Abstract
Background
Alzheimer’s disease (AD) is an irreversible neurodegenerative disease characterized by the hallmark finding of cerebral amyloid deposition. Many researchers have tried to predict the existence of cerebral amyloid deposition by using easily accessible blood plasma samples, but the effectiveness of such strategies remains controversial.
Methods
We developed a new multiplex kit, the QPLEX™ Alz plus assay kit, which uses proteomics-based blood biomarkers to prescreen for cerebral amyloid deposition. A total of 300 participants who underwent Pittsburgh compound B (PiB)-positron emission tomography (PET) which allows imaging of cerebral amyloid deposition were included in this study. We compared the levels of QPLEX™ biomarkers between patients who were classified as PiB-negative or PiB-positive, regardless of their cognitive function. Logistic regression analysis followed by receiver operating characteristic (ROC) curve analysis was performed. The kit accuracy was tested using a randomized sample selection method.
Results
The results obtained using our assay kit reached 89.1% area under curve (AUC) with 80.0% sensitivity and 83.0% specificity. Further validation of the QPLEX™ Alz plus assay kit using a randomized sample selection method showed an average accuracy of 81.5%.
Conclusions
Our QPLEX™ Alz plus assay kit provides preliminary evidence that it can be used as blood marker to predict cerebral amyloid deposition but independent validation is needed.
Similar content being viewed by others
Background
Alzheimer’s disease (AD) is a neurological disease accompanied by the pathological features of beta-amyloid (Aβ) plaques and neurofibrillary tangles. AD is the most prevalent dementia and has a much earlier pathological progress than the onset of clinical symptoms; thus, many research efforts have sought to discover bio-fluidic biomarkers in the blood or cerebral spinal fluid (CSF) that can be used for early detection of the disease. Although direct brain-imaging methods using Aβ-specific positron emission tomography (PET) ligands have been developed, such as Pittsburgh compound B (PiB) and florbetapir [1], PET is not an easily accessible method because of its high cost and radiation exposure. Especially in the early stages of the disease, when pathological hallmarks exist in the brain but no clinical symptom is seen, patients would be unlikely to undergo a brain PET scan [2, 3]. This a major obstacle when early diagnosis relies on PET. Many researchers and clinicians have noted that the use of efficient, early, and easily accessible diagnostic methods could prevent or delay the progress of AD pathology.
In a previous study, we revealed a novel blood-based biomarker panel for cerebral amyloid deposition consisting of galectin-3 binding protein (LGALS3BP), Aβ1–40, angiotensin-converting enzyme (ACE), and periostin (POSTN) [3, 4]. When assessed by logistic regression analysis and receiver operating characteristic (ROC) curve analysis, our biomarker panel exhibited a high area under the curve (AUC) and good sensitivity and specificity when blood levels were quantified through commercialized enzyme-linked immunosorbent assay (ELISA) kits or the xMAP technology. For clinical practice and large population screening, a readily accessible diagnostic kit capable of measuring these biomarkers could critically enable the quick prediction of cerebral amyloid deposition.
Here, we introduce a bioanalytical platform for AD diagnosis. We developed a new AD diagnostic kit, the QPLEX™ Alz plus assay kit, which predicts cerebral amyloid deposition using our previously developed blood biomarkers, LGALS3BP, Aβ1–40, ACE, and POSTN. In our prior work, these biomarkers were independently quantified using individual ELISA kits. Here, we combined them and developed a new multiplex kit with high efficiency and accuracy. We tested the diagnostic efficacy and accuracy of the kit for 300 cognitively diverse individuals who underwent PiB-PET scans. The new QPLEX™ Alz plus assay kit demonstrated high-level performance for prediction of PiB-PET positivity.
Methods
Participants
In total, 300 participants were included in this study. They consisted of 149 cognitively normal individuals (CN group), 87 patients with amnestic mild cognitive impairment (MCI group), and 64 patients with clinically diagnosed AD dementia (DEM group). These individuals were recruited as part of the Korean Brain Aging Study for the Early diagnosis and prediction of Alzheimer’s disease (KBASE). All participants were given appropriate clinical and neuropsychological assessments according to the KBASE assessment protocol. The details of participant recruitment, clinical diagnosis criteria, and further information were described in our previous report [5].
