Abstract
Introduction
People with subjective cognitive decline (SCD) report cognitive deterioration. However, their performance in neuropsychological evaluation falls within the normal range. The present study aims to analyse whether structural magnetic resonance imaging (MRI) reveals grey matter changes in the SCD population compared with healthy normal controls (HC).
Methods
Parallel systematic searches in PubMed and Web of Science databases were conducted, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Quality assessment was completed using the Newcastle-Ottawa Scale (NOS).
Results
Fifty-one MRI studies were included. Thirty-five studies used a region of interest (ROI) analysis, 15 used a voxel-based morphometry (VBM) analysis and 10 studies used a cortical thickness (CTh) analysis. Ten studies combined both, VBM or CTh analysis with ROI analysis.
Conclusions
Medial temporal structures, like the hippocampus or the entorhinal cortex (EC), seemed to present grey matter reduction in SCD compared with HC, but the samples and results are heterogeneous. Larger sample sizes could help to better determine if these grey matter changes are consistent in SCD subjects.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disease and the leading cause of dementia, accounting for an estimated 50–70% of cases [1]. AD is an age-related condition and its global worldwide prevalence is expected to be much greater with increasing in the ageing population, reaching 106.8 million people in 2050 [2]. The global annual economic cost of dementia supposes an amount of one billion US dollars and it will increase up to 2 billion in 2030 [3]. It is estimated that a 1-year delay on disease onset would reduce the number of cases in 12 million by 2050, being an early and precise diagnostic, an essential tool for it [2].
Nowadays, we know that the natural history of AD is divided into three phases: the preclinical phase, where the pathogenic mechanisms of the disease have started but no objective cognitive decline can be diagnosed; the prodromal phase, where mild objective cognitive symptoms can be identified, but they are not severe enough to meet dementia criteria; and the dementia phase, where cognitive decline interferes with daily activities [4]. Some subjects in the preclinical phase of AD declare mild cognitive symptoms with no clinical evidence of cognitive impairment as compared with age-, sex- and education-matched subjects. This clinical construct has historically received many names such as subjective cognitive impairment, subjective memory impairment or decline or memory complaints, although it is currently referred to as subjective cognitive decline (SCD) [5, 6].
SCD prevalence is noticeably high (25–50%) in the population over 65 years old, albeit not all causes are AD-related. In fact, the aetiology of SCD is heterogeneous and can also be related to normal ageing and psychiatric or non-degenerative neurological disorders such as depression, cerebrovascular diseases or concussions [5]. To decrease this heterogeneity, Jessen et al. proposed to exclude from this concept those subjects whose cognitive complaints could be accounted for by other disorders (psychiatric, neurological or systemic), drugs or their abuse [5].
Research in this field has been focused on tracking biomarkers that could define the preclinical AD phase in this population, characterising risk groups to start potential treatments that could delay disease progression [7, 8]. The most frequently used techniques are cerebrospinal fluid analysis and different neuroimaging modalities such as magnetic resonance imaging (MRI), fluorodeoxyglucose positron emission tomography (PET), amyloid PET or Tau PET.
The morphometric analysis of MRI images of the brain has become a widely used approach to investigate changes in brain structure in neurodegenerative disorders. Typically, changes in the grey matter have been assessed using T1-weighted images and the most frequently used methods to analyse them include the volumetric comparison of (manually, semi-automatically or automatically) delineated regions of interest (ROIs), whole-brain voxel-based comparison of grey matter (called voxel-based morphometry or VBM) and cortical surface-based comparison of cortical thickness. These methods of neuroimaging analyses have their own strengths and limitations and frequently show different results even with identical image sets [9, 10].
On the other hand, studies that evaluate changes in brain structure in subjects with SCD compared to control participants have shown heterogeneous results, in terms of areas affected and statistical significance, even in the AD-related structures such as the hippocampus [11,12,13]. The aim of this systematic review is to give an overview of studies examining the differences in the grey matter volume of the brain between individuals with a clinical diagnosis of SCD and cognitive unimpaired persons detected by MRI.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14].
Search strategy
We performed a literature search on PubMed and Web of Science (WoS) databases up to November 19, 2020. Combinations of the following terms were used in both searches: “subjective cognitive decline”, “subjective cognitive impairment”, “subjective cognitive complaints”, “subjective memory decline”, “subjective memory impairment”, “subjective memory complaints”, “self-reported memory complaints”, “self-reported memory decline”, “self-reported memory impairment”, “self-reported cognitive impairment”, “self-reported cognitive decline”, “self-reported cognitive complaints”, “MRI”, “magnetic resonance imaging”, “cortical thinning”, “atrophy”, “volume” and “cortical thickness”. The complete search syntax for each database is available in Supplementary Materials 1 and 2.
