Abstract
Background
Interactions between the gut microbiota, microglia, and aging may modulate Alzheimer’s disease (AD) pathogenesis but the precise nature of such interactions is not known.
Methods
We developed an integrated multi-dimensional, knowledge-driven, systems approach to identify interactions among microbial metabolites, microglia, and AD. Publicly available datasets were repurposed to create a multi-dimensional knowledge-driven pipeline consisting of an integrated network of microbial metabolite–gene–pathway–phenotype (MGPPN) consisting of 34,509 nodes (216 microbial metabolites, 22,982 genes, 1329 pathways, 9982 mouse phenotypes) and 1,032,942 edges.
Results
We evaluated the network-based ranking algorithm by showing that abnormal microglia function and physiology are significantly associated with AD pathology at both genetic and phenotypic levels: AD risk genes were ranked at the top 6.4% among 22,982 genes, P < 0.001. AD phenotypes were ranked at the top 11.5% among 9982 phenotypes, P < 0.001. A total of 8094 microglia–microbial metabolite–gene–pathway–phenotype–AD interactions were identified for top-ranked AD-associated microbial metabolites. Short-chain fatty acids (SCFAs) were ranked at the top among prioritized AD-associated microbial metabolites. Through data-driven analyses, we provided evidence that SCFAs are involved in microglia-mediated gut–microbiota–brain interactions in AD at both genetic, functional, and phenotypic levels.
Conclusion
Our analysis produces a novel framework to offer insights into the mechanistic links between gut microbial metabolites, microglia, and AD, with the overall goal to facilitate disease mechanism understanding, therapeutic target identification, and designing confirmatory experimental studies.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is the leading cause of dementia and the most common neurodegenerative disorder, affecting over 5.5 million people in the USA and 47 million people worldwide [1]. AD is complex, with genetic, epigenetic, and environmental factors contributing to disease susceptibility and progression [2].
Trillions of bacteria in the human body (human microbiota) may affect human health and diseases by modulating host functions through small molecule metabolites. Undigested dietary components are fermented by microbiota to produce a wide array of metabolites such as bile acids, choline, and short-chain fatty acids (SCFAs) that are essential for human health [3,4,5]. Metabolite activities of gut microbiota provide a mechanistic connection between environmental factors and brain function and behavior [6,7,8]. The gut microbiota of AD patients has altered microbial diversity and is compositionally distinct from control age- and sex-matched individuals [9,10,11]. Recent studies showed that altered serum levels of bile acids, lipopolysaccharide, SCFAs, and trimethylamine-N-oxide (TMAO) were associated with cognitive impairment in AD [12,13,14,15].
In AD, microglia are involved in amyloid-β (Aβ) clearance in the brain and many innate immunity genes are associated with the risk of sporadic AD [16]. Microglia are the main neuroimmune cells involved in the development, normal functioning, aging, and injury of the central nervous system [16,17,18]. Gene variants in TREM2 and CD33 that modulate macrophage and microglial function increase the risk for late-onset AD [19]. There is increasing evidence that the interactions between the gut microbiota and brain innate immune system (gut–immune–brain axis) may modulate AD pathogenesis through microglial maturation and function [20,21,22]. Short-chain fatty acids (SCFAs), the end products of fermentation of dietary fibers by gut microbiota, play a major role in the maintenance of gut and immune homeostasis [23, 24]. SCFAs may play a key role in microbiota–gut–brain crosstalk. In vitro administration of SCFAs (microbial fermentation metabolites) regulated microglia homeostasis and obstructed Aβ protein aggregation [25]. Supplementation of SCFAs in germ-free mice rescued the immature genetic and morphological phenotype of microglia [20]. Despite such growing links, the mechanisms underlying how gut microbial metabolites including SCFAs interact with microglia and host genetics in promoting or protecting against AD remain largely unknown.
We have previously demonstrated that data-driven computational systems approaches have the potential in uncovering mechanistic links between microbial metabolites and human diseases [26,27,28,29]. For example, in a prior study, we provided evidence that trimethylamine N-oxide (TMAO), a human gut microbial metabolite of dietary meat and fat, was linked to AD [27], a finding that was subsequently confirmed by an experimental study [15]. In this study, we significantly expanded our prior work to produce the first comprehensive, multi-dimensional, systems framework of analyzing and identifying complex interrelationships among gut microbial metabolites, microglia, and AD at both genetic and functional levels. We first constructed a multi-model context-sensitive network to model complex and heterogeneous interrelationships among microbial metabolites, genes, pathways, and disease phenotypes. Then network-based prioritization algorithm prioritized microbial metabolites based on their relevance to microglia physiology and functions in AD. The overall goal of this study was to identify potential microglia–microbial metabolite–gene–pathway–phenotype interactions in AD with supporting evidence at genetic, functional, and phenotypic levels, which can set a foundation for others to conduct hypothesis-driven studies to test these interactions in experimental models or clinical samples.
Methods
Overview
The overall experiment (Fig. 1) consisted of (1) network construction: an integrated network of metabolite–gene–pathway–phenotype (MGPPN) was constructed from multiple data resources to model and capture the complex and heterogeneous interrelationships among microbial metabolites, genes, pathways, and disease phenotypes; (2) network-based prioritization: microbial metabolites were prioritized from MGPPN based on their relevance to microglia physiology and functions. The algorithm was evaluated using known AD-specific phenotypes, genes, and microbial metabolites. (3) Identification of microglia–microbial metabolite–gene–pathway–phenotype interactions in AD: Based on the top-ranked microglia-associated microbial metabolites, we identified interactions among microglia, microbial metabolites, genes, pathways, and phenotypes (“microglia-microbial metabolite-gene-pathway-phenotype interactions”) in AD. (4) Case study of SCFAs: We analyzed how SCFAs are involved in the microglia–microbiota–brain interactions in AD.
