Abstract
Background
Although blood-based biomarkers have been identified as cost-effective and scalable alternatives to PET and CSF markers of neurodegenerative disease, little is known about how these biomarkers predict future brain atrophy and cognitive decline in cognitively unimpaired individuals. Using data from the Baltimore Longitudinal Study of Aging (BLSA), we examined whether plasma biomarkers of Alzheimer’s disease (AD) pathology (amyloid-β [Aβ42/40], phosphorylated tau [pTau-181]), astrogliosis (glial fibrillary acidic protein [GFAP]), and neuronal injury (neurofilament light chain [NfL]) were associated with longitudinal brain volume loss and cognitive decline. Additionally, we determined whether sex, APOEε4 status, and plasma amyloid-β status modified these associations.
Methods
Plasma biomarkers were measured using Quanterix SIMOA assays. Regional brain volumes were measured by 3T MRI, and a battery of neuropsychological tests assessed five cognitive domains. Linear mixed effects models adjusted for demographic factors, kidney function, and intracranial volume (MRI analyses) were completed to relate baseline plasma biomarkers to baseline and longitudinal brain volume and cognitive performance.
Results
Brain volume analyses included 622 participants (mean age ± SD: 70.9 ± 10.2) with an average of 3.3 MRI scans over 4.7 years. Cognitive performance analyses included 674 participants (mean age ± SD: 71.2 ± 10.0) with an average of 3.9 cognitive assessments over 5.7 years. Higher baseline pTau-181 was associated with steeper declines in total gray matter volume and steeper regional declines in several medial temporal regions, whereas higher baseline GFAP was associated with greater longitudinal increases in ventricular volume. Baseline Aβ42/40 and NfL levels were not associated with changes in brain volume. Lower baseline Aβ42/40 (higher Aβ burden) was associated with a faster decline in verbal memory and visuospatial performance, whereas higher baseline GFAP was associated with a faster decline in verbal fluency. Results were generally consistent across sex and APOEε4 status. However, the associations of higher pTau-181 with increasing ventricular volume and memory declines were significantly stronger among individuals with higher Aβ burden, as was the association of higher GFAP with memory decline.
Conclusions
Among cognitively unimpaired older adults, plasma biomarkers of AD pathology (pTau-181) and astrogliosis (GFAP), but not neuronal injury (NfL), serve as markers of future brain atrophy and cognitive decline.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is a debilitating neurodegenerative condition characterized by the presence of extracellular amyloid-β (Aβ) plaques, intracellular tau-containing neurofibrillary tangles (NFTs), neurodegeneration, and cognitive impairment [1, 2]. Traditionally, hallmarks of AD have been measured in vivo using positron emission tomography (PET) imaging or cerebral spinal fluid (CSF) measurement. Biomarkers of AD pathology as well as non-specific markers of neurodegeneration are now measurable in blood using ultrasensitive assays, providing a cost-effective, scalable, and minimally invasive alternative to PET and CSF measurement. Several studies demonstrate that plasma biomarkers of AD pathology (e.g., Aβ42/40, phosphorylated tau [pTau-181]), astrogliosis (glial fibrillary acidic protein [GFAP]), and to a lesser extent, neuronal injury (neurofilament light chain [NfL]) relate to – and can be used to predict – cortical Aβ and tau burden [3,4,5,6]. Further, plasma pTau-181, GFAP and NfL differentiate between AD, mild cognitive impairment, and control participants [7,8,9], and plasma pTau-181 and GFAP increase over time in preclinical AD [10]. Although plasma Aβ42/40 predicts Aβ-PET positivity [11, 12], its utility for differentiating clinically-defined disease stages is comparatively limited [13, 14]. These blood biomarkers are poised to play an important role in dementia research and clinical practice and are already being used to screen participants and as secondary endpoints in clinical trials for AD [15]. While these blood biomarkers have been extensively characterized in recent years, the prognostic significance of these measures among cognitively normal older adults remains largely unknown.
Prior cross-sectional studies have shown that that higher NfL [7, 16], GFAP [5, 17], pTau-181 [18], and lower Aβ42/40 [5] in blood were associated with smaller brain volume in regions vulnerable to AD (e.g., hippocampus, entorhinal cortex), as well as white matter volume [19]. Additionally, higher blood levels of NfL and GFAP [7, 17] and longitudinal increases blood levels of NfL [16, 20, 21] and pTau-181 [18] have been associated with decreased volume in AD-vulnerable brain regions.
Although previous studies have shown that lower Aβ42/40 and higher ptau-181 [18, 22], NfL [7, 17], and GFAP [17] in blood are associated with increased rate of cognitive decline [23], the vast majority of studies connecting these blood biomarkers to cognitive function have been cross-sectional [7, 17, 19, 24]. Accordingly, the extent to which these measures relate to future brain volume loss and cognitive decline in a cognitively normal community sample is not well understood. Additionally, whether demographic, genetic, and disease staging factors modify these associations is unknown. To address these questions, we used data from the Baltimore Longitudinal Study of Aging (BLSA) to examine the association of Aβ42/40, pTau-181, GFAP, and NfL with baseline and longitudinal measures of regional brain volume and domain-specific cognition. We further sought to determine whether sex, APOEε4 carrier status, and plasma amyloid status modified the association between plasma biomarkers and longitudinal brain volume and cognition.
