Abstract
Background
Neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinson’s disease with dementia (PDD), and dementia with Lewy bodies (DLB) share clinical and molecular features. Cerebrospinal fluid (CSF) biomarkers may help the characterization of these diseases, improving the differential diagnosis. We evaluated the diagnostic performance of five CSF biomarkers across a well-characterized cohort of patients diagnosed with AD, DLB, PDD, and Parkinson’s disease (PD).
Methods
A total of 208 patients were enrolled in 3 European centers. The diagnostic groups (AD, n = 48; DLB, n = 40; PDD, n = 20; PD, n = 54) were compared with cognitively healthy neurological control subjects (patients with other neurological diseases [OND], n = 46). CSF levels of fatty acid binding protein 3, heart type (FABP3), α-synuclein (α-syn), amyloid-β peptide 1–42, total tau (t-tau), and phosphorylated tau 181 (p-tau) were assessed with immunoassays. Univariate and multivariate statistical analyses were applied to calculate the diagnostic value of the biomarkers as well as their association with clinical scores.
Results
FABP3 levels were significantly increased in patients with AD and DLB compared with those with PD and OND (p < 0.001). CSF t-tau, p-tau, and α-syn were significantly higher in patients with AD than in patients with PDD, DLB, PD, and OND. Combination of FABP3 with p-tau showed high accuracy for the differential diagnosis between AD and DLB (AUC 0.92), whereas patients with AD were separated from those with PDD using a combination of p-tau, FABP3, and α-syn (AUC 0.96). CSF FABP3 was inversely associated with Mini Mental State Examination score in the whole cohort (r = −0.42, p < 0.001).
Conclusions
The combination of CSF biomarkers linked to different aspects of neurodegeneration, such as FABP3, α-syn, and AD biomarkers, improves the biochemical characterization of AD and Lewy body disorders.
Similar content being viewed by others
Background
Neurodegenerative disorders (NDDs) such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and dementia with Lewy bodies (DLB) share a central pathogenic theme: the accumulation, in extra- or intracellular deposits, of aggregated and misfolded proteins [1]. The type of protein, as well as the size, shape, and location of the deposits, is quite typical of each disorder and is used in the pathological examination for characterizing each disease. However, NDDs show remarkable similarities from the clinicopathological point of view, making accurate diagnosis difficult, especially at early stages of the disease [2, 3]. For instance, the clinical presentation of AD and DLB, two diseases considered the most common neurodegenerative forms of dementia, may overlap significantly, leading to low accuracy of the differential diagnosis [4]. Cognitive impairment can occur also in patients initially diagnosed with a prototypical movement disorder such as PD, generally at later stages of disease, and often leading to Parkinson’s disease with dementia (PDD). Apart from the temporal difference in the onset of cognitive deficits, PDD is remarkably similar to DLB in clinical terms, showing, beyond extrapyramidal signs, multidomain impairment and visual hallucinations [5].
Besides clinical similarities, the co-occurrence of different protein aggregates is also a prominent molecular feature of NDDs. On one hand, inclusion bodies composed of α-synuclein (α-syn), representing the major pathological determinants in PD and DLB, can also be detected in AD brains, especially in selected areas such as the amygdala [6]. On the other hand, tau and amyloid-β (Aβ) aggregation, considered the pathological hallmark of AD, is also found in DLB and PD brains, usually to different degrees [7,8,9].
The presence of similar molecular signatures across NDDs is also detectable in cerebrospinal fluid (CSF) [10]. CSF levels of the Aβ peptide 1–42 (Aβ1–42) are generally reduced in both AD and DLB compared with control subjects [11]. Patients with PD may show reduced CSF Aβ1–42 levels as well, a decrease often associated with cognitive decline [12, 13]. CSF α-syn is currently studied as a biomarker for PD and other synuclein-associated diseases, generally showing lower levels than control subjects and patients with AD [14,15,16,17,18]. Also, tau proteins in CSF may show a partial overlap between AD and DLB, with phosphorylated tau 181 (p-tau) being the most useful for differential diagnosis [19,20,21].