Selection criteria
We included studies that met the following inclusion criteria: (1) studies restricted to the English or Spanish language; (2) studies including a subjective cognitive impairment group, according to Jessen’s criteria [5]; (3) studies including healthy controls (HC); and (4) studies measuring grey volume by MRI. We discarded studies according to the following exclusion criteria: (1) single-sex studies, (2) fMRI studies, (3) studies performing any kind of clinical treatment (chemotherapy, drugs, memory training, physical exercise, etc.), (4) studies restricted to APOE carriers in their sample, (5) studies with a history of hypertension or vascular disease as a selection criterion or (6) systematic reviews, meta-analyses and letters.
Study selection
Two reviewers (PA and OEZ) independently performed the search up to November 2020. After the removal of duplicates, the titles and abstracts of the remaining articles were screened for eligibility. Additionally, we also screened the references cited in the relevant articles to include key studies that had not been previously detected following a snowball technique. Then, the full text of the elected articles was screened according to the selection criteria. Disagreements on study selection were resolved by a third independent reviewer (MR).
Data extraction
We performed a systematic extraction of the following variables from all eligible manuscripts: year of publication, journal, MRI field strength (1.5 or 3 Tesla), type of the study (retrospective vs prospective), sample size in each clinical group (SCI vs HC), age in each clinical group (mean and standard deviation), sample recruitment (population-based, mixed or memory-clinic sample), type of analysis, software used for the analysis, ROIs studied, segmentation applied in those studies based on ROI analysis, main results and statistical significance of the findings. All studies in which the SCD sample was not recruited exclusively from memory clinics were included in the category “mixed”. For statistical analysis purposes, we dichotomised the variable sample recruitment into memory clinic vs “other” (population-based and mixed) sample. The variables were previously defined and operationalised in an Excel template. A replicate of the data collection sheet can be found in Supplementary material S3.
Lastly, we further studied if any of the variables included were associated with the identification of statistically significant findings in the study. To compare continuous variables between the groups, we performed a one-way ANOVA or Kruskal-Wallis test and two-tailed Student’s T or Wilcoxon rank-sum test, when appropriate. To compare categorical variables, chi-squared or Fisher tests were used as appropriate.
Quality assessment
We used the Newcastle-Ottawa Scale (NOS) assessment to determine the quality of the studies selected [15].
Results
Eligible and included studies
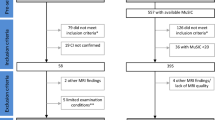
Our parallel searches yielded a total of 365 (PubMed) and 463 (WoS) articles. After removing duplicates, a total of 425 articles were screened by title/abstract reading. After further reading and applying inclusion/exclusion criteria, 51 studies were selected for review (Fig. 1).
Study characteristics
Most of the studies were published between 2010 and 2020 (n = 46). Only 5 studies were published during the first decade of the 2000s, being 2015 the year in which most studies were published (n = 8). The different ways of sample recruitment were patients or referrals from memory clinics (n =31), population-based cohort (n = 12) or a combination of both (n = 8). Three studies were based on pre-existing data banks. Regarding the design of the study, most of the articles included were retrospective (n = 42).
The studies used different techniques to study the volume of grey matter in the brain. Twenty-six studies exclusively performed a ROI analysis, being the most common analysis; 10 studies exclusively performed a VBM analysis and 6 exclusively analysed the cortical thickness. The remaining 9 studies performed a combination of two types of analysis (ROI and VBM analysis n = 5 or ROI and cortical thickness n = 4; results compared in Supplementary Table 1). Hence, 35 studies performed ROI analysis, 15 VBM analysis and 10 studies cortical thickness analysis.
Voxel-based morphometry analysis
Fifteen studies performed a VBM analysis (Table 1), and 8 found statistical differences between SCD and HC participants [16, 18, 21, 23, 24, 26,27,28]. Five of them found grey matter volume reduction in the hippocampus in SCD compared with HC [21, 24, 26,27,28]. Saykin et al. [27] found bilateral volume reduction in the whole hippocampus, Liang et al. [24] found bilateral volume reduction in the hippocampal tail and Perrotin et al. [26] found bilateral volume reduction in the CA1. Lastly, 2 studies only found unilateral volume reduction in the right hippocampal in the SCD group [21, 28]. Additional temporal areas were also involved in 3 studies. Volume reductions were found in the SCD group compared with HC in the right insula [18], the right amygdala [21] and the inferior temporal gyrus [23].
In the frontal lobe, Saykin et al. [27] found volume reductions in the whole lobe in the SCD group compared with HC. Other studies found differences in some specific frontal areas like the bilateral [16] and left [18] superior frontal, bilateral [22] or left [18] medial frontal, left inferior frontal [23], the bilateral anterior cingulate [21, 23] and the left orbitofrontal cortices [23]. In the parietal cortex, Choi et al. (2015) found SCD volume reductions in the left superior and inferior cortex and in the right precuneus. Hafkemeijer et al. [21] also found bilateral precuneus atrophy in the SCD group compared with HC. In the occipital lobe, volume reductions were found in the bilateral cuneus [21], right calcarine and lingual gyrus [23]. Finally, only one study found a higher volume in SCD compared with HC, located in the paracentral lobe [24].