Ethical approval
All participants and (where applicable) their legal representatives read and confirmed the informed consent documents. This project was approved by the Seoul National University Hospital Institutional Review Board.
PiB-PET
All participants underwent PiB-PET scans using a 3.0 T PET-MR scanner (Siemens Healthineers, Erlangen, Germany). Briefly, each individual was injected intravenously with 555 MBq of [11C] PiB (450–610 MBq) PET tracer, which enabled visualization of cerebral amyloid deposition. The degree of amyloid accumulation was calculated by the standardized uptake value ratio (SUVR), which was determined by the automatic anatomic algorithm. The four regions of interest (ROIs) were the lateral temporal, lateral parietal, posterior cingulate-precuneus, and frontal regions. If the SUVR value was 1.4 or higher for at least one of the four ROIs, the individual was defined as PiB-positive (PiB+). The details of the imaging protocols were described in our previous paper [5].
Blood sampling
All fasting blood samples were collected at 9:00 AM. Whole-blood samples were gathered in K2 EDTA tubes (BD Vacutainer Systems, Plymouth, UK) and centrifuged at 700 g for 5 min at room temperature (RT). The supernatants were collected, the centrifugation step was repeated, and the tubes were stored at − 80 °C.
QPLEX™ Alz plus assay (QPLEX™)
Our QPLEX™ kit utilized the Quantamatrix’s multiplex diagnostics platform (QMAP; Quantamatrix Inc., Seoul, Republic of Korea) with microdisk technology to perform multiplex analyses in a single well [6]. This suspension bead array system uses graphically coded beads to expose antigens to the 3D environment. Briefly, human plasma samples were diluted in the diluent buffer and incubated with the coded beads and biotin-conjugated detection antibodies in the provided black 96-well plate for 90 min at RT on a shaking incubator at 1000 rpm. The immunocomplexes, including the coded beads, were washed twice with washing buffer on a Biotek-510 magnetic wash station (Biotek, VT, USA). Fifty microliters of diluted R-phycoerythrin-conjugated streptavidin were added to each well, and the plate was incubated for 15 min at RT on the same shaking incubator. After three washes, the immunocomplexes were resuspended in 100 μl of washing buffer by tapping. Collected immunocomplexes were analyzed automatically by the QMAP™ system; approximately 30 beads were used for the calculation of each biomarker concentration.
In-house ELISA (IH-ELISA)
ELISA plates were coated with capture antibodies diluted to a working concentration in Dulbecco’s modified phosphate-buffered saline solution (DPBS; Invitrogen, Carlsbad, CA, USA) and incubated at RT. The remaining binding sites were blocked with 1 mg/ml bovine serum albumin (Sigma Aldrich, St. Louis, MO, USA) in DPBS. Human plasma samples were diluted in the same buffer that was provided with the QPLEX™ kit and incubated with the primary antibodies immobilized in the wells. Streptavidin-conjugated horseradish peroxidase was added to the wells, and the plate was incubated again for 20 min. The immunocomplexes were detected with a chromogenic substrate solution and the reaction was terminated by the addition of 0.5 M HCl. Absorption was read at 450 nm using an ELISA plate reader (Biotek, Winooski, VT, USA).
Statistical analyses
All statistical analyses were performed using the Medcalc 17.2 software (Ostend, Belgium) and GraphPad Prism 8 (San Diego, CA, USA). Comparison analyses between two variables were conducted by independent t test or analysis of covariance (ANCOVA) with correction for age and sex. Correlation analyses were performed using the Pearson’s correlation analysis method. To calculate the discriminatory power, sensitivity, and specificity for the biomarker panels, logistic regression, followed by receiver operating characteristic (ROC) curve analysis was performed. The basic formula of our algorithm used in our analyses was as follows:
(pi, predicted probabilities; an, coefficient values, e.g., a1 = 0.008 for Aβ40, a2 = − 0.0066 for ACE, a3 = − 0.0007 for LGALS3BP, a4 = 0.1322 for POSTN; C, constant, e.g., C = 1.2478. Each biomarker level of the samples was multiplied by coefficient values and pi was calculated).