Other proteins have been evaluated across NDDs for their potential value in differential diagnosis. Among them, several studies underlined the importance of the fatty acid binding protein 3, heart type (FABP3), a small cytosolic protein involved in lipid transport. In the brain, FABP3 regulates the lipid composition of the membrane [22], entailing possible roles in synapse formation [23] and in the activity of cholinergic and glutamatergic neurons [24]. Increased FABP3 levels were found in the CSF of patients with different neurological disorders, including Creutzfeldt-Jakob disease (CJD), AD in both its prodromal and dementia phases, and vascular dementia (VAD) [25,26,27,28,29,30,31]. Furthermore, in CSF, FABP3 strongly correlates with tau, the prototypical marker of neurodegeneration [30]. The role of FABP3 in NDDs related to α-syn aggregation is less defined. Increased levels of FABP3 have been found in the serum of patients diagnosed with DLB and PDD [26]. Furthermore, FABP3 is highly expressed in mouse dopaminergic neurons, and its overexpression has been linked to α-syn aggregation and PD pathogenesis [32].
Considering the different roles of FABP3, total α-syn, and AD core biomarkers across the AD-PD spectrum, we hypothesized that their combination would be of value for the differential diagnosis of NDDs. In this study, we measured this biomarker panel in a large, multicentric cohort composed of patients with AD, DLB, PDD, and PD compared with a group of subjects with other neurological diseases without dementia (OND). Additionally, we explored the associations of the CSF biomarker panel with cognitive decline and other clinical scores in the different diagnostic groups.
Methods
Patients and sample collection
A total of 208 subjects were included in this study. One-hundred forty-nine of them were consecutively enrolled at the Center for Memory Disturbances, University Hospital of Perugia (Italy), in the period 2006–2014; 20 patients were from the Reference Center for Biological Markers of Dementia, Institute Born-Bunge, University of Antwerp (Belgium), and 39 were from the Paracelsus-Elena-Klinik, Kassel (Germany). Details on the number of patients per condition are reported in Table 1. The AD group was composed of 48 patients diagnosed with probable AD according to National Institute of Aging-Alzheimer Association criteria [33]. The patients with PD (n = 54) were diagnosed with PD according to United Kingdom Brain Bank Society (UKBBS) criteria [34, 35]. Patients with PDD (n = 20) were diagnosed according to UKBBS criteria and criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. The diagnosis of DLB was made according to McKeith’s criteria [36] in all the centers.
As neurological controls, 46 subjects who underwent lumbar puncture (LP) for diagnostic reasons but without clinical evidence of dementia were enrolled (OND). The commonest OND diagnoses were headache, epilepsy, psychiatric disorders, and white matter lesions. All the diagnoses of the subjects with OND are reported in Additional file 1. The exclusion criteria for the control group were dementia disorders, atypical parkinsonism (i.e., multiple system atrophy, corticobasal syndrome, progressive supranuclear palsy), and systemic and neoplastic diseases.
The patients underwent a thorough clinical examination by experienced neurologists, including the following: (1) a neuropsychological evaluation, including screening tools such as the Mini Mental State Examination (MMSE) and the Montreal Cognitive Assessment, and extended cognitive batteries for the assessment of memory, language, attention, and executive functions; (2) evaluation of behavioral changes, functional status, and dementia staging by using the Neuropsychiatric Inventory, basic/instrumental activities daily living, and Clinical Dementia Rating; (3) brain magnetic resonance imaging; and (4) blood and CSF analysis. Patients included in the PD, PDD, and DLB groups were also evaluated by means of the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) and Hoehn and Yahr (H&Y) scores. The LP was performed from 8:00 a.m. to 10:00 a.m. after overnight fasting, following a standardized procedure and according to international guidelines [37]. CSF (10–12 ml) was taken from the L3-L4 or L4-L5 interspace, immediately collected in sterile polypropylene tubes, and gently mixed to avoid possible gradient effects. In Perugia and Kassel, the samples were centrifuged at 2000 × g for 10 minutes, aliquoted, and stored at −80 °C. CSF samples from Antwerp were collected in polypropylene vials, immediately frozen in liquid nitrogen, and subsequently stored at −80 °C. Blood-contaminated samples were excluded from the analysis (cutoff of 50 red blood cells per microliter).