Network construction
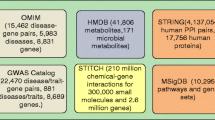
MGPPN consisted of four sub-networks: human metabolite–gene network (MGN), mouse phenotype–gene network (PhenGN), protein–protein interaction network (PPIN), and pathway–gene network (PathGN). The numbers of nodes (metabolites, genes, pathways, phenotypes) and edges (connections between nodes) of each sub-network are shown in Table 1.
Human microbial metabolite–gene network (MGN)
Metabolites found in the human body can originate from different sources, including human hosts, plants, foods, microbes, toxins, pollutants, cosmetics, and medications, among others. In this study, we focused on microbial metabolites that are present in the human body and that are produced (not necessarily exclusively) by microbes. Human Metabolome Database (HMDB) contains 114,304 small molecule metabolites found in the human body, among which 220 metabolites were originated in microbiota [30] (data accessed in October 2020). Based on manual curation effort in HMDB, 12 microbial metabolites are associated with AD (Table 2).
Microbial metabolite-associated genes were then obtained from the STITCH (Search Tool for Interactions of Chemicals) database by mapping microbial metabolites from HMDB to chemicals in STITCH. The STITCH database contains 15,473,939 chemical–gene associations found in the human body, representing 473,602 chemicals and 18,701 human genes [31] (data accessed in October 2020). Among the 220 microbial human metabolites, 216 were mapped to chemicals in STITCH. For example, three SCFAs (acetic acid, butyric acid, propionic acid) are associated with 3926, 815, and 459 human genes, respectively. Bile acids (deoxycholic acid, taurodeoxycholic acid, chenodeoxycholic acid) are associated with 258, 57, and 129 human genes, respectively. MGN is a weighted network consisting of 12,130 nodes (216 metabolite nodes, 11,914 gene nodes) and 67,012 edges (metabolite–gene associations), with edge weights directly derived from the chemical–gene association weights (ranging from 100 to 999).
Mutational phenotype–gene network (PhenGN)
PhenGN was constructed from MGD that contains The Mouse Genome Database (MGD) database large amounts of phenotypic descriptions of systematic genetic knockouts in mouse models [32]. A total of 517,381mutational/causal phenotype–gene annotations (9982 phenotypes and 11,021 mapped human genes) were obtained from MGD. We have used these strong causal gene–phenotype associations for screening and validating functional effects of drugs and microbial metabolites on disease phenotypes [33,34,35,36]. In this study, we developed a network-based model to model and assess phenotypic effects of the interactions of microglia, microbial metabolites, human genes, and pathways on AD-specific phenotypes. PhenGN allows us to interrogate causal microglia–gut–brain connections and examine how these interactions affect specific AD phenotypes such as “amyloid beta deposits,” “tau protein deposits,” or “neurodegeneration.” PhenGN is unweighted (same weights for all phenotype–gene connections on the network) and consisted of 21,003 nodes (9982 phenotype nodes, 11,021 gene nodes) and 517,381 edges.
Pathway–gene network (PathGN)
We obtained gene–pathway associations from the Molecular Signatures Database (MSigDB) (data accessed in June 2020). MSigDB is a comprehensive resource of annotated pathways and gene sets [37]. PathGN is unweighted (same weights for all pathway–gene connections on the network) and consisted of 10,197 nodes (8868 gene nodes, 1329 pathway nodes) and 66,293 edges (gene–pathway associations).
Protein–Protein Interaction Network (PPIN)
PPIN was directly constructed from the protein–protein interactions from the human protein reference database [38]. PPIN is weighted and consisted of 22,982 gene nodes and 382,256 gene–gene edges, with weights (ranging from 100 to 999) directly obtained from BioGrid.
Links between MGN, PhenGN, PathGN, and PPIN
These sub-networks were connected through common gene nodes. For example, MGN are linked to the PhenGN network through shared gene nodes.