On the contrary, 7 studies did not find any significant difference in SCD compared with HC [11, 12, 19, 20, 25, 29, 30].
ROI analysis
Hippocampus
A total of 35 studies performed a ROI analysis (Table 2), and 13 of them found a volume reduction in the hippocampus in SCD compared with HC (37.1%). Particularly, 6 of them found a volume reduction of the whole bilateral hippocampus [21, 26, 46, 51, 56, 57]. Focusing on the whole left hippocampus, 3 studies found it smaller in SCD compared with HC [33, 38, 58]. Heeding to some different left hippocampus subfields, CA1 [26, 33, 55], CA3 [55] CA4 [33, 55, 58], dentate gyrus [33], molecular layer [33, 55, 58], subiculum [26, 58], presubiculum [58] and hippocampal tail [55, 58] were smaller in SCD compared with HC. The whole right hippocampus was smaller in SCD compared with HC in 2 studies [28, 54]. Some right hippocampal subfields were also smaller in SCD, like the perirhinal area [35], dentate gyrus [35], presubiculum [58] (Zhao et al., 2019) and fimbria [58].
On the other hand, 20 studies did not find any significant difference in the hippocampal volume between SCD and HC (57.1%) [13, 23, 27, 31, 32, 34, 36, 39,40,41,42,43,44,45, 47, 49, 50, 52, 53, 59].
Entorhinal cortex
Three studies found a reduced volume in the entorhinal cortex (EC) bilaterally in SCD compared with HC [40, 47, 51]. On the contrary, one study also analysed this ROI, but did not find any significant difference [44].
Amygdala
Five studies found less grey matter volume in the amygdala in SCD compared with HC, 3 of them bilaterally [49, 56, 57] and 2 in the right hemisphere [51, 60]. Three studies did not find differences between groups [21, 37, 46].
Cingulate cortex
One study found grey matter atrophy in the posterior cingulate in SCD compared with HC [57]. Two studies did not find statistical differences between groups [23, 28].
Other
Scheef et al. [48] found the cholinergic basal forebrain (Ch1/2 and Ch 4p) smaller in SCD compared with HC. Zhao et al. [57] found the temporal lobe, the occipital lobe and the insular cortex smaller in SCD than in HC. Other studies analysed different brain areas like the thalamus, the putamen, the accumbens nucleus, the caudate nucleus, the globus pallidus [21], the corpus callosum [23], the precuneus, the parahippocampus [13], the inferior parietal, the middle temporal lobe or the retrosplenial cortex [13], but did not find any significant difference between SCD and HC.
Cortical thickness
Cortical thickness was analysed in 10 studies (Table 3). Six of them found increased thinning in SCD compared to HC in several regions such as the bilateral entorhinal cortex [49, 61], left entorhinal cortex [36, 64], right entorhinal cortex, bilateral parahippocampus, left perirhinal cortex [37], left medial orbitofrontal cortex [63] and whole frontal, temporal and parietal lobes [66]. Also, focal cortical thinning was found in fusiform, posterior cingulate and inferior parietal cortex [49]. On the other hand, 4 studies did not find differences in cortical thickness between groups [13, 44, 62, 65].
Factors determining the statistical significance of findings
We observed that the studies with a recruitment sample in a memory clinic tend to identify more frequently statistically significant findings compared with those with a population-based or mixed recruitment (70% vs 50%, p = 0.09). Moreover, articles that identify statistically significant findings were published earlier than those without statistically significant findings (median 2015 vs 2017, p = 0.03). However, we did not find any other variable related to the statistical significance of findings (type of the study, sample size, age, nor MRI strength, p > 0.05) (Supplementary Table 2).
Quality assessment
All 51 studies included in this review received quality assessment (Table 4) following the Newcastle-Ottawa Quality Assessment Scale [15]. Out of a maximum of 9 points, the average was 6.84, indicating good overall quality in the articles selected for review. However, only two studies correctly reported a non-response rate [25, 67], being Ivanoiu et al. [67] the only study obtaining the maximum score. The lowest score was 5 out of 9 points (n = 4).
Discussion
The main goal of this systematic review was to investigate whether individuals with SCD present volumetric or grey matter changes when compared with cognitively normal subjects. The main finding is that, among the reviewed studies, there is not a homogeneous and consistent structural change found in SCD compared with HC. The studies that found significant differences (disregarding the analysis method used) did so in the medial temporal lobe, a region implicated in AD [68, 69]. However, the results we have observed are heterogeneous in the different imaging analysis methods included in this review.