Immunoassays
FABP3, Aβ1–42, total tau (t-tau), and p-tau were measured using commercially available enzyme-linked immunosorbent assays (ELISAs) (FABP3 Human ELISA, Hycult Biotech, Uden, The Netherlands; INNOTEST β-AMYLOID(1–42)™, Fujirebio Europe, Gent, Belgium; Total Tau ELISA, Phosphorylated Tau 181 ELISA, EUROIMMUN AG, Lübeck, Germany) and according to previous reports [38, 39]. α-Syn was measured at ADx NeuroSciences (Gent, Belgium) using a new assay developed internally [40]. The ADx α-syn research ELISA is a colorimetry-based sandwich immunoassay (96-well microplate format) with a readout that can be measured in a conventional microplate reader using a 450-nm filter. α-Syn is captured by a C-terminal monoclonal antibody ADx301 (amino acid region 115–125). The (undiluted) sample or calibrator (recombinant full-length α-syn) and the detector monoclonal antibody ADx302 (amino acid region 95–110) are incubated simultaneously for 3 h at room temperature. After a subsequent wash step, addition of streptavidin-peroxidase, and then substrate incubation and reaction stop, the analyte concentration in the samples is calculated using a four-parameter logistic curve fitting the seven nonzero calibrator points (100–5000 pg/ml). Operators blinded to the diagnosis performed the measurements. CSF pools of patients with a positive (AD pool) or negative (non-AD pool) profile for the core AD biomarkers (Aβ1–42, t-tau, and p-tau) were run in each plate to check for run-to-run variability.
Data analysis
Statistical analyses were performed using R software version 3.1 [41]. Continuous variables were described as mean and SD, whereas categorical variables were reported as count and percent. Distribution of biomarkers was checked for normality with the Shapiro-Wilk test. Owing to nonnormality of the distribution of biomarkers, nonparametric analyses were carried out. All correlations were calculated using Spearman’s rho with the Benjamini-Hochberg correction. Nonparametric analysis of variance was used to compare biomarkers levels across all diagnostic groups, accounting for difference in age distribution; in cases of significant differences, pairwise group comparisons were performed using Tukey’s method. The diagnostic performance was assessed by the AUC of the ROC curve. Cutoff values were calculated using sensitivity and specificity that maximized Youden’s index. Ninety-five percent confidence intervals were calculated for the AUC. A backward elimination method was used for model selection by progressively eliminating predictors with the largest individual p value, one at a time at each step in the process, until only significant predictors remained. From these models, by using fitted probabilities, we derived ROC curves as well as estimates of the AUC, sensitivity, and specificity. p Values less than 0.05 were considered significant for all the analyses.
Results
Demographical and clinical features
The demographic and clinical data are reported in Table 1. There was a significant difference in the frequency of male sex among the groups (p = 0.019), with a higher percentage in the PD, PDD, and DLB groups than in the AD and OND groups. Also, there was a significant difference among groups in terms of age (p < 0.001), which was due to the higher mean age in the AD, DLB, and PDD groups than in the PD and OND groups. As expected, in patients with dementia (AD, DLB, and PDD), the MMSE scores were significantly lower than among subjects with PD and OND (p < 0.001). No significant difference was detected among subjects with PD, PDD, and DLB with regard to UPDRS-III score, whereas there was a significant difference among these groups in terms of H&Y stage (p < 0.001; PDD > DLB > PD).
Levels of the CSF biomarkers in the diagnostic groups
CSF levels of the five biomarkers in the diagnostic groups are reported in Table 2. Assay variability for Aβ1–42, FABP3, and t-tau was in line with previously published reports [30, 39], whereas for ADx p-tau and α-syn, both intra- and interassay coefficients of variation were below 10% (Additional file 2). Considering that our DLB group was composed of patients enrolled in two different centers (Kassel, n = 20; Antwerp, n = 20), we tested for the existence of possible center effects. There was no significant center effect for any of the measured biomarkers (Mann-Whitney U test; data not shown).