Network prioritization and evaluation
Network prioritization
The goal of this study is to identify microbial metabolites associated with abnormal microglia function and physiology that affect AD at the genetic, functional, and phenotypic levels. For a given input related to disrupted microglia physiology and function (i.e., microgliosis, abnormal microglial cell physiology, abnormal microglial cell morphology), the algorithm prioritized genes, phenotypes, and microbial metabolites from MGPPN based on the context-sensitive network-based ranking algorithm that we previously developed [39,40,41,42,43]. The random walk-based approach is briefly described below. More details are in our previous papers [23, 24, 30, 31, 44]. The movements of a random walker between any two sub-networks were regulated with jumping probabilities \({\lambda}_{N_i{N}_j}\) (Ni and Nj can be any of the four sub-networks). For example, if a random walker stands on a gene node on MGN, which is connected with both PhenGN, PathGN, and PPIN, it has the option to walk to PhenGN with the probability λ12, to PathGN with the probability λ13, and to PPIN with the probability λ14 or stay within MGN with the probability 1 − λ12 − λ13 − λ14. Given the seed node(s)/inputs, the ranking score for each node is iteratively updated by:
S k + 1 is the score vector at step k + 1, S0 is the initial vector, and 1 − α is the restarting probability, and M is the transition matrix. The transition matrix M was calculated as follows in Eq. 2 and 3):
M consisted of 16 sub-matrices, each contains the transition probabilities within or between four sub-networks. Each sub-matrix was calculated by normalizing the rows in the adjacency matrix of the corresponding sub-networks using the jumping probabilities. Specifically, the off-diagonal sub-matrices corresponded to the bipartite networks that connected each two networks. These sub-matrices were calculated by first normalizing the rows of the bipartite network \({A}_{N_i{N}_j}\) and then weighing each row by the jumping probability \({\lambda}_{N_i{N}_j}\):
The diagonal sub-matrices corresponded to the transition probabilities within each one of the four sub-networks and were calculated by first normalizing the rows of adjacency matrix for MGN, PhenGN, PathGN, and PPIN and then weighing the rows by the probability of staying in the same network:
In Eq. 4, \({A}_{N_i}\) is the adjacency matrix of the sub-matrix Ni, and \({I}_{N_j}\)is an indicator function, whose value is 1 if the kth row of \({A}_{N_i{N}_j}\)contains at least one non-zero element. The output from the context-sensitive network-based algorithm was a list of microbial metabolites, genes, and phenotypes prioritized based on their genetic, functional, phenotypic, and microbial relevance to disrupted microglia function based on the inputs: “microgliosis,” “abnormal microglial cell physiology,” and “abnormal microglial cell morphology.”
Evaluation
It is unknown which microbial metabolites are associated with microglia–brain interactions in AD. However, it is known that microglial function is closely associated with AD etiology. We evaluated the context-sensitive network-based ranking algorithm by showing that abnormal microglia function and physiology are indeed significantly involved in AD pathology at both genetic and phenotypic levels. We also showed that microbial metabolites are involved in AD through microglia functions by demonstrating the known AD-associated microbial metabolites were ranked highly by the algorithm for the inputs (“microgliosis,” “abnormal microglial cell physiology,” and “abnormal microglial cell morphology”).
Evaluate microglia–AD interactions at the phenotypic level
There is a large, ongoing effort to characterize AD models and identify core AD-related phenotypes. We obtained all core phenotypes from commonly used AD mouse models [45]. These phenotypes include a range of core AD-related phenotypes including plaques, tangles, neuronal loss, gliosis, synaptic loss, changes in LTP/LTD, and cognitive impairment (Table 2). We evaluated whether the prioritization algorithm ranked these AD phenotypes highly among a total of 9982 phenotypes on the network, when three microglia-related phenotypes (“abnormal microglial cell morphology,” “abnormal microglial cell physiology,” and “microgliosis”) were used as inputs separately and combined. Mean ranking and median rankings of these 13 phenotypes among the list of prioritized 9982 phenotypes were calculated and compared to the average ranking of 50% expected from random ranking. Significance was calculated using the two-sample t-test.
Evaluate microglia–AD interactions at the genetic level
Hundreds of genes are known to be associated with AD. In this study, we used genes known to be strongly associated with AD to evaluate microglia–AD interactions at the genetic level. We obtained all AD-associated genes (22 in total) from two well-established disease genetics databases Online Mendelian Inheritance in Man (OMIM) [46] and ClinVar [47] (Table 2). OMIM contains all known mendelian disorders and over 16,000 genes. ClinVar contains disease–gene associations with supporting evidence of clinical significance. While this list may not be complete, we used it to evaluate whether the prioritization algorithm ranked these AD genes highly among a total of 22,982 genes, when three microglia-related phenotypes (“abnormal microglial cell morphology,” “abnormal microglial cell physiology,” and “microgliosis”) were used as inputs separately and combined. Mean ranking and median rankings of these 22 AD genes among the list of prioritized 22,982 genes were calculated and compared to the average ranking of 50% expected from random ranking. Significance was calculated using the two-sample t-test.
Evaluate microglia–AD interactions at the microbial metabolism level
We obtained 12 AD–microbial metabolite associations from HMDB [30], which were manually curated from published biomedical research articles (Table 2). Note that these AD-associated microbial metabolites were produced by microbes, but not necessarily exclusively so. We evaluated whether the prioritization algorithm ranked these AD-associated microbial metabolites highly among a total of 216 microbial metabolites on the network, when three microglia-related phenotypes (“abnormal microglial cell morphology,” “abnormal microglial cell physiology,” and “microgliosis”) were used as inputs separately and combined. Mean ranking and median rankings of these 12 AD-associated microbial metabolites among the list of prioritized 216 microbial metabolites were calculated and compared to the average ranking of 50% expected from random ranking. Significance was calculated using the two-sample t-test.