Analysing VBM results from 15 studies, half of them (n = 8) found significant results between groups, and the other half (n = 7) did not. In those studies that found structural atrophy in SCD compared with HC, the hippocampus was the most affected area. Interestingly, the right hippocampus was found to be smaller more often than the left one, being the right hippocampus affected every time that hippocampal volume was decreased in SCD. This hippocampal asymmetry was analysed for mild cognitive impairment (MCI) and AD groups in a meta-analysis [70]. In contrast with our study, this meta-analysis found a left-less-than-right atrophy pattern and a poorer performance in episodic memory tests in subjects with less left than right hippocampal volume. Fewer studies found affected areas that are also part of the temporal lobe (the amygdala, the insula and the temporal gyrus). Interestingly, up to 5 studies observe decreased volume in different regions of the frontal lobe in participants with SCD. This structure is not typically affected in the early stages of AD and possibly represents the heterogeneous aetiology of this clinical syndrome.
Evaluating the results from 35 neuroimaging studies using ROI analysis, half of the sample found significant results (n = 18), and the other half did not (n = 17). The temporal lobe was also the most studied area of the brain in ROI studies. Specifically, the hippocampus, the amygdala, the entorhinal cortex and the posterior cingulate cortex were the most studied regions of interest. Although these areas are usually affected in mild and advanced stages of AD [71,72,73], there is no clear constant evidence of significant differences between SCD and HC individuals in these areas. One of the main limitations of ROI analysis may be the predetermination of the areas to be studied, especially when the underlying causes for SCD are not always AD-related. This selection bias can be avoided using other kinds of analyses such as voxel-based analysis. ROI segmentation is another possible source of bias, especially when manual segmentation is used. The distinction between manual versus automatic segmentation of ROIs could then be expected to be a determinant factor in the finding of significant differences. Manual segmentation was the gold standard for hippocampal volumetry [74, 75] but heterogeneity in anatomic definitions and tracing guidelines have hampered comparisons among different studies using hippocampal volumetry for diagnosis. Semiautomatic segmentation tries to solve this limitation and to reduce the inter- and intraobserver variability but fails to do so to the full extent [76]. Automatic segmentation is more consistent and time-efficient but needs larger samples to validate the technique. However, our review did not find this factor to be meaningful, possibly related with the sample size. Moreover, there is no clear evidence of volumetric changes in other ROIs. Nevertheless, this could be due to the reduced number of studies focusing on them.
Heterogeneity was also observed in studies measuring cortical thickness. Six studies showed statistically significant differences in the cortical thickness of participants with SCD compared to controls, while 4 did not. Although the entorhinal cortex is one of the most affected structures when significant cortical thinning was found, more studies are needed to consider it a reliable biomarker of preclinical AD.
Limitations
The main limitations of our study are the different research settings and operationalisation of SCD used in the studies, the heterogeneity inherent to this clinical syndrome and the small sample of the studies measuring structural changes. Methodologically, an explanation for these heterogeneous results could be that the term SCD was recently established by Jessen in 2014, unifying the diverse diagnostic criteria and terminology used until then to refer to this potential early state of AD. Additionally, SCD may include vastly diverse samples, since it may include patients who underly AD pathology, other kinds of neurodegenerative disorders or cases in which memory complaints are simply associated with normal ageing. More consistent results may be expected by selecting participants with SCD and specific features which increase the likelihood of the presence of preclinical AD (referred as SCD plus [5]). Moreover, participants are studied in different research environments such as clinical settings and population-based cohorts. Rodríguez-Gómez et al. [77] found that SCD patients who have been referred to a memory clinic had an increased risk of developing cognitive impairment than patients from the general population. In this line, we found a trend pointing towards the recruitment from memory clinics as a predictive factor of statistical differences. On the other hand, the use of larger samples and multimodal analysis techniques might help to establish regions associated to SCD and its progression to then develop an early and accurate diagnosis of preclinical AD. Finally, another possible limitation of our study might be the publication bias, given that studies that do not find significant differences are less likely to be published, thus reducing our sample.
Conclusion
As a conclusion, we have found that studies assessing volumetric or grey matter changes in subjects with SCD when compared with cognitively normal subjects showed heterogeneous results. Almost half of the studies do not find any significant difference between both groups, and when differences are observed, diverse structures are involved. However, the medial temporal lobe is the structure more frequently observed.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- ACC:
-
Anterior cingulate cortex
- AD:
-
Alzheimer disease
- BA35:
-
Broadman area 35 (perirhinal cortex)
- BA36:
-
Broadman area 36 (rhinal sulcus)
- chBFN:
-
Cholinergic basal forebrain nuclei
- CS:
-
Collateral sulcus
- CtH:
-
Cortical thickness
- DG:
-
Dentate gyrus
- EC:
-
Entorhinal cortex
- HATA:
-
Hippocampal-amygdaloid transition area
- HC:
-
Healthy control
- MCI:
-
Mild cognitive impairment
- ML:
-
Molecular layer
- mPFC:
-
Medial prefrontal cortex
- MRI:
-
Magnetic resonance imaging
- NOS:
-
Newcastle-Ottawa Scale
- PET:
-
Fluorodeoxyglucose positron emission tomography
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- ROI:
-
Region of interest
- SCD:
-
Subjective cognitive decline
- VBM:
-
Voxel-based morphometry
- WoS:
-
Web of Science
References
Ferri C. Global prevalence of dementia. Lancet London. 2005;1(9503):2112–7.
Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007;3(3):186–91.
Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu Y-T, Prina M. World Alzheimer Report 2015 - The Global Impact of Dementia: an analysis of prevalence, incidence, cost and trends. Alzheimer’s Dis Int. 2015.
Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–46.
Jessen F, Amariglio RE, Van Boxtel M, Breteler M, Ceccaldi M, Chételat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014;10(6):844–52.
Molinuevo JL, Rabin LA, Amariglio R, Buckley R, Dubois B, Ellis KA, et al. Implementation of Subjective Cognitive Decline criteria in research studies HHS Public Access Author manuscript. Alzheimers Dement. 2017;13(3):296–311 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5344703/pdf/nihms841894.pdf.
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7(3):280–92.
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–29.
Voormolen EHJ, Wei C, Chow EWC, Bassett AS, Mikulis DJ, Crawley AP. Voxel-based morphometry and automated lobar volumetry: the trade-off between spatial scale and statistical correction. Neuroimage. 2010;49(1):587–96.
Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48(2):371–80. https://doi.org/10.1016/j.neuroimage.2009.06.043.
Kiuchi K, Kitamura S, Taoka T, Yasuno F, Tanimura M, Matsuoka K, et al. Gray and white matter changes in subjective cognitive impairment, amnestic mild cognitive impairment and Alzheimer’s disease: a voxel-based analysis study. Chao L, editor. PLoS One. 2014;9(8):e104007. https://doi.org/10.1371/journal.pone.0104007.
Perrotin A, La Joie R, de La Sayette V, Barré L, Mézenge F, Mutlu J, et al. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: differential affective and imaging correlates. Alzheimer’s Dement. 2017;13(5):550–60. https://doi.org/10.1016/j.jalz.2016.08.011.
Selnes P, Fjell AM, Gjerstad L, Bjørnerud A, Wallin A, Due-Tønnessen P, et al. White matter imaging changes in subjective and mild cognitive impairment. Alzheimer’s Dement. 2012;8(SUPPL. 5):S112–21. https://doi.org/10.1016/j.jalz.2011.07.001.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013.
Chételat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, et al. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67(3):317–24.
Chételat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, et al. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67(3):317–24. https://doi.org/10.1002/ana.21955.
Choi Y, Yoon B-N, Choi SH, Lim MK, Kim H-J, Yang D-W. Reduced gray matter volume in subjective cognitive decline: a voxel-based morphometric study. Dement Neurocognitive Disord. 2015;14(4):143. https://doi.org/10.12779/dnd.2015.14.4.143.
Dong G, Yang L, Li C, Shan R, Wang X, Zhang Y, et al. Dynamic network connectivity predicts subjective cognitive decline: the Sino-Longitudinal Cognitive impairment and dementia study. Brain Imaging Behav. 2020;14(6):2692–707.
Erk S, Spottke A, Meisen A, Wagner M, Walter H, Jessen F. Evidence of neuronal compensation during episodic memory in subjective memory impairment. Arch Gen Psychiatry. 2011;68(8):845–52.
Hafkemeijer A, Altmann-Schneider I, Oleksik AM, van de Wiel L, Middelkoop HAM, van Buchem MA, et al. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect. 2013;3(4):353–62. https://doi.org/10.1089/brain.2013.0144.
Hafkemeijer A, Altmann-Schneider I, Oleksik AM, van de Wiel L, Middelkoop HAM, van Buchem MA, et al. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect. 2013;3(4):353–62.
Hong YJ, Yoon B, Shim YS, Ahn KJ, Yang DW, Lee J-H. Gray and white matter degenerations in subjective memory impairment: comparisons with normal controls and mild cognitive impairment. J Korean Med Sci. 2015;30(11):1652. https://doi.org/10.3346/jkms.2015.30.11.1652.
Liang L, Zhao L, Wei Y, Mai W, Duan G, Su J, et al. Structural and functional hippocampal changes in subjective cognitive decline from the community. Front Aging Neurosci. 2020;17:12. https://doi.org/10.3389/fnagi.2020.00064/full.
Parker AF, Smart CM, Scarapicchia V, Gawryluk JR. Identification of earlier biomarkers for Alzheimer’s disease: a multimodal neuroimaging study of individuals with subjective cognitive decline. J Alzheimer’s Dis. 2020;77(3):1067–76.