Methods
Participants
The current study used data from the BLSA, an ongoing community-based longitudinal study of physical and psychological aging [25]. Recruitment and enrollment for the BLSA have been previously described [26, 27]. BLSA study visits occur every four years for participants aged < 60 years, every two years for participants aged 60–79 years, and every year for participants aged ≥ 80 years. At each visit participants receive a clinical and physiological assessment, health and lifestyle questionnaires, and a comprehensive cognitive exam. BLSA participants received 3T MRI scans at each visit (beginning in 2008) as part of the Neuroimaging Substudy. Blood samples were collected at each participant’s first 3T MRI visit, and for a set of participants, during the first PET visit as a part of a separate protocol. We selected the first biomarker visit (on and after age 50 at the time of collection) that also had a concurrent 3T MRI scan as the baseline for brain volume analyses. For the cognition sample, we selected the first biomarker visit (on and after age 50 at the time of collection) that had a concurrent cognitive assessment as the baseline for the cognition analyses. Participants were included if they were cognitively unimpaired at baseline, had 3T MRI data or neuropsychological data, were at least 50 years old, and did not have neurological health conditions (e.g., stroke, seizures, previous brain surgery) or MRI contraindications (Fig. 1). Mild cognitive impairment (MCI) and dementia classification were determined by consensus diagnostic conference, as described previously [28]. Briefly, a participant’s cognitive status was adjudicated at consensus diagnostic conference if their score on the Blessed Information-Memory-Concentration Test [29] score was ≥ 4, if their self or informant Clinical Dementia Rating (CDR) scale [30] was ≥ 0.5, and/or if concerns were raised about their cognition by study staff. Impairment was determined using selected longitudinal neuropsychological assessment and CDR scores. Cognitive tests are listed elsewhere [28]. Mild cognitive impairment was diagnosed using the Petersen criteria: 1) when cognitive impairment was present for a single domain (typically memory), or 2) when cognitive impairment occurred without significant functional loss in activities of daily living [31]. Diagnoses of dementia are consistent with the Diagnostic and Statistical Manual, third edition, revised. [32]
Study design and participant selection. Participants completed 3T MRI beginning in 2008, and the standardized cognitive battery beginning in 2005. The majority of plasma biomarker measurements occurred in 2009; however, some participant samples were as early as 2005. For MRI analyses, the first plasma biomarker visit for participants on or after age 50 at the time of collection, that also had a concurrent 3T MRI scan was selected as the baseline visit for brain volume analyses. Similarly, for cognition analyses, the first plasma biomarker visit for participants on or after age 50 at the time of collection, that also had a concurrent cognitive assessment was selected as the baseline visit for cognition analyses. Fig.created with BioRender.com
MRI
Participants in the BLSA Neuroimaging Substudy completed T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) scans (TR = 6.8 ms, TE = 3.2 ms, flip angle = 8◦, image matrix = 256 × 256 × 170, voxel size = 1.0 × 1.0 × 1.2 mm3) on a 3 T (Philips Achieva) over an average of 4.7 years (Table 1). Preprocessing procedures have been described elsewhere [27]. Anatomical regions of interest (ROIs) were segmented using the Multi-atlas Region Segmentation Utilizing Ensembles (MUSE) anatomic labeling method to create voxelwise tissue maps reflecting local volumes (RAVENS maps) [33, 34]. MUSE ROI labels were used for the primary analysis in the current study. ROIs for the present study included total brain, gray matter, and white matter volumes, ventricular volume, superior, middle, and inferior temporal gyrus volume, as well as parahippocampal gyrus, hippocampal, entorhinal cortex, and amygdala volumes. Whole brain RAVENS gray matter maps (smoothed using a 2 mm Gaussian filter) were used for secondary voxel-based morphometry (VBM) analyses. VBM analyses were completed using AFNI 16.3.13, FSL 5.0.6, and MatLab 2018b.
Neuropsychological assessment
Cognitive performance was assessed using composite scores across five cognitive domains: attention, verbal fluency, visuospatial skills, verbal memory, and executive functioning. Scores from individual cognitive measures were z-scored using the baseline mean and standard deviation and averaged to create each domain composite score. The attention composite was comprised of scores from Trail Making Test Part A (total seconds)35 and the Digit Span Forward subtest (total score) from the Wechsler Adult Intelligence Scale-Revised [36]. The verbal fluency composite included both letter (F,A,S) and category fluency (fruits, animals, vegetables), which each consisted of total words recalled in 60-s. The visuospatial composite included a modified version of the Educational Testing Service Card Rotations Test (total score = total number correct – total number incorrect) [37]. The verbal memory composite was created using immediate recall (sum of 5 trials) and long-delay free recall from the California Verbal Learning Test-I [38]. Long delay free recall included a 20-min delay. The executive function composite included Trail Making Test Part B (total seconds)35 and the Digit Span Backward subtest (total score) from the Wechsler Adult Intelligence Scale-Revised [36]. Prior to computing the composites, Trails scores (in seconds) were inverted so higher scores reflect better performance.