Identify microglia–microbial metabolite–gene–pathway–phenotype interactions in AD
We identified microbial metabolite–microglia–gene–pathway–phenotype interactions in AD based on top-ranked microbial metabolites (top 20% in this study). For each top-ranked microglia-associated microbial metabolite (e.g., acetic acid), we obtained its associated genes from the STITCH (Search Tool for Interactions of Chemicals) database [31]. The confidence score of chemical–gene associations in STITCH ranges from 100 to 999. In this study, we used the cutoff score of 500. Since a microbial metabolite can target many genes and pathways, many of which are not involved in AD, we then filtered microbial metabolite-associated genes using the 22 known AD risk genes (Table 2) in order to find AD genes targeted by the microbial metabolite. For example, at the cutoff value of 500, butyric acid is associated with 1787 genes, among which three are strong AD genes (APP, NOS3, VEGFA). From microbial metabolite-associated AD genes, microbial metabolite–gene–pathway–phenotype associations were identified by linking microbial metabolite-associated AD genes to their pathways based on gene–pathway associations from Molecular Signatures Database (MSigDB) [37] and gene–phenotype annotations from the Mouse Genome Database (MGD) database [32]. For example, based on the acetic acid–APP association, we obtained “acetic acid-APP-inflammasomes-amyloid beta deposits” and “acetic acid-APP-GPCR ligand binding-amyloid beta deposits” interactions that are mediated by microglia.
Case study of SCFAs
Short-chain fatty acids (SCFAs) are the end products of fermentation of dietary fibers by gut microbiota and play a major role in the maintenance of gut and immune homeostasis [23, 24]. Both in vitro and in vivo studies suggested that SCFA may play a key role in microbiota–gut–brain crosstalk [20, 25]; however, the mechanisms through which SCFAs might influence brain functioning in AD have not been fully elucidated [44]. We first examined whether three SCFAs (acetic acid, butyric acid, and propionic acid) were ranked highly by the network-based ranking algorithm given the input microglia-associated phenotypes. We then examined top AD-associated genes, pathways, and phenotypes targeted by each of these three SCFAs.
Results
Validation: disrupted microglia function and physiology are significantly associated with AD at both genetic and phenotypic levels
As a validation step, we examined how abnormal microglial function and physiology were associated with AD-specific pathological phenotypes and causal genes. Using three seeds (“microgliosis,” “abnormal microglial cell physiology,” “abnormal microglial cell morphology”) alone or combined, the network-based ranking algorithm prioritized a total of 10,122 mouse mutational phenotypes. Among the prioritized phenotypes, the 13 AD-associated phenotypes (described in the “Methods” section) ranked significantly highly than random expectation (Table 3). For example, when the seed “microgliosis” was used as the input, the AD-associated phenotype “neurodegeneration,” “amyloid beta deposits,” and “tau protein deposits” ranked at the top 0.30%, 2.36%, and 10.42%, respectively. These findings are consistent with published findings that microglia are involved in AD pathologies, including tau protein spreading [48]. These results confirmed the validity of our network-based ranking algorithms in studying etiologies of AD and that microglia dysfunction is mechanistically involved in AD pathologies (“microglia-AD axis”).
We then examined how abnormal microglia function and physiology was associated with AD-specific causal genes. Using three seeds (“microgliosis,” “abnormal microglial cell physiology,” “abnormal microglial cell morphology”) alone or combined, the network-based ranking algorithm prioritized a total of 23,995 human genes. The 22 AD-associated genes (Table 2) ranked significantly highly among the list of prioritized human genes (Table 4). For example, when the seed “microgliosis” was used as the input, AD-associated genes “APP,” “PSEN1,” “PRNP,” “PSEN2,” “APOE,” “TNF,” and “VEGFA” ranked at the top 0.004%, 0.02%, 0.04%, 0.10%, 0.38%, 0.39%, and 0.59% among a total of 23,995 prioritized genes, respectively. The fact that many AD-associated genes ranked highly for all inputs indicates that abnormal microglial function is involved in multiple AD-related genetic functions. However, not all AD-associated genes ranked highly. For example, when the seed “microgliosis” was used as the input, gene MT-ND1, the NADH dehydrogenase subunit 1 gene, ranked at the top 60.23%, suggesting that microgliosis may contribute to AD etiology independent of oxidative phosphorylation in mitochondria.
Microbial metabolites underlie microglia–brain interactions in AD
The 12 known AD-associated microbial metabolites (described in the “Methods” section) ranked significantly highly among a total of 220 prioritized microbial metabolites for three inputs (“abnormal microglial cell morphology,” “abnormal microglial cell physiology,” and “microgliosis”) separately or combined (Table 5). These results supported our hypothesis that microbial metabolites are involved in microglia-mediated AD pathologies.
Among the 220 microbial metabolites present in human analyzed, the top 44 microbial metabolites for the combined input (“microgliosis,” “abnormal microglial cell physiology,” “abnormal microglial cell morphology”) are listed in Table 6. The entire list of prioritized microbial metabolites for the combined input “microgliosis,” “abnormal microglial cell physiology,” and “abnormal microglial cell morphology” is in Supplement_S1. Among the top-ranked microbial metabolites that were predicted to be associated with microglia–AD interactions include three short-chain fatty acids or SCFAs (acetic acid, propionic acid, butyric acid) that are metabolic products of bacterial dietary fiber fermentation. Many others are produced by both microbes and human hosts, such as dopamine and gamma-aminobutyric acid. Our results are consistent with the recently published finding of protective roles of SCFAs in AD-type beta-amyloid neuropathological mechanisms [16].