The formulas, coefficients, and constants could be optimized since there were appropriate outliers and various logistic regression models. Multicollinearities were checked using the values of variance inflation factors (VIF). Finally, the accuracy of each model was calculated using a randomized sample selection method. Randomizing analysis was used to create random groups with even distributions of age (the variable for case identification was ‘age’). In other words, each group had almost the same average age (group 1, average 71.8, range 56 to 86; group 2, 72.3, range 61 to 84; group 3, 70.1, range 55 to 85; etc.). After the randomized sample selection and regrouping, each group was validated by the logistic regression models to calculate the accuracy of each biomarker panel. The average accuracy was calculated as shown in Fig. 3. All statistical outliers were excluded from the cohort according to the Grubb’s double-side outlier test (p < 0.05).
Results
Categorization of participants
This study included 300 participants (age, 55–88 years): 113 PiB− CN (CN−), 36 PiB+ CN (CN+), 31 PiB− MCI (MCI−), 56 PiB+ MCI (MCI+), 6 PiB− DEM (DEM−), and 58 PiB+ DEM (DEM+). The demographic details are described in Table 1.
Comparison between IH-ELISA and QPLEX™ kit performance
We previously reported a novel blood-based biomarker panel identified from the results of a proteomic analysis [4]. In the prior work, we used four different commercially available ELISA kits to assess the individual biomarkers. Here, we developed a new multiplex strategy. Before seeking to validate our QPLEX™ kit using the main cohort samples, we compared the results obtained for these markers between IH-ELISA and our kit. All four biomarkers included in the kit showed a high correlation coefficient and low intra- or inter-coefficient of variation (CV, < 10%) (Fig. 1a). This indicated that our QPLEX™ kit could show high performance similar to that achieved with IH-ELISA of the individual biomarkers.
The levels of QPLEX™ biomarkers and their correlation to cerebral amyloid deposition. a Correlation between IH-ELISA and QPLEX™ biomarkers and inter- or intra-CV. Significant outliers were excluded by the Grubb’s test method. b Classification of participants. Participants were divided into two groups (cerebral amyloid deposition; − or + means PiB− vs. PiB+) or three groups (depending on their cognitive status; CN normal, MCI mild cognitive impaired, Dementia). Comparison analyses for PiB− vs. PiB+ were conducted. c The levels of QPLEX™ biomarkers. *p < 0.05, **p < 0.01, independent t test; #p < 0.10, ###p < 0.001, #####p < 0.0001, ANCOVA test with the correction of age, sex, ApoE, and/or MMSE score. d Correlation between cerebral amyloid deposition and QPLEX™ biomarkers. e Inter-correlations between the markers (Pearson’s correlation coefficient, p values, and variance inflation factor)
Validation of QPLEX™ biomarkers for predicting PiB-PET positivity
We divided all participants into two groups, PiB− (CN−, MCI−, and DEM−) and PiB+ (CN+, MCI+, DEM+), and compared the levels of QPLEX™ biomarkers (Fig. 1b). Similar to the results reported in our previous paper [4], all QPLEX™ biomarkers showed significant differences between the PiB− and PiB+ groups (Fig. 1c). There were several intra-subgroup differences (parentheses indicate markers that showed significant between-subgroup differences): CN− vs. CN+ (Aβ1–40, LGALS3BP, and POSTN), MCI− vs. MCI+ (Aβ1–40, LGALS3BP, and POSTN) and DEM− vs. DEM+ (LGALS3BP) (Fig. 1c). Moreover, the QPLEX™ biomarkers exhibited correlations with the degree of cerebral amyloid deposition (PiB-PET standardized uptake value ratio; PiB-PET SUVR) with the exception of ACE, which exhibited a non-significant tendency (p = 0.2) (Fig. 1d). ACE did, however, show a significant association with PiB-PET SUVR in the multiple regression analysis (Table 2). Finally, we found that all of the QPLEX™ biomarkers were associated with each other (Fig. 1e, left and middle). The low variance inflation factors (VIF < 2) revealed that there was no problem with multicollinearity (Fig. 1e, right). Together, these results confirmed that our QPLEX™ biomarkers appear appropriate for use in prescreening patients for cerebral amyloid deposition.