Plasma biomarkers
Aβ40, Aβ42, GFAP, NfL and pTau-181 concentrations were measured using the Single Molecule Array (Simoa®) Neurology 4-Plex E (N4PE) and pTau-181 (V2) assays on the Simoa HD-X instrument (Quanterix™ Corporation). Assays were run in duplicate, and the values were averaged. Intra-assay coefficients of variation (CVs) for Aβ40, Aβ42, GFAP, NfL and pTau-181 were 1.5%, 1.0%, 4.9%, 4.8%, and 4.4%, respectively, while inter-assay CVs were 5.2%, 5.9%, 8.1%, 7.8%, and 13.1%, respectively. Outliers were defined as 5 standard deviation outside the mean, and were excluded. Aβ42/40 status (low/high) was determined using a cutoff value (0.05259) derived from an ROC analysis to determine the optimal threshold (Youden’s Index) for predicting amyloid (11C-Pittsburgh compound-B [PiB]) PET positivity in a subsample of participants (n = 212) from the present study. All BLSA (Neuroimaging Substudy) participants with available PiB PET data (n = 212) were used for the analysis. Participants from the BLSA were eligible to undergo PiB PET imaging if they were not diagnosed with CNS disease, severe cardiac (myocardial infarction, coronary artery disease requiring angioplasty or coronary artery bypass surgery) or pulmonary disease, and metastatic cancer [39]. Full details of PET acquisition and preprocessing have been described elsewhere [40]. The cutoff to determine PiB status was a mean cortical distribution volume ratio (DVR) of 1.064 derived from a Gaussian mixture model. Participants with DVR > 1.064 were considered PiB positive, while those below were considered PiB negative.
Genotyping
APOEε4 carrier status was determined by either polymerase chain reaction (PCR) with restriction isotyping using the Type IIP enzyme Hhai [41] or the Taqman method [42]. Participants were classified as ε4 non-carrier (0 ε4 alleles), or carrier (1–2 ε4 alleles).
Covariates
Baseline age, sex (male/female), race (white/non-white), and education level (total years) were defined based on participant self-report. Estimated glomerular filtration rate (eGFR)-creatinine, used as a proxy for kidney function, was defined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) criteria [43, 44]. Total intracranial volume (ICV) at age 70 was computed using methods previously described [45] and included as a covariate in ROI and VBM analyses.
Statistical analyses
Separate linear mixed effects (LME) models were used to examine the associations of baseline values of each plasma biomarker of AD pathology and neurodegeneration (predictor variables) with baseline and longitudinal change in brain volumes and cognitive performance (outcome variables). Each model adjusted for baseline age, sex, race, education, and eGFR, as well as the interaction of each covariate with time (of follow up) to adjust for longitudinal effects of confounders. Random effects included intercept and time with an unstructured covariance matrix. Analyses of brain volumes also adjusted for intracranial volume defined at age 70. Plasma biomarkers and brain volumes were standardized (mean = 0 and standard deviation = 1). Secondary analyses examined the modifying effect of sex, APOEε4 status, and Aβ42/40 status on the association of plasma biomarkers with brain volume and cognitive trajectories by including additional two-way (modifier*biomarker) and three-way (biomarker*modifier*time) interactions in models. Modifier-specific estimates were derived within the same model using linear combinations of relevant beta coefficients. Additional sensitivity analyses were performed without eGFR as a covariate even though kidney function has been previously shown to impact plasma biomarker concentration [46,47,48] likely due to poor renal clearance [49]. All continuous covariates were mean centered, sex was coded 0.5 for males and -0.5 for females. Race was coded using 3 categories: White, Black and other race, where White was used as the reference group. All participants were cognitively unimpaired at baseline. A false discovery rate (FDR) method was used to correct for multiple comparisons. Statistical significance was defined as FDR-corrected P < 0.05 for primary analyses and as uncorrected P < 0.05 for secondary analyses. All statistical analyses were performed using SAS statistical software version 9.4 (SAS institute). Linear mixed effects models conducted for VBM mirrored ROI analyses.
Results
Baseline plasma biomarkers and brain volume
Participant characteristics are described in Table 1 (Aβ42/40, GFAP, and NfL sample: N = 622, mean age = 70.9 ± 10.2, 55.8% female, 67.0% White; pTau-181 sample: N = 574, mean age = 69.7 ± 10.6, 55.4% female, 64.8% White). Follow up time was 4.7 years (SD = 2.9) for Aβ42/40, GFAP, and NfL and 4.5 years (SD = 2.9) for pTau-181. Thirty-nine (6%) participants developed cognitive impairment during follow up. Kidney function, as estimated by eGFR, was inversely associated with pTau-181 (ρ = -0.23, P < 0.0001) and NfL (ρ = -0.21, P < 0.0001) levels, but did not correlate with Aβ42/40 (ρ = 0.02, P = 0.64) and GFAP (ρ = -0.07, P = 0.06).
Cross-sectional analyses showed no associations of plasma Aβ42/40, GFAP, and NfL with total or regional brain volumes (Supplemental Table 1; Supplemental Table 2 [model without eGFR as a covariate]; Supplemental Table 3 [full model]); however, higher plasma pTau-181 was associated with smaller inferior temporal gyrus volume (P < 0.0001) at baseline. pTau-181 levels were additionally associated with faster declines in total gray matter volume (P = 0.005), and in the superior temporal gyrus (P = 0.001), middle temporal gyrus (P = 0.001), inferior temporal gyrus (P = 0.005), parahippocampal gyrus (P = 0.002), and amygdala (P = 0.009) regions after correcting for multiple comparisons (Fig. 2a; Supplemental Table 1). Higher baseline GFAP was associated with faster longitudinal increases in ventricular volume (P < 0.0001), and a nominally significant association with steeper declines in amygdala volume which did not survive FDR correction for multiple comparisons. Aβ42/40 and NfL levels were not associated with brain volume change (Supplemental Table 1; Fig. 2). The results were similar in sensitivity analyses that did not adjust for eGFR (Supplementary Table 2). Secondary whole brain VBM analyses were completed to confirm the regional specificity of biomarker-associated changes in volume. As shown in Fig. 3, significant (uncorrected at P = 0.001) longitudinal associations with gray matter volume were observed for both pTau-181 (left inferior frontal gyrus, left precuneus) and NfL (left superior temporal gyrus, left cuneus).