Microglia–microbial metabolite–gene–pathway–phenotype interactions in AD
A total of 8094 microglia–microbial metabolite–gene–pathway–phenotype interactions in AD were identified for the top 20% (top 44) ranked microbial metabolites (Supplement_S2). For example, acetic acid, the top microglia-associated microbial metabolite, is involved in 446 microglia–microbial metabolite–gene–pathway–phenotype interactions in AD, including microglia-acetic acid-APP-inflammasomes-tau protein deposits, microglia-acetic acid-NOS3-calcium signaling-abnormal long-term potentiation, microglia-acetic acid-VEGFA-cytokine-cytokine interaction-astrocytosis, among others. The top 10 most frequently targeted AD genes, pathways, and phenotypes by top 44 microbial metabolites are shown in Table 7. For example, APP and TNF are targeted by 21 and 9 of the top 44 microbial metabolites, respectively. Pathways “Alzheimer’s disease” and “Amyloids” are targeted by 22 and 21 of the top 44 microbial metabolites, respectively. Phenotypes “abnormal synaptic transmission” and “amyloid beta deposits” are targeted by 27 and 22 of the top 44 microbial metabolites, respectively.
Short-chain fatty acids (SCFAs)
Three SCFAs ranked in the top 44 microglia-associated microbial metabolites: acetic acid (top 1), butyric acid (top 23), and propionic acid (top 36) (Table 6). APP gene is targeted by three SCFAs; a total of 8 (out of 12) AD phenotypes (abnormal long-term potentiation, abnormal synaptic transmission, amyloid beta deposits, amyloidosis, astrocytosis, gliosis, microgliosis, and tau protein deposits) are associated with three SCFAs. The 30 shared pathways targeted by three SCFAs (Table 8) include known AD-related genetic pathways, including “Alzheimer’s disease,” amyloids, and immune systems. These results indicated that SCFAs are mechanistically involved in microglia-mediated gut–microbiota–brain interactions in AD at both genetic, functional, and phenotypic levels.
Discussion
We developed a knowledge-driven context-sensitive network-based framework to identify microglia–microbial metabolite–gene–pathway–phenotype interactions in AD. The approach innovatively repurposed publicly available datasets collected for other purposes to study the gut–microbiota–microglia–brain interactions in AD. We validated this computational framework by showing that abnormal microglia function/physiology are indeed significantly associated with AD at both the genetic, phenotypic, and microbial metabolism levels. We identified a total of 8094 potential microglia–microbial metabolite–gene–pathway–phenotype interactions in AD for the top-ranked microbial metabolites.
There is evidence in the literature associating many of the top-ranked 44 microglia-associated microbial metabolites with AD. For example, in vitro administration of SCFAs (microbial fermentation metabolites) regulated microglia homeostasis and obstructed Aβ protein aggregation [25]. Supplementation of SCFAs in germ-free mice rescued the immature genetic and morphological phenotype of microglia [20]. Three SCFAs may play a key role in microbiota–gut–brain crosstalk [44] and have protective roles in AD-type beta-amyloid neuropathological mechanisms [48]. Despite such growing links, the mechanisms underlying how gut microbial metabolites interact with microglia and host genetics in promoting or protecting against AD remain largely unknown. Our analysis provided potential mechanistic insights into how microbial metabolites SCFAs (acetic acid, butyric acid, and propionic acid) are involved in microglia functions in AD at genetic, functional, and phenotypic levels: they are among top microbial metabolites prioritized for microglia functions, all target APP gene and AD-related pathways including “Alzheimer’s disease,” “Amyloids,” and “Immune systems” and are associated with multiple AD-related phenotypes including “abnormal long term potentiation,” “abnormal synaptic transmission,” “amyloid beta deposits,” “amyloidosis,” “astrocytosis,” “gliosis,” “microgliosis,” and “tau protein deposits.” Details of these links are provided in Supplement_S2.
Many studies reported associations among alcohol consumption, dementia, and cognitive decline [49,50,51], but the findings remain controversial [52]. The effect of alcohol on cognitive functions and AD pathogenesis and the underlying molecular mechanisms remain elusive. In our study, ethanol ranked at top 2 among microglia-associated microbial metabolites. It targets NOS3 and VEGFA and is associated with multiple AD core phenotypes including abnormal long-term potentiation, abnormal synaptic transmission, astrocytosis, gliosis, and neuron degeneration (see Supplement_S2). It remains an open question as to in what degree ethanol produced by microbiota play a role in AD pathogenesis and whether previous controversial findings of alcohol–AD associations could be partially explained by different levels of microbial ethanol production among different individuals.
The roles of glutamic acid in AD remain unclear. Our analysis suggests that glutamic acid targets APP and may be associated with amyloidosis, amyloid beta deposits, tau protein deposits, and neurodegeneration. The dopaminergic system and dopaminergic deficit may be involved in cognitive decline in AD [53, 54]. Concentrations of several monoaminergic neurotransmitters, their precursors, and metabolites such as dopamine and serotonin were significantly reduced in AD versus control patients [55]. Dopamine and serotonin were ranked at top 6 and 25 among 220 prioritized microglial-associated microbial metabolites. However, it remains unclear how gut microbiota-produced dopamine and serotonin, which normally are unable to cross the blood–brain barrier, exert their effects on the brain by restoring deficits of human dopaminergic transmitter systems.
A higher plasma level of lactic acid was found in significantly higher levels in Down syndrome patients with AD than Down syndrome patients without AD, suggesting that lactic acid may be involved in AD [56]. Our results showed that lactic acid targets APP and AD-associated pathways and are associated with amyloidosis, amyloid beta deposits, tau protein deposits, neurodegeneration, and other core AD phenotypes. These results are consistent with a prior study showing that increased levels of lactic acid lead to amyloidogenesis in AD by inducing APP aggregates [57].