Discrimination power of QPLEX™ biomarkers
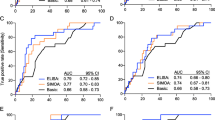
To verify the usefulness of the QPLEX™ biomarkers, we performed logistic regression analysis and receiver operating characteristics (ROC) curve analysis (Fig. 2a, Table 3). We generated three regression models (ApoE variable only, model I; QPLEX™ markers only, model II; ApoE + QPLEX™ markers, model III) with correction for age and sex (Fig. 2b). Model II showed a higher AUC (0.834 with 76.6% sensitivity and 73.5% specificity) than model I (0.783 with 76.7% sensitivity and 68.0% specificity), and model III showed a significantly higher AUC (0.891 with 80.0% sensitivity and 83.0% specificity) than models I and II (Fig. 2c, d). Furthermore, we performed the same analyses for CN or MCI group separately, it also showed the consistent pattern of high performances (for CN group, 0.786 AUC for model I, 0.896 AUC for model II, 0.907 AUC for model III; for MCI group, 0.781 AUC for model I, 0.791 AUC for model II, 0.900 AUC for model III) and similar tendency of AUC with Fig. 2c (model I < model II < model III) (Supplementary Fig. 1). The results indicated that the model combining our QPLEX™ biomarkers and ApoE genotypic characteristics yielded a significantly higher AUC value than that generated using only ApoE characteristics.
Receiver operating characteristic (ROC) curve analysis for PiB positivity. a ROC curve analyses for PiB positivity. Age and sex were used as covariates. b Interactive dot diagram and plot versus criterion values showing sensitivity, specificity, and Youden index cutoff criteria. c, d Comparison of ROC curve analyses. Age sex were used as covariates. Model III had the highest area under curve (AUC) value (0.891). PV, predictive values; SE, standard error; CI, confidence interval
Accuracy test using randomized sample selection
Next, we tested the accuracy of our regression models by using randomized sample validation (Fig. 3a). First, we randomly divided the participants into 10 groups with even distributions of ages 55 to 88 (variable for case identification = “age”; for more detail on the randomized sample selection process, see Fig. 3b). We confirmed that the average ages were almost the same (data not shown). We then tested how many samples could be predicted accurately by models I, II, and III, in each group (e.g., for model I, correct predictions were obtained for 25 out of 30, 83%, for group 1; 21 out of 30, 70%, for group 2; 19 out of 30, 63%, for group 3;… 19 out of 30, 63%, for group 10). Finally, we calculated average accuracies for models I, II, and III. Consistent with the results for the whole-population ROC curve analysis (Fig. 2c, d), model III showed the highest average accuracy (81.5%) compared to model I (70.6%) and model II (75.0%). The results obtained from our QPLEX™ prediction models suggest that this new method can effectively discriminate PiB-PET-positive and thus is ready to be put to practical use for the general public.
Accuracy validation of QPLEX™ kit by using randomized sample selection. a Random groups were generated (sorted by age) and 10 groups were blind-tested according to our QPLEX™ algorithm. Three models were tested: model I, 70.6% accuracy; model II, 75.0% accuracy; model III, 81.5% accuracy. b The detailed algorithm process to validate our biomarker models by using randomized sample selection. Random groups were generated by randomizing analysis (create random groups using “age” with case identification). Three hundred participants were classified as 10 groups and validated using their predicted probabilities (P). Accuracy (correct number of participants/total number of participants, %) of each group were calculated and averaged
Discussion
Blood-based biomarkers for AD theoretically should enable early-stage disease detection or screening while also offering increased accessibility, greater convenience, and reduced cost [7]. Various blood biomarkers for AD diagnosis have been published in the last few decades [7,8,9,10], but few have proven useful for the development of diagnostic kits. Indeed, no blood-based biomarker kit for diagnosing AD has yet been launched in the market. This likely reflects the technical difficulty of composing a multiplex kit with detection accuracies that are consistently equivalent to those of the individual ELISA experiments used during the discovery and validation stages of a given biomarker.