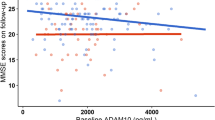
Association between baseline plasma biomarkers and longitudinal brain volume change. a. Results are derived from linear mixed effect models adjusted for baseline age, sex, race, education level, estimated glomerular filtration rate (eGFR), total intracranial volume at age 70, and the interaction between covariates and time. Estimates, derived from the plasma biomarker × time interaction terms, represent the difference in annual change in brain volume (β estimates) per standard deviation (SD) increase in baseline biomarker. Blue bars reflect significant associations between plasma biomarkers and change in brain volume at an FDR corrected threshold (P < 0.05). b. Results are derived from linear mixed effect models that examined Aβ42/40 as a moderator of the association between plasma biomarkers and longitudinal change in brain volume (models were also adjusted for, baseline age, sex, race, education level, eGFR, total intracranial volume at age 70, and the interaction between covariates and time). Each figure (blue lines: low pTau-181 or -1.5 standard deviations below the mean; orange lines: high pTau-181 or + 1.5 standard deviations above the mean) shows the marginal effects for the following interaction term pTau-181 × time × Aβ42/40 status on brain volume. Abbreviations: Aβ: amyloid-β; CSF: cerebral spinal fluid; GFAP: glial fibrillary acidic protein; NfL: neurofilament light chain; pTau-181: tau phosphorylated at threonine-181
Association between plasma biomarkers and longitudinal brain volume change defined using voxel-based morphometry (VBM). Plasma biomarker effects on the difference in annual change in gray matter volume (VBM). Results are derived from linear mixed effects models examining the association of baseline plasma biomarkers with longitudinal change in whole brain volume. Models were adjusted for baseline age, sex, race, education level, estimated glomerular filtration rate (eGFR), and total intracranial volume at age 70, as well as time × covariate interactions. T values reflect estimates derived from the plasma biomarker × time interactions term. Warm colors reflect positive t values while cool colors reflect negative t values. An uncorrected P < 0.001 threshold was used
Examination of effect modification by sex found an isolated GFAP × sex interaction on longitudinal change in middle temporal gyrus volume (P-interaction = 0.03). Post hoc analyses showed that the association of GFAP with faster declines in middle temporal gyrus volume was much stronger among men than it was among women (Supplemental Tables 4 and 5). Sex did not modify the association of Aβ42/40, pTau-181, and NfL with brain volume change. However, there was evidence for effect modification by sex on cross-sectional associations for each of these biomarkers (see Supplemental Table 4).
There was a significant pTau181 × APOEε4 interaction on longitudinal change in parahippocampal gyrus volume (P-interaction = 0.02). The association of higher pTau-181 with faster parahippocampal gyrus volume loss was considerably stronger among APOEε4-negative participants compared to APOEε4-positive participants (Supplemental Tables 5 and 6). Modification effects of APOEε4 status on brain volume change were not observed for Aβ42/40, GFAP, or NfL (Supplemental Table 6).
Amyloid status (defined by plasma Aβ42/40) modified the association between baseline pTau-181 and longitudinal brain volume changes (ventricular volume: P-interaction = 0.001; hippocampal volume: P-interaction = 0.04; total brain volume: P-interaction = 0.05; Supplemental Table 7). Post hoc analyses demonstrated that among those with higher Aβ burden (low Aβ42/40), higher baseline pTau-181 was associated with faster ventricular volume increase; however, no relationship was observed among those with lower Aβ burden (high Aβ42/40) (Supplemental Table 5 and 7; Fig. 2b). Surprisingly, among those with lower Aβ burden (high Aβ42/40), higher baseline pTau-181 was associated with accelerated declines in total brain volume and hippocampal volume, while no association was observed among those with higher Aβ burden (Supplemental Tables 5 and 7; Fig. 2b). We further show that age-related brain volume trajectories by age group vary by pTau-181 concentration and amyloid status (Supplemental Fig. 1).
Baseline plasma biomarkers and cognitive performance
Participant characteristics are described in Table 1 (Aβ42/40, GFAP, and NfL: N = 674, mean age = 71.2 ± 10.1, 54.2% female, 68.6% White; pTau-181 subset: N = 602, mean age = 70.0 ± 10.6, 55.0% female, 65.6% White). Follow up time was 5.7 years (SD = 3.2) for Aβ42/40, GFAP, and NfL and 5.6 years (SD = 3.3) for pTau-181. Fifty-seven (8.5%) participants developed cognitive impairment during follow-up.
Lower Aβ42/40 was associated with higher verbal memory scores (P = 0.01), but was not related to baseline attention, executive function, verbal fluency, and visuospatial abilities. Similarly, pTau-181, GFAP, and NfL and were not associated with cognition in cross-sectional analyses (Supplemental Table 8; Supplemental Table 9 [model without eGFR as a covariate]; Supplemental Table 10 [full model]). In longitudinal analyses, lower Aβ42/40 was associated with faster declines in verbal memory (P < 0.0001) and visuospatial performance (P = 0.02), while higher GFAP was associated with accelerated declines in verbal fluency (P = 0.002; Fig. 4a) after correcting for multiple comparisons. pTau-181 and NfL levels were not associated with changes in cognition (Supplemental Table 8). The results were similar in sensitivity analyses that did not adjust for eGFR (Supplementary Table 9).
Consistent with our brain volume analyses, examination of effect modification by sex found an isolated GFAP × sex interaction on change in visuospatial skills (P-interaction = 0.01; Supplemental Table 11. Among men, higher baseline GFAP was associated with faster declines in visuospatial performance, but this was not the case for women (Supplemental Table 11 and 12).
