143-4.
Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–22. https://doi.org/10.1073/PNAS.1015950108/-/DCSUPPLEMENTAL.
Hanslmayr S, Axmacher N, Inman CS. Modulating human memory via entrainment of brain oscillations. Trends Neurosci. 2019;42:485–99. https://doi.org/10.1016/J.TINS.2019.04.004.
Sameiro-Barbosa CM, Geiser E. Sensory entrainment mechanisms in auditory perception: neural synchronization cortico-striatal activation. Front Neurosci. 2016;10:361. https://doi.org/10.3389/FNINS.2016.00361.
Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540:230–5. https://doi.org/10.1038/nature20587.
Singer AC, Martorell AJ, Douglas JM, Abdurrob F, Attokaren MK, Tipton J, et al. Noninvasive 40-Hz light flicker to recruit microglia and reduce amyloid beta load. Nature Protocols. 2018;13:1850–68. https://doi.org/10.1038/s41596-018-0021-x.
Ning S, Jorfi M, Patel SR, Kim DY, Tanzi RE. Neurotechnological approaches to the diagnosis and treatment of Alzheimer’s disease. Front Neurosci. 2022;16:854992. https://doi.org/10.3389/FNINS.2022.854992/FULL.
Sahu PP, Tseng P. Gamma sensory entrainment for cognitive improvement in neurodegenerative diseases: opportunities and challenges ahead. Front Integr Neurosci. 2023;17:1146687. https://doi.org/10.3389/FNINT.2023.1146687.
Acknowledgements
Not Applicable.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal
Author information
Authors and Affiliations
Contributions
L.T., K.G., H.P. and S.P. conceptualized the proposal and formulated the research question. L.T. and D.S.P. created a search strategy and conducted a thorough search. L.T. and K.G. reviewed the titles and abstracts independently, and in case of any conflicts, HP resolved them. The same process was followed for the full-text screening. L.T. wrote the initial version of the paper, and all authors collaborated to revise and finalize the draft to its current form. The final version was approved by all the authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Thamizhmani, L., Ganapathy, K., Palaniswamy, H.P. et al. Efficacy of acoustic stimulation techniques on cognitive functions in individuals with Alzheimer’s disease—a scoping review. Alz Res Therapy 16, 174 (2024). https://doi.org/10.1186/s13195-024-01544-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-024-01544-2