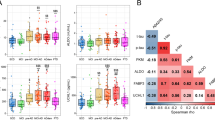
CSF FABP3 levels (Fig. 1) were significantly higher in the AD group than in the PD and OND groups (p < 0.001) (Fig. 1a). Also, patients with DLB showed higher levels of FABP3 than those with PD (p < 0.01) and OND (p < 0.001). Patients with PDD showed an increase of CSF FABP3 levels compared with subjects with OND and PD, without reaching statistical significance. Subjects with PD and OND showed similar levels of FABP3 in CSF. Aβ1–42 was significantly lower in the AD and PDD groups than in the OND and PD groups (Table 2). No significant difference was noted between patients with AD and patients with DLB regarding Aβ1–42. Both t-tau and p-tau CSF levels were higher in the AD group than in the DLB, PDD, PD, and OND groups (p < 0.001). Furthermore, CSF t-tau levels were significantly higher in the DLB group than in the PD group (p < 0.01). α-Syn levels were not significantly different in the PD, PDD, and DLB groups compared with the control group. Interestingly, increased levels of α-syn were found in the AD group compared with the other diagnostic groups and with the OND group (Table 2 and Fig. 1a).
Cerebrospinal fluid (CSF) levels of fatty acid binding protein 3, heart type (FABP3), and tau across the diagnostic groups. a Box plots of the five biomarkers across the diagnostic groups. b, and c Correlation plots (Spearman) for the CSF five-biomarker panel in the whole cohort and in the diagnostic groups. OND Other neurological diseases, PD Parkinson’s disease, PDD Parkinson’s disease with dementia, DLB Dementia with Lewy bodies, AD Alzheimer’s disease, Aβ 1–42 Amyloid-β peptide 1–42, p-tau Phosphorylated tau 181, α-syn α-Synuclein, t-tau Total tau
We next explored more deeply the role of Aβ1–42 across the diagnostic groups. We dichotomized the cohort according to Aβ1–42 positivity with the cutoff used in our clinic for supporting AD diagnosis (500 pg/ml). Interestingly, besides the AD group, there was also an increase in the percentage of the Aβ1–42-positive cases in the PD, PDD, and DLB groups compared with the OND group (Table 2). In the whole cohort, FABP3, t-tau, and p-tau were significantly increased in Aβ1–42-positive cases (Additional file 3). In the single diagnostic groups, this trend was confirmed but did not reach statistical significance. Instead, there was a significant decrease (approximately 50%) of α-syn CSF levels in subjects with OND and PD who were Aβ1–42-positive (Additional file 3).
A complete correlation analysis was carried out for the five biomarkers in the whole cohort and in each diagnostic group (Fig. 1b and Additional file 4). In the whole cohort, FABP3 correlated significantly with t-tau and p-tau (r = 0.65, p < 0.0001; and r =0.58, p < 0.001, respectively), confirming previous reports [30]. FABP3 also correlated positively with α-syn (r = 0.56, p < 0.001), whereas a weak but significant and inverse correlation was found with Aβ1–42 (r = −0.21, p < 0.01). In the whole cohort, we noted a strong correlation between α-syn and tau proteins (r = 0.68, p < 0.001 for t-tau; r = 0.60, p < 0.0001 for p-tau), which was generally confirmed also in each diagnostic group to different degrees (Fig. 1b, Additional file 2). When we analyzed the correlations in the single groups, we also calculated the similarity of the biomarker correlation matrices across the different groups. Interestingly, the most similar groups according to the correlation of the CSF biomarkers were PDD and DLB, with a correlation of 0.99, followed by DLB and AD (r = 0.97) (Fig. 1c).
Diagnostic performance of cerebrospinal fluid biomarkers. a Heat map of AUCs of the single biomarkers for all diagnostic comparisons. b ROC analysis of the logistic regression results for differential diagnosis of neurodegenerative disorders. Aβ 1–42 Amyloid-β peptide 1–42, AD Alzheimer’s disease, DLB Dementia with Lewy bodies, FABP3 Fatty acid binding protein 3, heart type, OND Other neurological diseases, PD Parkinson’s disease, PDD Parkinson’s disease with dementia, p-tau Phosphorylated tau 181, α-syn α-Synuclein, t-tau Total tau
Diagnostic performance of the single biomarkers
The diagnostic values of the five biomarkers vs. the control group (OND) and for differential diagnosis were first calculated using univariate ROC analysis. Figure 2a reports the AUC for each of the tested comparisons, and the complete analysis is reported in Additional file 5, including also sensitivity and specificity for each biomarker.