APOEε4 status did not modify the association between baseline plasma biomarkers and longitudinal change in cognitive performance. However, cross-sectional analyses did show evidence for APOEε4 as a modifier of the cross-sectional associations of Aβ42/40 and NfL with measures of attention (Supplemental Table 13).
Amyloid status (defined by Aβ42/40) modified the associations of GFAP and pTau-181 with longitudinal change in cognitive performance (GFAP: P-interaction = 0.02; pTau-181: P-interaction = 0.03; Supplemental Tables 12 and 14). Post hoc analyses demonstrated that among participants with high Aβ burden (low Aβ42/40), higher baseline GFAP and pTau-181 were associated with steeper declines in verbal memory, while no associations were observed among those with a low Aβ burden (Fig. 4b). We further show that age-related verbal memory trajectories vary by pTau-181and GFAP concentration and amyloid status (Supplemental Fig. 1).
Association between baseline plasma biomarkers and longitudinal cognitive change. a. Results are derived from linear mixed effect models adjusted for baseline age, sex, race, education level, and estimated glomerular filtration rate (eGFR), and the interaction between covariates and time. Estimates, derived from the plasma biomarker × time interaction terms, represent the difference in annual change in cognition (β estimates) per standard deviation increase in baseline biomarker. Blue bars reflect significant associations between plasma biomarkers and cognition by domain at an FDR corrected threshold (P < 0.05). b. Results are derived from separate linear mixed effect models that examined Aβ42/40 as a moderator of the association between plasma biomarkers and longitudinal cognition (models were also adjusted for baseline age, sex, race, education level, eGFR, and the interaction between covariates and time). Each figure (blue lines: low biomarker [e.g., pTau-181 or GFAP] or -1.5 standard deviations below the mean; orange lines: high biomarker [e.g., pTau-181 or GFAP] or + 1.5 standard deviations above the mean) shows the marginal effects for the following interaction terms pTau-181 × time × Aβ42/40 status or GFAP × time × Aβ42/40 status on verbal memory. Abbreviations: Aβ: amyloid-β; GFAP: glial fibrillary acidic protein; NfL: neurofilament light chain; pTau-181: tau phosphorylated at threonine-181.
Discussion
The present study examined whether plasma markers of AD pathology (Aβ42/40, pTau-181), astrogliosis (GFAP), and neuronal injury (NfL) predict longitudinal changes in brain volume and cognitive performance in a sample of cognitively unimpaired adults over a median follow-up of five to six years. We found that higher plasma pTau-181 was a robust predictor of brain volume loss, particularly in gray matter structures, while higher plasma GFAP showed a strong association with longitudinal ventricular enlargement and accelerated declines in verbal fluency. Although plasma Aβ42/40 and NfL were not associated with brain volumetric changes, lower plasma Aβ42/40 (indicative of greater Aβ burden) was associated with faster declines in verbal memory and visuospatial performance. Overall, our results suggest that higher pTau-181 and GFAP are indicative of future brain volume loss in cognitively normal older adults, while Aβ42/40 and GFAP levels are associated with faster subsequent cognitive declines in specific cognitive domains.
Given that declines in brain volume typically precede cognitive impairment [50], early predictors of brain atrophy may aid in identifying individuals at risk for age-related cognitive decline and cognitive impairment, including dementia. The finding that plasma pTau-181 was associated with steeper longitudinal declines in brain volume aligns with a previous study that found baseline pTau-181 to be associated with greater brain atrophy in cognitively unimpaired individuals [18]. In contrast to other studies [7, 17] which have examined both participants with and without cognitive impairment, we found that higher abundance of plasma NfL was not associated with faster subsequent declines in brain volume. One reason for this discrepancy may be disease stage. Given our focus on cognitively unimpaired older adults, participants may not be advanced enough from a neuropathological perspective to detect significant associations with a marker of neuronal injury. Previous work has demonstrated that plasma NfL does not increase in those with AD compared to healthy controls until approximately 10 years before dementia diagnosis [51]. In the present study, only 8.5% of participants went on to develop any type of dementia or mild cognitive impairment during the follow-up period. Increases in plasma NfL are thought to coincide with brain atrophy and occur after elevations in pTau-181, Aβ42, and GFAP [50, 52, 53]. The lack of NfL associations with brain volume change in the context of associations of pTau-181 and GFAP with brain volume change suggests that, among cognitively unimpaired older adults, NfL is comparatively less informative as a prognostic indicator of neurodegeneration.
The lack of an association between Aβ42/40 levels and subsequent brain changes is surprising, particularly in the context of the robust pTau-181 associations. In a recent analysis using the same (BLSA) cohort, we found that Aβ42/40 predicted amyloid PET status with good – but less than optimal – levels of accuracy (AUC = 0.72), with pTau-181 showing similar accuracy for prediction of amyloid status (AUC = 0.72) [12]. The divergent associations with brain volume loss despite similar predictive accuracy for cortical amyloid suggest that plasma pTau-181, in addition to acting as a measure of amyloid status [54], captures a broader range of neuropathological processes linked to neurodegeneration compared to plasma Aβ42/40 at this point in the disease process [18]. There are at least three additional explanations for the null association between plasma Aβ42/40 and brain volume. First, accuracy of the Aβ42/40 quantification may be limited by assay-specific factors, as the Simoa Aβ42/40 assay has been shown to be inferior to mass spectrometry and other immune-assays for prediction of cortical Aβ [55, 56]. Second, plasma Aβ42/40 has a limited dynamic range with a group level difference between healthy control and AD patients of only 10–20%, making discrimination between AD and non-AD challenging, perhaps even more so among cognitively normal individuals [50, 57]. Lastly, the accumulation of Aβ42 peptides, which has been proposed to be an initiating factor in AD, may be too distal in the disease processes from the neurodegenerative processes underlying brain atrophy [12].