Limitations
Our study has several limitations that warrant further discussion. First, our study is purely “in silico” and generated a large number of possible hypotheses (8094 potential microglia–microbial metabolite–gene–pathway–phenotype interactions in AD). We robustly evaluated the knowledge-driven network-based ranking algorithm by showing that microglia are involved in AD at both genetic and phenotypic levels and that microbial metabolites are involved in AD through microglial function. However, we could not directly evaluate the identified microglia–microbial metabolite–gene–pathway–phenotype interactions in AD since we currently have limited knowledge of how gut microbial metabolites contribute to AD through microglial functions at genetic, functional, and phenotype levels. Future studies on the role of top-ranked microglia-related genes and microbial metabolites in an experimental rodent model of AD may provide a better understanding of the impact of microglia–microbial metabolite–gene–pathway–phenotype interactions in the pathogenesis of AD.
Second, our findings are largely associational. Although the networks incorporated strong causal/mutational gene–phenotype relationships from MGD, other relationships on the network including chemical–gene, gene–gene, and gene–pathway are associational. In addition, network-based ranking algorithms that prioritized entities on the network for a given input are based on associational analysis. To translate these findings into AD diagnosis, prevention, and treatment, it is necessary to establish cause–effect relationships of the identified microglia–microbial metabolite–gene–pathway–phenotype interactions in experimental models of AD and identify specific gut bacteria that produce the metabolites. Furthermore, the identified top-ranked AD–microbial metabolites and their associated pathways are not necessarily specific for AD. For example, SCFAs and associated immune functions and pathways are also involved in other diseases including cancers [58].
Third, HMDB is currently the most comprehensive human metabolome database. However, it is not specific for microbial metabolism and contains only 220 metabolites that originated from gut microbiota. The field of human microbial metabolism research is expanding rapidly, with an increasing number of microbial metabolites being identified or predicted by computational methods. For example, AGORA (assembly of gut organisms through reconstruction and analysis) is a resource of genome-scale metabolic reconstructions semi-automatically generated for 773 human gut bacteria [59]. The computationally predicted (while not experimentally confirmed) microbial metabolites in AGORA may significantly broaden our current list of 220 microbial metabolites.
Another rich resource for a list of validated microbial metabolites is the 30 million published biomedical literature. Many microbial metabolites already reported in the literature are not included in the list of 220 microbial metabolites from HMDB [60, 61]. We have recently developed natural language processing, text classification, and network-based approaches to automatically extract and prioritize microbial metabolites from 28 million biomedical articles [60, 61]. Currently, we are manually curating top-ranked microbial metabolites extracted from biomedical literature, in order to update the analysis of microglia–microbial metabolite–gene–pathway–phenotype interactions in AD using an updated list of microbial metabolites.
Fourth, this study used 22 AD risk genes obtained from OMIM and ClinVar as one of the evaluation datasets. Both OMIM and ClinVar contain disease–gene associations with known clinical significance. However, this list of AD genes is not complete. For example, studies showed that rare variants of PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in AD and the list of AD genes did not include these three genes. However, this limitation did not affect the prioritization algorithm as the input to the prioritization algorithm was microglia-associated phenotypes and the list of AD genes was used for evaluation purpose.
In this study, we did not explicitly differentiate early-onset from late-onset AD. The inputs to the network-based algorithms to identify AD-associated microbial metabolites were three phenotypes related to abnormal microglial functions. Studies showed that proliferation and activation of microglia around amyloid plaques is a canonical feature of AD, including both early-onset and late-onset AD [62,63,64]. While findings from our study suggest that microbial metabolites are involved in microglia-mediated AD pathophysiology in both early- and late-onset AD, future experimental studies are warranted to further tease out how and which microbial metabolites contribute to different aspects of AD.
Fifth, we used 517,381 mouse mutational phenotype–gene annotations from the MGD to construct the PhenGN network and to infer effects of microbial metabolites on AD-related phenotypes. The advantages of MGD data are that these gene–phenotype associations are strong and often causal and that they contain a large number of genes and phenotypes (11,021 human gene homologs, 9982 phenotypes). However, it is known that phenotypes in mouse models do not necessarily resemble the human phenotypes, especially in AD and other neurological disorders. Future work will include incorporating known phenotype–gene associations from human (though limited availability) into the networks to further improve the human relevance of the predicted gut–microbiota–brain–AD interactions.
Conclusions
We constructed a context-sensitive network that integrates and models our existing knowledge of semantic relationships among tens of thousands of genes, phenotypes, metabolites, and pathways. We prioritized and identified 8094 potential microglia–microbial metabolite–gene–pathway–phenotype interactions in AD, which provide evidence that multiple gut microbial metabolites are involved in microglia-mediated AD pathophysiology through different genetic and functional mechanisms that are finally converged on several AD-specific phenotypes including “amyloid beta deposits,” “amyloidosis,” “microgliosis,” and “tau protein deposits.” To the best of our knowledge, our study represents the first computational approach to comprehensively characterize the complex gut–microbial metabolite–microglia–gene–pathway–phenotype–brain connections in AD, by innovatively repurposing large amounts of publicly available data collected for other purposes. As our knowledge of genetics and genomics of diseases and chemicals evolve, the network structures that capture our current knowledge of gene–phenotype, chemical–gene, gene–gene, gene–pathway, and metabolite–disease associations will surely also change. Consequently, microglia–microbial metabolite–gene–pathway–phenotype–AD interactions identified based on network prioritization will be further improved. As new data coming in, the context-sensitive network-based approach is highly flexible and dynamic in incorporating new biomedical knowledge into the network. The identification of gut microbial metabolites and the understanding of their role in AD has potential in providing new insights into the basic mechanisms of AD etiology and enable new possibilities for AD prevention and treatment.