ELISA has long been used for biomarker detection in diverse body fluids, including blood and CSF [11, 12]; however, it requires intensive labor and typically shows intra- and inter-assay variability [13]. The newly developed xMAP technology exhibits high sensitivity and low variability thanks to its use of pre-made calibrators [14]. Comparative analysis showed that the detection values of ELISA and xMAP were generally (but not always) correlated, the results differed in terms of the absolute concentrations identified [15,16,17]. Comparison of the CSF Aβ42, t-tau, and p-tau181 levels detected by ELISA and xMAP [14, 18] showed that the levels were well correlated, supporting the idea that multiplex xMAP is an appropriate and potentially clinically relevant platform for biomarker detection [14].
Quantamatrix’s multiplexed diagnostics platform (QMAP™) uses a proprietary microdisk technology to perform 1000 reactions in a single well. The microdisk is formed of a polymer that comprises a large amount of 50-μm particles applied by a semiconductor process, and can easily be formed into various shapes [6]. The microdisk is further coated with silica to improve its physical and chemical stability, and to facilitate various chemical surface treatments [19]. This microdisk-based multiplex technology enhanced the detection ability of our QPLEX™ Alz plus assay kit for rare or volume-limited samples. Moreover, the graphical code used by our group is more stable than the fluorescence code which can be affected by light and heat. This high-level stability and the magnetic character make the QMAP™ system suitable for application in automated analysis equipment. The QMAP™-based kit measures blood biomarkers by sandwich ELISA using capture antibodies and biotinylated detection antibodies. The system can perform up to 1000 reactions in a single well with a suspension bead array system that enables 3D incubation and provides fast results within 2 h (excluding sample preparation).
Our QPLEX™ Alz plus assay kit was developed using QMAP™ technology and four blood biomarkers we previously identified as being useful for distinguishing cerebral amyloid deposition [3, 4]: LGALS3BP, ACE, POSTN, and Aβ1–40. We validated the diagnostic accuracy of this diagnostic kit in a clinical trial with a 300-person validation cohort of individuals who were imaged using PiB-PET. In the previous discovery stage, we utilized ELISA to detect LGALS3BP, ACE, and POSTN, and used xMAP to detect Aβ1–40; with this strategy, we used the developed kit to validate that there were differences in the blood levels of the four biomarkers between the PiB− and PiB+ groups [3, 4]. In the QPLEX™ Alz plus assay kit, we simultaneously detected LGALS3BP, ACE, POSTN, and Aβ1–40 using QMAP technology, which helped maximize the detection efficiency for low-abundance blood proteins.
When assessed with the new assay kit, the blood levels of LGALS3BP, POSTN, and Aβ1–40 showed fairly distinct and significant differences between the PiB− and PiB+ groups and significant correlations with cerebral amyloid deposition (SUVR); the level of ACE tended to be decreased and was correlated with cerebral amyloid deposition in the PiB+ group (Fig. 2b, c). Notably, previous studies showed that aspects of ACE other than the blood concentration appear to be tightly related to AD pathology. For example, decreased ACE activity was found in CSF from AD patients [20] and the ACE D allele was associated with an increased risk for AD in two distinct population cohorts [21]. This increased risk for AD was independent of ApoE4 genotype and was equal to or greater in magnitude than the ApoE4 genotype-related risk of AD [21]. Here, we found that the blood ACE level was also significantly correlated with the levels of two other AD-related blood biomarkers: LGALS3BP and Aβ1–40 (Fig. 2d). Given this, we propose that ACE is associated with AD pathology and that the blood concentration and activity level of ACE should be investigated further in a larger population cohort. We screened the medication history of all participants and confirmed that there was no current use of an ACE-related medication, such as an ACE inhibitor. As ACE inhibitors are known to influence the activity or concentration of ACE in the blood [22], future studies are warranted to examine the possible effects associated with taking ACE inhibitors.