In the current study, we found that lower plasma Aβ42/40 and higher GFAP were associated with faster declines in verbal memory and fluency, respectively, over approximately 6 years. The association of Aβ42/40 with accelerated decline in verbal memory supports the clinical utility and specificity of this biomarker in asymptomatic AD. The association of higher GFAP with faster declines in verbal fluency aligns with prior studies that suggest astrogliosis promotes synaptic dysfunction and subsequent cognitive impairment [58]. While the association of Aβ42/40 and GFAP with cognitive decline has been reported previously, the lack of association of NfL and pTau-181 with cognitive decline runs counter to previous findings [23]. Despite finding that higher baseline pTau-181 was associated with accelerated declines in temporal lobe brain volume, pTau-181 was not associated with cognitive decline. Given that soluble pTau-181 levels are known to rise prior to significant increases in cortical tau [52], and cortical tau is strongly associated with cognitive decline [59], associations of plasma pTau-181 with cognitive decline may be observed only at a later disease stage or with a longer follow-up period. Notably, we did see nonsignificant trends in pTau-181 associations with cognitive change, suggesting that higher levels may relate to declines in several cognitive domains.
While only 8.5% of participants went on to develop mild cognitive impairment or dementia during the follow-up period of the current study, 40% of participants were estimated to be amyloid positive at the time plasma biomarkers were measured based on plasma Aβ42/40 levels. We found that amyloid-positive status – an indicator of the presence of AD pathology – modified the association of plasma pTau-181 with brain volume loss such that higher pTau-181 was more strongly associated with increased ventricular volume among those with higher Aβ burden. In contrast to these results, we also found that higher pTau-181 was more strongly associated with accelerated declines in total brain and hippocampal volume among participants with a lower Aβ burden. The reason for this seemingly contradictory finding is unknown. However, these results suggest that the association of pTau-181 with total brain and hippocampal volume loss may be magnified early in the course of AD pathogenesis (ahead of any major elevations in cortical amyloid), whereas pTau-181 and GFAP are associated with faster declines in verbal memory later in the course of AD pathogenesis, in the context of high Aβ burden.
This study has several strengths, including a large sample, the availability of longitudinal outcome data from a comprehensive neuropsychological examination and repeated neuroimaging assessments on a 3T MRI, and the focus on cognitively unimpaired (asymptomatic) older adults. Despite these strengths, the current study has several limitations. First, the BLSA is a relatively healthy sample and mean level of education in the present sample was 17 years. While the sample is relatively large and diverse, it is not sufficiently powered for stratified analyses to determine whether findings are consistent across included racial and ethnic groups. Therefore, the findings derived from the present study may not be generalizable to the broader population. Second, only a small percentage of participants progressed to cognitive impairment, which may have limited our ability to detect significant associations among some plasma biomarkers (e.g., NfL) and brain volume. Finally, the present study did not include measurement of the pTau-217 isoform, which compared to pTau-181, has demonstrated greater accuracy for prediction of cortical AD pathology [60, 61]. Further, the addition of pTau-217 may have enabled us to detect differences in brain volume and cognition as a function of sex, as prior work has demonstrated that higher pTau-217 is related to declines in verbal memory and brain atrophy for cognitively unimpaired women but not men [62].
Conclusions
The current study demonstrates that plasma biomarkers of AD pathology (pTau-181) and astrogliosis (GFAP), but not neuronal injury (NfL), may serve as markers of future brain atrophy and cognitive decline among cognitively unimpaired older adults in the community. In addition to demonstrating that these plasma biomarkers are associated with future neurodegenerative and cognitive trajectories, we show that the extent to which GFAP and pTau-181 predict brain atrophy and cognitive decline depends on the burden of brain amyloid.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
Amyloid-β
- BLSA:
-
Baltimore Longitudinal Study of Aging
- CVs:
-
Coefficients of variation
- eGFR:
-
Estimated glomerular filtration rate
- FDR:
-
False discovery rate
- GFAP:
-
Glial fibrillary acidic protein
- ICV:
-
Total intracranial volume
- LME:
-
Linear mixed effects
- MPRAGE:
-
Magnetization-prepared rapid gradient echo
- MUSE:
-
Multi-atlas Region Segmentation Utilizing Ensembles
- N4PE:
-
Neurology 4-Plex E
- NfL:
-
Neurofilament light chain
- NFT:
-
Neurofibrillary tangles
- PCR:
-
Polymerase chain reaction
- PET:
-
Positron emission tomography
- PiB:
-
11C-Pittsburgh compound-B
- pTau-181:
-
Tau phosphorylated at threonine-181
- ROIs:
-
Regions of interest
- VBM:
-
Voxel-based morphometry
References
Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505–8.
Busche MA, Hyman BT. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat Neurosci. 2020;23(10):1183–93.
Asken BM, Elahi FM, La Joie R, et al. Plasma glial fibrillary acidic protein levels differ along the spectra of amyloid burden and clinical disease stage. J Alzheimers Dis. 2020;78(1):265–76.
Janelidze S, Palmqvist S, Leuzy A, et al. Detecting amyloid positivity in early Alzheimer’s disease using combinations of plasma Aβ42/Aβ40 and p-tau. Alzheimers Dement. 2022;18(2):283–93.