Availability of data and materials
All the data are provided in the Supplement files.
References
Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–83.
Citron M. Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discov. 2010;9(5):387–98.
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–7.
Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242.
Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701.
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–63.
Smith PA. The tantalizing links between gut microbes and the brain. Nat News. 2015;526(7573):312.
Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241–55.
Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1):13537.
Zhuang ZQ, Shen LL, Li WW, Fu X, Zeng F, Gui L, et al. Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis. 2018;63(4):1337–46.
Saji N, Niida S, Murotani K, Hisada T, Tsuduki T, Sugimoto T, et al. Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci Rep. 2019;9(1):1–9.
MahmoudianDehkordi S, Arnold M, Nho K, Ahmad S, Jia W, Xie G, et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease—an emerging role for gut microbiome. Alzheimers Dement. 2019;15(1):76–92.
Marizzoni M, Cattaneo A, Mirabelli P, Festari C, Lopizzo N, Nicolosi V, et al. Short-chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in Alzheimer’s disease. J Alzheimers Dis. 2020;78(2):683–97.
Del Rio D, Zimetti F, Caffarra P, Tassotti M, Bernini F, Brighenti F, et al. The gut microbial metabolite trimethylamine-N-oxide is present in human cerebrospinal fluid. Nutrients. 2017;9(10):1053.
Vogt NM, Romano KA, Darst BF, Engelman CD, Johnson SC, Carlsson CM, et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res Ther. 2018;10(1):124.
Rogers J, Lue LF. Microglial chemotaxis, activation, and phagocytosis of amyloid β-peptide as linked phenomena in Alzheimer’s disease. Neurochem Int. 2001;39(5-6):333–40.
Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21(10):1359–69.
Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T, et al. Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci. 2018;12:488.
Robinson M, Lee BY, Hane FT. Recent progress in Alzheimer’s disease research, part 2: genetics and epidemiology. J Alzheimers Dis. 2017;57(2):317–30.
Erny D, de Angelis ALH, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965.
Mosher KI, Wyss-Coray T. Go with your gut: microbiota meet microglia. Nat Neurosci. 2015;18(7):930.
Cryan JF, Dinan TG. Gut microbiota: microbiota and neuroimmune signaling—Metchnikoff to microglia. Nat Rev Gastroenterol Hepatol. 2015;12(9):494.
Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119.
Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3(10):858–76.
Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother. 2018;18(1):83–90.
Xu R, Wang Q, Li L. A genome-wide systems analysis reveals strong link between colorectal cancer and trimethylamine N-oxide (TMAO), a gut microbial metabolite of dietary meat and fat. BMC Genomics. 2015;16(7):S4.
Xu R, Wang Q. Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst Biol. 2016;10(3):63.
Wang Q, Li L, Xu R. A systems biology approach to predict and characterize human gut microbial metabolites in colorectal cancer. Sci Rep. 2018;8(1):6225.
Wang Q, Xu R. Data-driven multiple-level analysis of gut-microbiome-immune-joint interactions in rheumatoid arthritis. BMC Genomics. 2019;20(1):124.
Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vázquez-Fresno R, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2017;46(D1):D608–17.
Szklarczyk D, Santos A, von Mering C, Jensen LJ, Bork P, Kuhn M. STITCH 5: augmenting protein–chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2015;44(D1):D380–4.
Bult CJ, Blake JA, Smith CL, Kadin JA, Richardson JE. Mouse genome database (MGD) 2019. Nucleic Acids Res. 2018;47(D1):D801–6.
Nagaraj AB, Wang QQ, Joseph P, Zheng C, Chen Y, Kovalenko O, et al. Using a novel computational drug-repositioning approach (DrugPredict) to rapidly identify potent drug candidates for cancer treatment. Oncogene. 2018;37(3):403–14.
Nagaraj AB, Joseph P, Kovalenko O, Wang QQ, Xu R, DiFeo A. Evaluating class III antiarrhythmic agents as novel MYC targeting drugs in ovarian cancer. Gynecol Oncol. 2018;151(3):525–32.
Chen Y, Gao Z, Wang B, Xu R. Towards precision medicine-based therapies for glioblastoma: interrogating human disease genomics and mouse phenotypes. BMC Genomics. 2016;17(7):251–7.
Chen Y, Cai X, Xu R. Combining human disease genetics and mouse model phenotypes towards drug repositioning for Parkinson’s disease. In: AMIA annual symposium proceedings (Vol. 2015). San Francisco: American Medical Informatics Association; 2015. p. 1851.
Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27(12):1739–40. https://doi.org/10.1093/bioinformatics/btr260.
Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, et al. Human protein reference database—2009 update. Nucleic Acids Res. 2008;37(suppl_1):D767–72.
Chen Y, Xu R. Context-sensitive network-based disease genetics prediction and its implications in drug discovery. Bioinformatics. 2016;33(7):1031–9.
Zhou M, Chen Y, Xu R. A drug-side effect context-sensitive network approach for drug target prediction. Bioinformatics. 2018;35(12):2100–7.
Wang Q, Xu R. Disease comorbidity-guided drug repositioning: a case study in schizophrenia. AMIA Annu Symp Proc. 2018;2018:1300–9.