Perrotin A, de Flores R, Lamberton F, Poisnel G, La Joie R, de la Sayette V, et al. Hippocampal subfield volumetry and 3D surface mapping in subjective cognitive decline. Tales A, Jessen F, Butler C, Wilcock G, Phillips J, Bayer T, editors. J Alzheimer’s Dis. 2015;48(s1):S141–50. https://doi.org/10.3233/JAD-150087.
Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–42.
Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kolsch H, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79(13):1332–9. https://doi.org/10.1212/WNL.0b013e31826c1a8d.
Sun Y, Dai Z, Li Y, Sheng C, Li H, Wang X, et al. Subjective cognitive decline: mapping functional and structural brain changes—a combined resting-state functional and structural MR imaging study. Radiology. 2016;281(1):185–92. https://doi.org/10.1148/radiol.2016151771.
Xue C, Sun H, Hu G, Qi W, Yue Y, Rao J, et al. Disrupted patterns of rich-club and diverse-club organizations in subjective cognitive decline and amnestic mild cognitive impairment. Front Neurosci. 2020;14:575652. https://doi.org/10.3389/fnins.2020.575652/full.
Beckett LA, Donohue MC, Wang C, Aisen P, Harvey DJ, Saito N. The Alzheimer’s Disease Neuroimaging Initiative phase 2: increasing the length, breadth, and depth of our understanding. Alzheimer’s Dement. 2015;11(7):823–31. https://doi.org/10.1016/j.jalz.2015.05.004.
Caillaud M, Hudon C, Boller B, Brambati S, Duchesne S, Lorrain D, et al. Evidence of a relation between hippocampal volume, white matter hyperintensities, and cognition in subjective cognitive decline and mild cognitive impairment. J Gerontol Ser B Psychol Sci Soc Sci. 2020;75(7):1382–92.
Cantero JL, Iglesias JE, Van Leemput K, Atienza M. Regional hippocampal atrophy and higher levels of plasma amyloid-beta are associated with subjective memory complaints in nondemented elderly subjects. J Gerontol Ser A Biol Sci Med Sci. 2016;71(9):1210–5. https://doi.org/10.1093/gerona/glw022.
Cherbuin N, Sargent-Cox K, Easteal S, Sachdev P, Anstey KJ. Hippocampal atrophy is associated with subjective memory decline: the PATH through life study. Am J Geriatr Psychiatry. 2015;23(5):446–55 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1064748114002255.
Cong S, Risacher SL, West JD, Wu Y-C, Apostolova LG, Tallman E, et al. Volumetric comparison of hippocampal subfields extracted from 4-minute accelerated vs. 8-minute high-resolution T2-weighted 3T MRI scans. Brain Imaging Behav. 2018;12(6):1583–95. https://doi.org/10.1007/s11682-017-9819-3.
Fan LY, Lai YM, Chen TF, Hsu YC, Chen PY, Huang KZ, et al. Diminution of context association memory structure in subjects with subjective cognitive decline. Hum Brain Mapp. 2018;39(6):2549–62.
Fan L-Y, Lai Y-M, Chen T, Hsu Y-C, Chen P, Huang K-Z, et al. Diminution of context association memory structure in subjects with subjective cognitive decline. Hum Brain Mapp. 2018;39(6):2549–62. https://doi.org/10.1002/hbm.24022.
van der Flier WM, van Buchem MA, Weverling-Rijnsburger AWE, Mutsaers ER, Bollen ELEM, Admiraal-Behloul F, et al. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol. 2004;251(6):671–5.
Ivanoiu A, Dricot L, Gilis N, Grandin C, Lhommel R, Quenon L, et al. Classification of non-demented patients attending a memory clinic using the new diagnostic criteria for Alzheimer’s disease with disease-related biomarkers. J Alzheimer’s Dis. 2015;43(3):835–47.
Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27(12):1751–6 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0197458005003416.
Lindberg O, Mårtensson G, Stomrud E, Palmqvist S, Wahlund L-O, Westman E, et al. Atrophy of the posterior subiculum is associated with memory impairment, tau- and Aβ pathology in non-demented individuals. Front Aging Neurosci. 2017;20:9. https://doi.org/10.3389/fnagi.2017.00306/full.
López-Sanz D, Garcés P, Álvarez B, Delgado-Losada ML, López-Higes R, Maestú F. Network disruption in the preclinical stages of Alzheimer’s disease: from subjective cognitive decline to mild cognitive impairment. Int J Neural Syst. 2017;27(08):1750041. https://doi.org/10.1142/S0129065717500411.
López-Sanz D, Bruña R, Garcés P, Camara C, Serrano N, Rodríguez-Rojo IC, et al. Alpha band disruption in the AD-continuum starts in the subjective cognitive decline stage: a MEG study. Sci Rep. 2016;6(1):37685 Available from: http://www.nature.com/articles/srep37685.