Verberk IM, Thijssen E, Koelewijn J, et al. Combination of plasma amyloid beta (1–42/1-40) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimers Res Ther. 2020;12(1):1–14.
Dark HE, Duggan MR, Walker KA. Plasma biomarkers for Alzheimer’s and related dementias: a review and outlook for clinical neuropsychology. Arch Clin Neuropsychol. 2024:acae019. https://doi.org/10.1093/arclin/acae019.
Mattsson N, Andreasson U, Zetterberg H, Blennow K, Iniative AsDN. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA. 2017;74(5):557–66.
Rauchmann BS, Schneider-Axmann T, Perneczky R. Associations of longitudinal plasma p-tau181 and NfL with tau-PET, Aβ-PET and cognition. J Neurol Neurosurg Psychiatry. 2021;92(12):1289–95.
Kivisäkk P, Carlyle BC, Sweeney T, et al. Plasma biomarkers for diagnosis of Alzheimer’s disease and prediction of cognitive decline in individuals with mild cognitive impairment. Front Neurol. 2023;14:1069411.
Yakoub Y, Ashton NJ, Strikwerda-Brown C, et al. Longitudinal blood biomarker trajectories in preclinical Alzheimer’s disease. Alzheimers Dement. 2023;19(12):5620–31.
Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93(17):e1647–59.
Bilgel M, An Y, Walker KA, et al. Longitudinal changes in Alzheimer’s-related plasma biomarkers and brain amyloid. Alzheimers Dement. 2023;19(10):4335–45.
Benussi A, Cantoni V, Rivolta J, et al. Classification accuracy of blood-based and neurophysiological markers in the differential diagnosis of Alzheimer’s disease and frontotemporal lobar degeneration. Alzheimers Res Ther. 2022;14(1):1–12.
Feinkohl I, Schipke CG, Kruppa J, et al. Plasma Amyloid concentration in alzheimer’s disease: Performance of a high-throughput amyloid assay in distinguishing alzheimer’s disease cases from controls. J Alzheimers Dis. 2020;74(4):1285–94.
Pontecorvo MJ, Lu M, Burnham SC, et al. Association of donanemab treatment with exploratory plasma biomarkers in early symptomatic Alzheimer disease: a secondary analysis of the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 2022;79(12):1250–9.
Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76(7):791–9.
Rajan KB, Aggarwal NT, McAninch EA, et al. Remote blood biomarkers of longitudinal cognitive outcomes in a population study. Ann Neurol. 2020;88(6):1065–76.
Moscoso A, Grothe MJ, Ashton NJ, et al. Longitudinal associations of blood phosphorylated Tau181 and neurofilament light chain with neurodegeneration in Alzheimer disease. JAMA Neurol. 2021;78(4):396–406.
Asken BM, VandeVrede L, Rojas JC, et al. Lower white matter volume and worse executive functioning reflected in higher levels of plasma GFAP among older adults with and without cognitive impairment. J Int Neuropsychol Soc. 2022;28(6):588–99.
Benedet AL, Leuzy A, Pascoal TA, et al. Stage-specific links between plasma neurofilament light and imaging biomarkers of Alzheimer’s disease. Brain. 2020;143(12):3793–804.
Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11(1):812.
Chen Y-H, Lin R--R, Huang H-F, Xue Y-Y, Tao Q-Q. Microglial activation, tau pathology, and neurodegeneration biomarkers predict longitudinal cognitive decline in Alzheimer’s disease continuum. Front Aging Neurosci. 2022;14.
Chatterjee P, Pedrini S, Doecke JD, et al. Plasma Aβ42/40 ratio, p-tau181, GFAP, and NfL across the Alzheimer’s disease continuum: a cross-sectional and longitudinal study in the AIBL cohort. Alzheimers Dement. 2023;19(4):1117–34.
Bettcher BM, Olson KE, Carlson NE, et al. Astrogliosis and episodic memory in late life: higher GFAP is related to worse memory and white matter microstructure in healthy aging and Alzheimer’s disease. Neurobiol Aging. 2021;103:68–77.
Shock NW, Greulich RC, Aremberg D, Costa PT, Lakatta EG, Tobin JD. Normal human aging: the Baltimore longitudinal study of aging. Washington, D.C.: NIH Publication; 1984.
Ferrucci L. The Baltimore Longitudinal Study of Aging (BLSA): a 50-year-long journey and plans for the future. J Gerontol A Biol Sci Med Sci. 2008;63(12):1416–9.
Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3295–301.
Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54(11):2072–7.
Fuld P. Psychological testing in the differential diagnosis of the dementias. Alzheimer’s disease: senile dementia and related disorders. 1978;7:185–93.
Morris JC. The Clinical Dementia Rating (CDR) current version and scoring rules. Neurology. 1993;43(11):2412–2412-a.
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Int Psychogeriatr. 1997;9(S1):65–9.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (3rd ed., revised). Washington, DC: American Psychiatric Association; 1987.
Davatzikos C, Genc A, Xu D, Resnick SM. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage. 2001;14(6):1361–9.
Shen D, Davatzikos C. Very high-resolution morphometry using mass-preserving deformations and HAMMER elastic registration. Neuroimage. 2003;18(1):28–41.
Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8(3):271–6.
Wechsler D. Wechsler Adult Intelligence Scale-Revised. Psychological Corporation; 1981.
Wilson JR, De Fries J, Mc Clearn G, Vandenberg S, Johnson R, Rashad M. Cognitive abilities: Use of family data as a control to assess sex and age differences in two ethnic groups. The International Journal of Aging and Human Development. 1975;6(3):261–76.