The ApoE genotype currently stands as the strongest known risk factor for late-onset AD [23], but this genetic risk factor does not reflect the risk an aging individual acquires related to the accumulation of life pattern- and environment-related factors. The biomarkers assessed in the QPLEX™ Alz plus assay kit represent a real-time risk assessment for the initiation and progression of AD pathology. Therefore, the combined screening of ApoE and blood biomarkers can be used to improve disease diagnosis. Indeed, the results of our ROC curve analysis revealed that the AUC value of the QPLEX™ Alz plus assay kit was higher than that of ApoE alone (0.834 and 0.783, respectively) and that their use in combination significantly increased the AUC value (up to 0.891) compared to that obtained with either strategy alone (Fig. 3). Furthermore, the diagnostic accuracy of our developed kit is comparable or superior to those from other tests using well-known plasma AD biomarkers such as plasma Aβ42/40 ratio, Aβ40/42 ratio, or plasma phosphorylated-tau (p-tau) (Supplementary Table 1) [3, 24,25,26,27,28,29,30,31,32]. First, the AUC value from Model III (0.891 with 80.0% sensitivity and 83.0% specificity; the model with ApoE covariate) was the highest rank among the models using ApoE as a covariate (1st out of 3 biomarkers). Next, when we compared our AUC value from model II (0.834 with 76.6% sensitivity and 73.5% specificity; the model without ApoE covariate) to those of the tests using these known biomarkers without ApoE covariate, it was also comparable to them (4th out of 10 biomarkers). Our result is fairly promising in that this kit could be easily commercialized and utilized with high accuracy, considering the following limitations of the four tests with higher AUC than that of us: (i) immunoprecipitation-mass spectrometry (IP-MS) method is not accessible to most clinical laboratories [31], (ii) Perez-Grijalba’s approach has a limitation in that the number of participants is too small (n = 59) [29], and (iii) it is still necessary to discuss practicality of p-tau, since its level in the blood is too low [24]. Of course, our model also needs to be replicated in an independent cohort, but instead, the results from ROC curve analysis were supported by another validation study in which we used randomized subgroups (see Fig. 3). We found that the accuracy of the QPLEX™ Alz plus assay kit (75.0%) was better than that of the ApoE genotype (70.6%), and the highest accuracy was achieved using both together (81.5%). The results provide preliminary evidence that this new assay kit can be used as a blood biomarker panel to predict cerebral amyloid deposition if further validation using an independent cohort is completed.
Limitations
Although the efficacy of our assay kit was further validated by the randomized selection methods (Fig. 3), our new QPLEX Alz plus assay kit should be screened on other independent cohorts with the goal of putting it to practical use. Going forward, we plan to apply the QPLEX Alz plus assay kit to a larger patient population and potentially seek to identify a better algorithm. Also, we need to test the QPLEX Alz plus assay kit in other neurodegenerative diseases in our future study to validate the selective effects of discriminating PiB-PET-positivity regardless of disease types.
Conclusions
Our QPLEX™ Alz plus assay kit shows high performance and provides the possibility of clinical application for prediction of cerebral amyloid deposition but independent validation is needed.
Availability of data and materials
Not applicable.
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
Beta-amyloid
- CSF:
-
Cerebral spinal fluid
- PET:
-
Positron emission tomography
- PiB:
-
Pittsburgh compound B
- LGALS3BP:
-
Galectin-3 binding protein
- ACE:
-
Angiotensin-converting enzyme
- POSTN:
-
Periostin
- ROC:
-
Receiver operating characteristics
- AUC:
-
Area under curve
- PV:
-
Predictive value
- ELISA:
-
Enzyme-linked immunosorbent assay
- CN:
-
Cognitively normal
- MCI:
-
Mild cognitive impairment
- DEM:
-
Clinically diagnosed AD dementia
- KBASE:
-
Korean Brain Aging Study for the Early diagnosis and prediction of Alzheimer’s disease
- SUVR:
-
Standardized uptake value ratio
- ROI:
-
Region of interest
- QMAP:
-
Quantamatrix’s multiplex diagnostics platform
- ANCOVA:
-
Analysis of covariance
- VIF:
-
Variance inflation factors
- ApoE:
-
Apolipoprotein E
References
Uzuegbunam BC, Librizzi D, Hooshyar Yousefi B. PET radiopharmaceuticals for Alzheimer’s disease and Parkinson’s disease diagnosis, the current and future landscape. Molecules. 2020;25(4):977. https://pubmed.ncbi.nlm.nih.gov/32098280/.
Armstrong RA. Beta-amyloid (Abeta) deposition in cognitively normal brain, dementia with Lewy bodies, and Alzheimer’s disease: a study using principal components analysis. Folia Neuropathol. 2012;50(2):130–9.