Disease vs. control group (OND)
Tau proteins were globally the best biomarkers in distinguishing NDDs from the OND group, reaching a very high accuracy, especially in AD diagnosis (AUC 0.96 for t-tau and 0.97 for p-tau). Indeed, for AD diagnosis vs. subjects with OND, all five biomarkers showed an AUC >0.7, with FABP3 being the biomarker with the lowest performance (AUC 0.75). Four of the five biomarkers (FABP3, t-tau, p-tau, Aβ1–42) had a similar accuracy when discriminating patients with DLB from the OND group (AUC approximately 0.7), whereas no biomarker was able to achieve an adequate discrimination of PD from OND (t-tau AUC 0.54). For patients with PDD vs. subjects with OND, the decrease of Aβ1–42 was the best biomarker (AUC 0.74).
Differential diagnosis among neurodegenerative disorders
Clinically, the differential diagnosis between AD and DLB is of utmost importance. In our cohort, tau proteins confirmed their importance in the characterization of these two dementias. p-tau was the best single biomarker (AUC 0.89), followed by t-tau and α-syn (AUC 0.85 and 0.78, respectively), with all of them increased in AD. A similar pattern was found for the comparison AD vs. PDD (Fig. 2a and Additional file 5). ROC analysis confirmed the similarity between DLB and PDD groups, with no biomarkers showing very high accuracy in distinguishing the two diseases; a decrease of α-syn in the PDD group showed the best performance, reaching an AUC of 0.63 with a specificity of 69% and a sensitivity of 65%. In the comparison between the DLB and PD groups, t-tau was the best biomarker, with an AUC of 0.83, followed by p-tau, FABP3, and Aβ1–42, all of them with AUCs ranging from 0.77 to 0.70. Finally, Aβ1–42 confirmed its important role in the differentiation between the PDD and PD groups [12], showing an AUC of 0.73 (decreased in PDD), followed by FABP3 (AUC 0.70, increased in PDD).
Combination of biomarkers for the differential diagnosis of NDDs
To further assess both the effect of potential confounders such as age and the value of biomarker combinations, a multivariate logistic regression approach was used. Several biomarker combinations were tested in different models. Table 3 shows a summary of the biomarkers retained by the best model for each comparison, while in Fig. 2b the ROC analysis of the best model for the differential diagnosis of NDDs is depicted. For the differentiation between AD and OND groups, the best model retained the core CSF AD biomarkers (Aβ1–42, t-tau, p-tau) and resulted in the correct classification of 98% of the subjects with a specificity of 88% and a sensitivity of 100%. DLB diagnosis vs. OND was related to CSF Aβ1–42 and age; the model was able to correctly classify 79% of the subjects with a specificity of 90% and sensitivity of 69%. The logistic regression analysis of patients with PD vs. subjects with OND included t-tau and age in the final model, correctly classifying 72% of the subjects with a specificity of 58% and a sensitivity of 90%. Also, in the comparison between patients with PDD and the OND group, age played a significant role, together with Aβ1–42; this model resulted in the correct classification of 81% of the subjects (specificity 69%, sensitivity 95%).
The differential diagnosis of AD from DLB was again dependent on p-tau; however, in the final model, FABP3 was also retained, improving the AUC up to 0.92 but with no significant difference compared with the univariate analysis (p = 0.283) (Table 3 and Fig. 2b). A similar improvement in diagnostic accuracy was found for the comparison of patients with AD and patients with PDD, where the inclusion of p-tau, α-syn, and FABP3 led to an AUC of 0.96 with a specificity of 88% and a sensitivity of 100%. Also, the multivariate model was not significantly different from the model including only p-tau (p = 0.256 by DeLong test).
Differential diagnosis across Lewy body disorders was significantly improved by our biomarker panel. The inclusion of FABP3 and α-syn for the differentiation of DLB from PD led to an AUC of 0.92 (p = 0.022 vs. t-tau alone). A significant improvement was also noted for the comparison between PD and PDD (Table 3) (p = 0.017 vs. Aβ1–42 alone). The latter was also the only comparison in which age played a significant role and was included in the final model. This was expected because dementia is a feature of PD at later stages, and patients with PDD in our cohort were significantly older than those with PD. The exception for differential diagnosis across Lewy body disorders, was the comparison of DLB vs. PDD, where our panel of biomarkers obtained accuracy similar to that of the univariate analysis (Table 3 and Additional file 5).