Delis D, Kramer J, Kaplan E, Ober B. California verbal learning test research edition manual. San Antonio: The Psychological Corporation; 1987.
Bilgel M, Beason-Held L, An Y, Zhou Y, Wong DF, Resnick SM. Longitudinal evaluation of surrogates of regional cerebral blood flow computed from dynamic amyloid PET imaging. J Cereb Blood Flow Metab. 2020;40(2):288–97.
Bilgel M, Bannerjee A, Shafer A, An Y, Resnick SM. Vascular risk is not associated with PET measures of Alzheimer’s disease neuropathology among cognitively normal older adults. Reports. 2021;1(4):100068.
Hixson JE, Vernier D. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–8.
Koch W, Ehrenhaft A, Griesser K, et al. TaqMan systems for genotyping of disease-related polymorphisms present in the gene encoding apolipoprotein E. Clin Chem Lab Med. 2002;40(11):1123–31.
Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79(2):268–88 (e1).
Inker LA, Eneanya ND, Coresh J, et al. New creatinine-and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–49.
Doshi J, Erus G, Habes M, Davatzikos C. DeepMRSeg: A convolutional deep neural network for anatomy and abnormality segmentation on MR images. arXiv preprint arXiv:190702110. 2019;
Dark HE, Paterson C, Daya GN, et al. Proteomic Indicators of Health Predict Alzheimer’s Disease Biomarker Levels and Dementia Risk. Ann Neurol. 2023;95(2):260–73.
O’Bryant SE, Petersen M, Hall J, Johnson LA, Team HHS. Medical comorbidities and ethnicity impact plasma Alzheimer’s disease biomarkers: important considerations for clinical trials and practice. Alzheimers Dement. 2023;19(1):36–43.
Janelidze S, Stomrud E, Palmqvist S, et al. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci Rep. 2016;6(1):1–11.
Cheng Y, Tian D-Y, Wang Y-J. Peripheral clearance of brain-derived Aβ in Alzheimer’s disease: pathophysiology and therapeutic perspectives. Translational Neurodegeneration. 2020;9:1–11.
Hansson O. Biomarkers for neurodegenerative diseases. Nat Med. 2021;27(6):954–63.
de Wolf F, Ghanbari M, Licher S, et al. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain. 2020;143(4):1220–32.
Guo Y, Huang Y-Y, Shen X-N, et al. Characterization of Alzheimer’s tau biomarker discordance using plasma, CSF, and PET. Alzheimers Res Ther. 2021;13(1):1–12.
Mattsson-Carlgren N, Andersson E, Janelidze S, et al. Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer’s disease. Science advances. 2020;6(16):eaaz2387.
McGrath ER, Beiser AS, O’Donnell A, et al. Blood phosphorylated tau 181 as a biomarker for amyloid burden on brain PET in cognitively healthy adults. J Alzheimers Dis. 2022;87(4):1517–26.
Hirtz C, Busto GU, Bennys K, et al. Comparison of ultrasensitive and mass spectrometry quantification of blood-based amyloid biomarkers for Alzheimer’s disease diagnosis in a memory clinic cohort. Alzheimers Res Ther. 2023;15(1):1–12.
Janelidze S, Teunissen CE, Zetterberg H, et al. Head-to-head comparison of 8 plasma amyloid-β 42/40 assays in Alzheimer disease. JAMA Neurol. 2021;78(11):1375–82.
Karikari TK, Ashton NJ, Brinkmalm G, et al. Blood phospho-tau in Alzheimer disease: analysis, interpretation, and clinical utility. Nat Rev Neurol. 2022;18(7):400–18.
De Sousa RAL. Reactive gliosis in Alzheimer’s disease: a crucial role for cognitive impairment and memory loss. Metab Brain Dis. 2022;37(4):851–7.
Hanseeuw BJ, Betensky RA, Jacobs HI, et al. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. 2019;76(8):915–24.
Therriault J, Servaes S, Tissot C, et al. Equivalence of plasma p‐tau217 with cerebrospinal fluid in the diagnosis of Alzheimer’s disease. Alzheimers Dement. 2023;19(11):4967–77.
Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324(8):772–81.
Saloner R, VandeVrede L, Asken BM, et al. Plasma phosphorylated tau-217 exhibits sex-specific prognostication of cognitive decline and brain atrophy in cognitively unimpaired adults. Alzheimers Dement. 2024;20(1):376–87. https://doi.org/10.1002/alz.13454.
Acknowledgements
The authors would like to thank the BLSA participants and staff for their participation and continued dedication.
Funding
Open access funding provided by the National Institutes of Health This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Author information
Authors and Affiliations
Contributions
HED: study design, drafted manuscript, statistical analysis, figure preparation, results interpretation. YA: statistical analysis, study design, results interpretation. MRD: edited manuscript. CJ: literature review. CD and GE: neuroimaging data processing and analyses. AL and AM: plasma biomarker data processing and quality control. SMR: direction of BLSA study, study design, results interpretation. KW: drafted manuscript, study design, study supervision, results interpretation. All authors reviewed and approved the manuscript prior to submission.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The BLSA protocol was approved by the Institutional Review Board (IRB) responsible for oversight of the National Institute on Aging, National Institutes of Health (03AG0325). All participants provided written informed consent prior to participation, and deidentified BLSA data were used for analyses.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dark, H.E., An, Y., Duggan, M.R. et al. Alzheimer’s and neurodegenerative disease biomarkers in blood predict brain atrophy and cognitive decline. Alz Res Therapy 16, 94 (2024). https://doi.org/10.1186/s13195-024-01459-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-024-01459-y