Park JC, Han SH, Cho HJ, Byun MS, Yi D, Choe YM, et al. Chemically treated plasma Abeta is a potential blood-based biomarker for screening cerebral amyloid deposition. Alzheimers Res Ther. 2017;9(1):20.
Park JC, Han SH, Lee H, Jeong H, Byun MS, Bae J, et al. Prognostic plasma protein panel for Abeta deposition in the brain in Alzheimer's disease. Prog Neurobiol. 2019;183:101690.
Byun MS, Yi D, Lee JH, Choe YM, Sohn BK, Lee JY, et al. Korean brain aging study for the early diagnosis and prediction of Alzheimer’s disease: methodology and baseline sample characteristics. Psychiatry Investig. 2017;14(6):851–63.
Chung SE, Park W, Park H, Yu K, Park N, Kwon S. Optofluidic maskless lithography system for real-time synthesis of photopolymerized microstructures in microfluidic channels. Appl Phys Lett. 2007;91(4):043014. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3026036/.
Hampel H, O’Bryant SE, Molinuevo JL, Zetterberg H, Masters CL, Lista S, et al. Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol. 2018;14(11):639–52.
Han SH, Park JC, Mook-Jung I. Amyloid beta-interacting partners in Alzheimer's disease: from accomplices to possible therapeutic targets. Prog Neurobiol. 2016;137:17–38.
Zetterberg H, Burnham SC. Blood-based molecular biomarkers for Alzheimer’s disease. Mol Brain. 2019;12(1):26.
Park JC, Han SH, Mook-Jung I. Peripheral inflammatory biomarkers in Alzheimer’s disease: a brief review. BMB Rep. 2020;53(1):10–9.
Bruggink KA, Jongbloed W, Biemans EA, Veerhuis R, Claassen JA, Kuiperij HB, et al. Amyloid-beta oligomer detection by ELISA in cerebrospinal fluid and brain tissue. Anal Biochem. 2013;433(2):112–20.
Han SH, Park JC, Byun MS, Yi D, Lee JH, Lee DY, et al. Blood acetylcholinesterase level is a potential biomarker for the early detection of cerebral amyloid deposition in cognitively normal individuals. Neurobiol Aging. 2019;73:21–9.
Schoonenboom NS, Mulder C, Vanderstichele H, Van Elk EJ, Kok A, Van Kamp GJ, et al. Effects of processing and storage conditions on amyloid beta (1-42) and tau concentrations in cerebrospinal fluid: implications for use in clinical practice. Clin Chem. 2005;51(1):189–95.
Wang LS, Leung YY, Chang SK, Leight S, Knapik-Czajka M, Baek Y, et al. Comparison of xMAP and ELISA assays for detecting cerebrospinal fluid biomarkers of Alzheimer’s disease. J Alzheimers Dis. 2012;31(2):439–45.
de Jager W, Prakken BJ, Bijlsma JW, Kuis W, Rijkers GT. Improved multiplex immunoassay performance in human plasma and synovial fluid following removal of interfering heterophilic antibodies. J Immunol Methods. 2005;300(1–2):124–35.
Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38(4):317–23.
Ray CA, Bowsher RR, Smith WC, Devanarayan V, Willey MB, Brandt JT, et al. Development, validation, and implementation of a multiplex immunoassay for the simultaneous determination of five cytokines in human serum. J Pharm Biomed Anal. 2005;36(5):1037–44.
Reijn TS, Rikkert MO, van Geel WJ, de Jong D, Verbeek MM. Diagnostic accuracy of ELISA and xMAP technology for analysis of amyloid beta(42) and tau proteins. Clin Chem. 2007;53(5):859–65.
Kim LN, Kim M, Jung K, Bae HJ, Jang J, Jung Y, et al. Shape-encoded silica microparticles for multiplexed bioassays. Chem Commun (Camb). 2015;51(60):12130–3.
Zubenko GS, Volicer L, Direnfeld LK, Freeman M, Langlais PJ, Nixon RA. Cerebrospinal fluid levels of angiotensin-converting enzyme in Alzheimer's disease, Parkinson's disease and progressive supranuclear palsy. Brain Res. 1985;328(2):215–21.