Marcotte C, Potvin O, Collins DL, Rheault S, Duchesne S. Brain atrophy and patch-based grading in individuals from the CIMA-Q study: a progressive continuum from subjective cognitive decline to AD. Sci Rep. 2019;9(1):13532 Available from: http://www.nature.com/articles/s41598-019-49914-3.
Risacher SL, WuDunn D, Tallman EF, West JD, Gao S, Farlow MR, et al. Visual contrast sensitivity is associated with the presence of cerebral amyloid and tau deposition. Brain Commun. 2020;2(1):1–14.
Rogne S, Vangberg T, Eldevik P, Wikran G, Mathiesen EB, Schirmer H. Magnetic resonance volumetry: prediction of subjective memory complaints and mild cognitive impairment, and associations with genetic and cardiovascular risk factors. Dement Geriatr Cogn Dis Extra. 2016;6(3):529–40 Available from: https://www.karger.com/Article/FullText/450885.
Ryu SY, Lim EY, Na S, Shim YS, Cho JH, Yoon B, et al. Hippocampal and entorhinal structures in subjective memory impairment: a combined MRI volumetric and DTI study. Int Psychogeriatrics. 2017;29(5):785–92 Available from: https://www.cambridge.org/core/product/identifier/S1041610216002349/type/journal_article.
Scheef L, Grothe MJ, Koppara A, Daamen M, Boecker H, Biersack H, et al. Subregional volume reduction of the cholinergic forebrain in subjective cognitive decline (SCD). NeuroImage Clin. 2019;21:101612 Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213158218303656.
Schultz SA, Oh JM, Koscik RL, Dowling NM, Gallagher CL, Carlsson CM, et al. Subjective memory complaints, cortical thinning, and cognitive dysfunction in middle-age adults at risk of AD. Alzheimer’s Dement Diagn Assess Dis Monit. 2015;1(1):33–40. https://doi.org/10.1016/j.dadm.2014.11.010.
Shu N, Wang X, Bi Q, Zhao T, Han Y. Disrupted topologic efficiency of white matter structural connectome in individuals with subjective cognitive decline. Radiology. 2018;286(1):229–38. https://doi.org/10.1148/radiol.2017162696.
Striepens N, Scheef L, Wind A, Popp J, Spottke A, Cooper-Mahkorn D, et al. Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement Geriatr Cogn Disord. 2010;29(1):75–81 Available from: https://www.karger.com/Article/FullText/264630.
Tepest R, Wang L, Csernansky JG, Neubert P, Heun R, Scheef L, et al. Hippocampal surface analysis in subjective memory impairment, mild cognitive impairment and Alzheimer’s dementia. Dement Geriatr Cogn Disord. 2008;26(4):323–9 Available from: https://www.karger.com/Article/FullText/161057.
van Rooden S, van den Berg-Huysmans AA, Croll PH, Labadie G, Hayes JM, Viviano R, et al. Subjective cognitive decline is associated with greater white matter hyperintensity volume, Zhou J, editor. J Alzheimer’s Dis. 2018;66(3):1283–94. https://doi.org/10.3233/JAD-180285.
Yue L, Wang T, Wang J, Li G, Wang J, Li X, et al. Asymmetry of hippocampus and amygdala defect in subjective cognitive decline among the community dwelling Chinese. Front Psychiatry. 2018;9:226. https://doi.org/10.3389/fpsyt.2018.00226/full.
Zajac L, Koo B-B, Tripodis Y, Mian A, Steinberg E, Mez J, et al. Hippocampal resting-state functional connectivity patterns are more closely associated with severity of subjective memory decline than whole hippocampal and subfield volumes. Cereb Cortex Commun. 2020;1(1):tgaa019. https://doi.org/10.1093/texcom/tgaa019/5847849.
Kim M-J, Seo SW, Kim GH, Kim ST, Lee J-M, Qiu A, et al. Less depressive symptoms are associated with smaller hippocampus in subjective memory impairment. Arch Gerontol Geriatr. 2013;57(1):110–5 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167494313000216.
Zhao W, Luo Y, Zhao L, Mok V, Su L, Yin C, et al. Automated brain MRI volumetry differentiates early stages of Alzheimer’s disease from normal aging. J Geriatr Psychiatry Neurol. 2019;32(6):354–64. https://doi.org/10.1177/0891988719862637.
Zhao W, Wang X, Yin C, He M, Li S, Han Y. Trajectories of the hippocampal subfields atrophy in the Alzheimer’s disease: a structural imaging study. Front Neuroinform. 2019;22:13. https://doi.org/10.3389/fninf.2019.00013/full.
Platero C, López ME, del CTM, Yus M, Maestu F. Discriminating Alzheimer’s disease progression using a new hippocampal marker from T1-weighted MRI: the local surface roughness. Hum Brain Mapp. 2019;40(5):1666–76. https://doi.org/10.1002/hbm.24478.