Chen Y, Xu R. Context-sensitive network analysis identifies food metabolites associated with Alzheimer’s disease: an exploratory study. BMC Med Genet. 2019;12(1):17.
Zhou M, Zheng C, Xu R. Combining phenome-driven drug-target interaction prediction with patients’ electronic health records-based clinical corroboration toward drug discovery. Bioinformatics. 2020;36(Supplement_1):i436–44. https://doi.org/10.1093/bioinformatics/btaa451.
Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–78.
Alzforum. Alzheimer’s disease commonly used mouse models. https://www.alzforum.org/research-models/alzheimers-disease/commonly-used (data Accessed in June 2020).
Hamosh A, Scott AF, Amberger JS, Bocchini CA, McKusick VA. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33(suppl_1):D514–7.
Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2015;44(D1):D862–8. https://doi.org/10.1093/nar/gkv1222.
Ferrarelli LK. Microglia spread tau. Sci Signal. 2015;8:ec329. https://doi.org/10.1126/scisignal.aad8159.
Piazza-Gardner AK, Gaffud TJ, Barry AE. The impact of alcohol on Alzheimer’s disease: a systematic review. Aging Ment Health. 2013;17(2):133–46.
Wang J, Ho L, Zhao Z, Seror I, Humala N, Dickstein DL, et al. Moderate consumption of Cabernet Sauvignon attenuates A neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006;20(13):2313–20.
Deng J, Zhou DH, Li J, Wang YJ, Gao C, Chen ME. A 2-year follow-up study of alcohol consumption and risk of dementia. Clin Neurol Neurosurg. 2006;108(4):378–83.
Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. 2009;17(7):542–55.
Nobili A, Latagliata EC, Viscomi MT, Cavallucci V, Cutuli D, Giacovazzo G, et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat Commun. 2017;8(1):1–14.
Martorana A, Koch G. Is dopamine involved in Alzheimer’s disease? Front Aging Neurosci. 2014;6:252.
Storga D, Vrecko K, Birkmayer JGD, Reibnegger G. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci Lett. 1996;203(1):29–32.
Gross TJ, Doran E, Cheema AK, Head E, Lott IT, Mapstone M. Plasma metabolites related to cellular energy metabolism are altered in adults with Down syndrome and Alzheimer’s disease. Dev Neurobiol. 2019;79(7):622–38.
Xiang Y, Xu G, Weigel-Van Aken KA. Lactic acid induces aberrant amyloid precursor protein processing by promoting its interaction with endoplasmic reticulum chaperone proteins. PLoS One. 2010;5(11):e13820.
Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr. 2002;132(5):1012–7.
Magnúsdóttir S, Heinken A, Kutt L, Ravcheev DA, Bauer E, Noronha A, et al. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat Biotechnol. 2017;35(1):81–9.
Wang Q, Xu R. CoMNRank: an integrated approach to extract and prioritize human microbial metabolites from MEDLINE records. J Biomed Inform. 2020;109:103524. https://doi.org/10.1016/j.jbi.2020.103524 Epub 2020 Aug 11.
Wang Q, Xu R. Automatic extraction, prioritization and analysis of gut microbial metabolites from biomedical literature. Sci Rep. 2020;10(1):9996. https://doi.org/10.1038/s41598-020-67075-6.
Efthymiou AG, Goate AM. Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Mol Neurodegener. 2017;12(1):1–2.
Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol. 2018;217(2):459–72.
Sarlus H, Heneka MT. Microglia in Alzheimer’s disease. J Clin Investig. 2017;127(9):3240–9.
Acknowledgements
Not applicable
Funding
RX and QW are supported by the NIH National Institute on Aging R01 AG057557, R01 AG061388, R56 AG062272, National Institute on Drug Addiction UG1DA049435, American Cancer Society Research Scholar Grant RSG-16-049-01 – MPC, The Clinical and Translational Science Collaborative (CTSC) of Cleveland 1UL1TR002548-01. XQ is supported by NIH R01AG065240, R01NS115903, R21NS107897 and Dr. Ralph and Marian Falk Medical Research Trust-Transformative Award. PMD is supported by the Cure Alzheimer’s Fund and the Karen L. Wrenn Trust.
Author information
Authors and Affiliations
Contributions
Study design and conception: QW and RX; analysis and evaluation: QW; paper writing: QW and RX; paper review and critical edits: PBD, QX, SGC, MEG, GP, and PMD. Study supervision: RX. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
There are no disclosures for other authors. MC is an employee of Tetra Therapeutics and owns shares. PMD has received research grants/advisory fees from companies for other projects; PMD owns stock in health companies whose products are not discussed here; PMD is a co-inventor on unlicensed patents in this field; PMD serves on the health system and non-profit boards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplement S1.
The list of prioritized microbial metabolites for the combined input microgliosis”, “abnormal microglial cell physiology” and “abnormal microglial cell morphology”. Supplement S2. A total of 8,094 microglia-microbial metabolite-gene-pathway-phenotype interactions in AD identified for top 20% (top 44) ranked microbial metabolites.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Q., Davis, P.B., Qi, X. et al. Gut–microbiota–microglia–brain interactions in Alzheimer’s disease: knowledge-based, multi-dimensional characterization. Alz Res Therapy 13, 177 (2021). https://doi.org/10.1186/s13195-021-00917-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-021-00917-1