Correlation of the CSF biomarkers with clinical scores
The complete correlation analysis between CSF biomarkers and clinical scores is reported in Additional file 6. All biomarkers were associated to various degrees with baseline MMSE scores, with the exception of α-syn. FABP3 inversely correlated with MMSE score in the whole cohort (r = −0.42, p < 0.001) (Fig. 3). Tau proteins were strongly and negatively associated with baseline MMSE score (r = −0.47 for t-tau, r = −0.43 for p-tau, p < 0.001), whereas Aβ 1–42 correlated positively (r = 0.46, p < 0.001) (Fig. 3). Motor (UPDRS-III and H&Y) scores were available for the PD, PDD, and DLB groups. The correlations of the CSF biomarkers with motor scores were generally weaker than the correlations with cognitive scores. In the whole cohort, FABP3 and t-tau were weakly associated with the H&Y score (r = 0.26, p < 0.05; and r = 0.29, p < 0.05, respectively), whereas Aβ1–42 was inversely associated with H&Y (r = −0.37, p < 0.01). In the single diagnostic groups, FABP3 correlated with MMSE only in the PDD group (r = −0.49, p < 0.05), but after correction for multiple comparisons, no significant correlation was retained, possibly owing to the relatively limited size of the groups.
Correlation of cerebrospinal fluid (CSF) biomarkers with cognitive decline. CSF fatty acid binding protein 3, heart type (FABP3), total tau (t-tau), phosphorylated tau 181 (p-tau) and amyloid-β peptide 1–42 (Aβ1–42) levels significantly correlated with Mini Mental State Examination (MMSE) scores in the whole cohort. Correlations were calculated using Spearman’s rho
Discussion
In this study, we show that the combination of CSF biomarkers linked to different aspects of neurodegeneration may improve the characterization of NDDs, namely AD, DLB, PDD, and PD. In particular, we report the following findings:
-
1.
Tau protein and α-syn levels were significantly increased in patients with AD compared with the other diagnostic groups.
-
2.
FABP3 CSF levels were increased in the AD and DLB groups compared with the OND and PD groups.
-
3.
Combination of FABP3 with p-tau showed excellent performance in the discrimination between AD and DLB, whereas the inclusion of α-syn in the logistic models improved the discrimination of PDD and DLB from PD.
-
4.
FABP3 showed a significant inverse correlation with MMSE score in the whole cohort.
To our knowledge, this is the largest study analyzing the combination of FABP3 and α-syn with AD core biomarkers for differential diagnosis of NDDs, also including patients with PD without dementia. The core CSF AD biomarkers have become an important tool to support the diagnosis of AD and have been included in the new diagnostic criteria for AD [33, 42]. However, these three biomarkers have not shown enough accuracy in the differential diagnosis of dementias [43]. This is possibly related to the existence of a disease continuum across the neurodegeneration spectrum, at both the molecular and phenotypical levels [2].
Our results show that tau proteins are fundamental biomarkers, not only for distinguishing patients with AD from those with OND but also in differential diagnosis across dementias. In our cohort, tau proteins showed a different degree of increase in dementia groups (AD > DLB > PDD). This trend has been found previously, with patients with AD generally showing higher CSF levels of tau proteins than patients with DLB and patients with PDD [43,44,45]. On one hand, our data show a high discriminative power of p-tau in distinguishing patients with AD from those with DLB and patients with PDD, confirming previous results [46, 47]. On the other hand, researchers in some studies have found substantial overlap of the CSF AD profile between subjects with DLB and AD [11,49,, 44, 48–50]. Because of the current uncertainty about the real potential of classical AD biomarkers in the differential diagnosis of NDDs, the inclusion of additional biomarkers with the core AD panel is mandatory in order to improve neurochemical dementia diagnostics.