Yue L, Hu D, Zhang H, Wen J, Wu Y, Li W, et al. Prediction of 7-year’s conversion from subjective cognitive decline to mild cognitive impairment. Hum Brain Mapp. 2020;42:25216. https://doi.org/10.1002/hbm.25216.
Eliassen CF, Reinvang I, Selnes P, Grambaite R, Fladby T, Hessen E. Biomarkers in subtypes of mild cognitive impairment and subjective cognitive decline. Brain Behav. 2017;7(9):e00776. https://doi.org/10.1002/brb3.776.
Hong JY, Yun HJ, Sunwoo MK, Ham JH, Lee J-M, Sohn YH, et al. Cognitive and cortical thinning patterns of subjective cognitive decline in patients with and without Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(9):999–1003 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1353802014002314.
Lauriola M, Esposito R, Delli Pizzi S, de Zambotti M, Londrillo F, Kramer JH, et al. Sleep changes without medial temporal lobe or brain cortical changes in community-dwelling individuals with subjective cognitive decline. Alzheimer’s Dement. 2017;13(7):783–91 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1552526016331053.
Meiberth D, Scheef L, Wolfsgruber S, Boecker H, Block W, Träber F, et al. Cortical thinning in individuals with subjective memory impairment. J Alzheimer’s Dis. 2015;45(1):139–46. https://doi.org/10.3233/JAD-142322.
Niemantsverdriet E, Ribbens A, Bastin C, Benoit F, Bergmans B, Bier J-C, et al. A retrospective Belgian multi-center MRI biomarker study in Alzheimer’s disease (REMEMBER). Mecocci P, editor. J Alzheimer’s Dis. 2018;63(4):1509–22. https://doi.org/10.3233/JAD-171140.
Lim EY, Shim YS, Hong YJ, Ryu SY, Cho AH, Yang DW. Different cortical thinning patterns depending on their prognosis in individuals with subjective cognitive decline. Dement Neurocognitive Disord. 2019;18(4):113. https://doi.org/10.12779/dnd.2019.18.4.113.
Ivanoiu A, Dricot L, Gilis N, Grandin C, Lhommel R, Quenon L, et al. Classification of non-demented patients attending a memory clinic using the new diagnostic criteria for Alzheimer’s disease with disease-related biomarkers. J Alzheimer’s Dis. 2014;43(3):835–47. https://doi.org/10.3233/JAD-140651.
Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease ☆ ☆This research was supported by grants P01 AG09466 and P30 AG10161 from the National Institute on Aging, National Institutes of Health. Neurobiol Aging. 2001;22(5):747–54.
Visser PJ, Verhey FRJ, Hofman PAM, Scheltens P, Jolles J. Medial temporal lobe atrophy predicts Alzheimer’s disease in patients with minor cognitive impairment. J Neurol Neurosurg Psychiatry. 2002;72(4):491–7.
Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: meta-analyses of MRI studies. Hippocampus. 2009;19(11):1055–64.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. https://doi.org/10.1007/BF00308809.
Frisoni GB, Beltramello A, Weiss C, Geroldi C, Bianchetti A, Trabucchi M. Linear measures of atrophy in mild Alzheimer disease. Am J Neuroradiol. 1996;17(5):913–23.
Laakso MP, Soininen H, Partanen K, Helkala EL, Hartikainen P, Vainio P, et al. Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer’s disease: correlation with memory functions. J Neural Transm Park Dis Dement Sect. 1995;9(1):73–86.
Bonilha L, Kobayashi E, Cendes F, Li LM. Protocol for volumetric segmentation of medial temporal structures using high-resolution 3-D magnetic resonance imaging. Hum Brain Mapp. 2004;22(2):145–54.
Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10(4):433–42.
Tobias H, Merkle EM, Reiner CS, Davenport MS, Horvath JJ, Feuerlein S, et al. Reproducibility of dynamic part II. Comparison of intra- and interobserver variability with manual region of interest placement versus. Radiology. 2013;266(3):812–21.
Rodríguez-Gómez O, Abdelnour C, Jessen F, Valero S, Boada M. Influence of sampling and recruitment methods in studies of subjective cognitive decline. J Alzheimer’s Dis. 2015;48(S1):S99–107.
Acknowledgements
We are grateful to Dr. Álvaro Murillo and Dr. Verónica Moreno-Juan for advice on manuscript preparation.
Funding
No funding was received for this publication.
Author information
Authors and Affiliations
Contributions
P. A., O. E-Z and M. R. designed the study and prepared and wrote the manuscript. M. A. F-S prepared and wrote the manuscript. P. A. and O. E-Z performed and analysed the parallel searches and their resulting data. G. M. A. designed, performed and analysed statistical analyses. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Arrondo, P., Elía-Zudaire, Ó., Martí-Andrés, G. et al. Grey matter changes on brain MRI in subjective cognitive decline: a systematic review. Alz Res Therapy 14, 98 (2022). https://doi.org/10.1186/s13195-022-01031-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-022-01031-6