Farrer LA, Sherbatich T, Keryanov SA, Korovaitseva GI, Rogaeva EA, Petruk S, et al. Association between angiotensin-converting enzyme and Alzheimer disease. Arch Neurol. 2000;57(2):210–4.
Krasowski MD, Savage J, Ehlers A, Maakestad J, Schmidt GA, La'ulu S, et al. Ordering of the serum angiotensin-converting enzyme test in patients receiving angiotensin-converting enzyme inhibitor therapy: an avoidable but common error. Chest. 2015;148(6):1447–53.
Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–18.
Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387–97.
Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422–33.
Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018;14(8):989–97.
Risacher SL, Fandos N, Romero J, Sherriff I, Pesini P, Saykin AJ, et al. Plasma amyloid beta levels are associated with cerebral amyloid and tau deposition. Alzheimers Dement (Amst). 2019;11:510–9. https://pubmed.ncbi.nlm.nih.gov/31384662/.
Palmqvist S, Janelidze S, Stomrud E, Zetterberg H, Karl J, Zink K, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related beta-amyloid status. JAMA Neurol. 2019;76(9):1060–9.
Perez-Grijalba V, Arbizu J, Romero J, Prieto E, Pesini P, Sarasa L, et al. Plasma Abeta42/40 ratio alone or combined with FDG-PET can accurately predict amyloid-PET positivity: a cross-sectional analysis from the AB255 study. Alzheimers Res Ther. 2019;11(1):96.
Vergallo A, Megret L, Lista S, Cavedo E, Zetterberg H, Blennow K, et al. Plasma amyloid beta 40/42 ratio predicts cerebral amyloidosis in cognitively normal individuals at risk for Alzheimer’s disease. Alzheimers Dement. 2019;15(6):764–75.
Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature. 2018;554(7691):249–54.
Feinkohl I, Schipke CG, Kruppa J, Menne F, Winterer G, Pischon T, et al. Plasma amyloid concentration in Alzheimer’s disease: performance of a high-throughput amyloid assay in distinguishing Alzheimer's disease cases from controls. J Alzheimers Dis. 2020;74(4):1285–94.
Acknowledgements
The authors thank the patients, staff of hospitals, and volunteers for the participation in this project.
Funding
This work was supported by grants from the National Research Foundation (NRF) of Korea (NRF2019R1I1A1A01063525 to S-H.Han), from the Korea Health Technology R&D Project through Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI18C0630 and HI19C1132 to I. Mook-Jung), from the NRF (2018R1A5A2025964 to I. Mook-Jung, 2014M3C7A1046042 to D.Y.Lee), and from KHIDI (HI18C0630 and HI19C0149 to D.Y.Lee).
Author information
Authors and Affiliations
Contributions
J-C.P, S-H.H, and I.M-J conceptualized this study. J-C.P, K.S.J, J. K, J.S.J, and S. K carried out experiments. M.S.B, D. Y, G. B, and G. J obtained blood samples from the subjects and collected the cognitive and PiB-PET data. PiB-PET scan data were obtained, collected, and analyzed by Y.K.K, M.S.B, and D.Y. J-C.P analyzed statistics. J-C.P organized all figures and tables. D.Y.L provided the resources. J-C.P, S-H.H, and I.M-J wrote the original draft. D.Y.L and I.M-J reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This project was approved by the Institutional Review Board (IRB) of the Seoul National University Hospital, South Korea, and the subjects of their legal representatives gave their written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Fig. 1.
Comparison of PiB− vs. PiB+ among CN or MCI. (A) Logistic regression analysis followed by ROC curve analysis among the CN group and comparison of ROC curve analysis. (B) Logistic regression analysis followed by ROC curve analysis among the MCI group and comparison of ROC curve analysis. AUC, area under curve; PV, predictive values; SE, standard error; CI, confidence interval.
Additional file 2: Supplementary Table 1.
Recent studies (< 5 years) published on data using plasma p-tau or Aβ42/40 or 40/42 ratio biomarkers, for brain Aβ positivity.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Park, JC., Jung, K.S., Kim, J. et al. Performance of the QPLEX™ Alz plus assay, a novel multiplex kit for screening cerebral amyloid deposition. Alz Res Therapy 13, 12 (2021). https://doi.org/10.1186/s13195-020-00751-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-020-00751-x