α-Syn is currently studied for its possible value as a PD biomarker and in the differential diagnosis of NDDs [51,52,53]. Researchers in previous studies found that α-syn species, also in combination with tau proteins, may be useful in improving diagnostic accuracy across dementia disorders, especially for DLB [14, 49, 50]. In our cohort, total α-syn levels were not significantly changed between the OND group and the Lewy body disorders groups, showing only a trend toward reduction in patients with PDD and patients with DLB. However, we found significantly increased levels of α-syn in patients with AD, and the final models for differential diagnosis of PD and PDD vs. AD included α-syn. This finding confirms the potential role of α-syn as a biomarker also in AD, where α-syn CSF levels are usually increased compared with those of control subjects and patients with parkinsonism. The alteration of α-syn in AD has been linked to synaptic damage [54] or to an underlying Lewy body pathology [55, 56]. In our cohort, the strong correlation between α-syn and tau proteins without specific group differences may underline an association with neuronal damage, as recently reported [57].
FABP3 measurement in serum and CSF has previously been tested as a biomarker for the differential diagnosis of NDDs, including DLB and PDD, in relatively small-scale studies [26, 58]. In the present study, increased FABP3 CSF levels were linked to AD and DLB, whereas patients with PD showed levels similar to those of subjects with OND. The value of FABP3 as an AD biomarker was moderate compared with the core AD biomarkers, confirming the results of a recent meta-analysis [59]. Previous studies have shown a high correlation between FABP3 and tau proteins in CSF [30, 31], supporting the role of FABP3 as a neurodegeneration biomarker. This is confirmed by the parallel increase in FABP3 and tau in other conditions, such as CJD [25], subarachnoid hemorrhage [60], and VAD [61]. However, some findings may endorse specific roles of FABP3 in AD dementia development, because elevated CSF FABP3 levels have been shown to correlate with atrophy of the entorhinal cortex and amyloid pathology in AD-vulnerable brain regions [62]. The association with amyloid pathology was also found in our study, where CSF levels of FABP3 and tau proteins were increased in Aβ1–42-positive subjects, similarly to a recent report [63].
Patients with PD without dementia had levels of FABP3 similar to those of the OND group and were characterized only by reduced t-tau CSF levels. In a recent study, Bäckström and colleagues found that high levels of FABP3 and neurofilament light chain protein, together with low Aβ1–42, were significantly associated with the development of dementia after 5–9 years of follow-up in a large cohort of patients with PD [64]. In our study, FABP3 CSF levels were inversely correlated with MMSE scores in the whole cohort. The difference from the above-mentioned study may be due to the shorter follow-up available for patients with PD in our cohort (mean 5.2 months, maximum 1 year). Also, although dementia usually occurs in advanced phases [65], not all patients with PD develop dementia along the disease course. In patients with PDD, CSF FABP3 levels showed a trend toward an increase and were inversely correlated with MMSE score. This evidence supports the idea that FABP3 is linked to the neurodegeneration process and cognitive impairment occurring at later stages [26] and is more evident in patients with PDD. Furthermore, the lack of any association with motor and progression scores in patients with PD and patients with PDD may indirectly support the hypothesis of FABP3 as a degenerative marker not linked to pathogenic mechanisms specific to PD.
Despite the high predictive value of the biomarker combinations for the differentiation between AD and the other dementias, the five biomarkers we tested did not improve the distinction between patients with DLB and patients with PDD, with the best biomarker, α-syn, showing a relatively low discriminatory capability (AUC 0.63). This finding, together with the high correlation between the CSF profile of the five biomarkers between DLB and PDD (r = 0.99), confirms the molecular and clinical similarities between these two conditions, which can be considered as a continuum across the pathogenesis of Lewy body disorders [66].
Our study has some limitations. Some of the diagnostic groups were enrolled only in one center (AD, OND, and PD in Perugia), possibly introducing a source of variability in the results linked to CSF processing. However, the three clinics are experienced reference centers for CSF biomarker measurement and follow international guidelines for CSF collection [37]. Another limitation is the heterogeneity of the control group, which was composed of different neurological disorders and not of healthy control subjects. Nonetheless, the OND group represents a population ordinarily assayed for CSF biomarkers in a neurology and memory clinic, thus exemplifying the use of CSF biomarkers in routine clinical practice. A third limitation might be related to the disease stage, because most of the patients included in this study were enrolled at quite advanced stages of the disease. Therefore, the value of this panel of biomarkers at early stages of neurodegeneration remains to be determined, even though FABP3 has already shown some diagnostic value in early AD [30, 31].
Conclusions
Our results show that the inclusion of FABP3 and α-syn in the core panel of AD CSF biomarkers can improve the molecular characterization of NDDs encompassing AD dementia and Lewy body disorders. Researchers in other studies have investigated the combination of different biomarkers for the differential diagnosis of dementia and parkinsonism, highlighting how measuring panels of proteins linked to different facets of neurodegeneration will be essential for the biochemical characterization of NDDs and to support clinical diagnosis [19, 67, 68]. Further longitudinal studies including different cohorts of patients with NDDs are necessary to verify the role of this biomarker panel across the neurodegeneration spectrum.
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ1–42 :
-
Amyloid-β peptide 1–42
- CJD:
-
Creutzfeldt-Jakob disease
- CSF:
-
Cerebrospinal fluid
- DLB:
-
Dementia with Lewy bodies
- ELISA:
-
Enzyme-linked immunosorbent assay
- FABP3:
-
Fatty acid binding protein 3, heart type
- H&Y:
-
Hoehn and Yahr scale
- LP:
-
Lumbar puncture
- MMSE:
-
Mini Mental State Examination
- MoCA:
-
Montreal Cognitive Assessment
- NDD:
-
Neurodegenerative disorder
- OND:
-
Other neurological diseases
- PD:
-
Parkinson’s disease
- PDD:
-
Parkinson’s disease with dementia
- p-tau:
-
Phosphorylated tau 181
- t-tau:
-
Total tau
- UKBBS:
-
United Kingdom Brain Bank Society
- UPDRS-III:
-
Unified Parkinson’s Disease Rating Scale part III
- VAD:
-
Vascular dementia
- α-syn:
-
α-Synuclein
References
Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011;70:532–40.
Jellinger KA. Interaction between pathogenic proteins in neurodegenerative disorders. J Cell Mol Med. 2012;16:1166–83.
McMillan PJ, Leverenz JB. From model system to clinical medicine: pathophysiologic links of common proteinopathies. Alzheimers Res Ther. 2010;2:26.
Doubleday EK, Snowden JS, Varma AR, Neary D. Qualitative performance characteristics differentiate dementia with Lewy bodies and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;72:602–7.
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–707. quiz 1837.
Mikolaenko I, Pletnikova O, Kawas CH, O’Brien R, Resnick SM, Crain B, et al. Alpha-synuclein lesions in normal aging, Parkinson disease, and Alzheimer disease: evidence from the Baltimore Longitudinal Study of Aging (BLSA). J Neuropathol Exp Neurol. 2005;64:156–62.
Hepp DH, Vergoossen DLE, Huisman E, Lemstra AW, Netherlands Brain Bank, Berendse HW, et al. Distribution and load of amyloid-β pathology in Parkinson disease and dementia with Lewy bodies. J Neuropathol Exp Neurol. 2016;75:936–45.
Gomperts SN, Locascio JJ, Makaretz SJ, Schultz A, Caso C, Vasdev N, et al. Tau positron emission tomographic imaging in the Lewy body diseases. JAMA Neurol. 2016;73:1334–41.
Donaghy P, Thomas AJ, O’Brien JT. Amyloid PET imaging in Lewy body disorders. Am J Geriatr Psychiatry. 2015;23:23–37.
Toledo JB, Brettschneider J, Grossman M, Arnold SE, Hu WT, Xie SX, et al. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol. 2012;124:23–35.
Struyfs H, Van Broeck B, Timmers M, Fransen E, Sleegers K, Van Broeckhoven C, et al. Diagnostic accuracy of cerebrospinal fluid amyloid-β isoforms for early and differential dementia diagnosis. J Alzheimers Dis. 2015;45:813–22.
Parnetti L, Farotti L, Eusebi P, Chiasserini D, De Carlo C, Giannandrea D, et al. Differential role of CSF α-synuclein species, tau, and Aβ42 in Parkinson’s disease. Front Aging Neurosci. 2014;6:53.
Alves G, Lange J, Blennow K, Zetterberg H, Andreasson U, Førland MG, et al. CSF Aβ42 predicts early-onset dementia in Parkinson disease. Neurology. 2014;82:1784–90.
Parnetti L, Chiasserini D, Bellomo G, Giannandrea D, de Carlo C, Qureshi MM, et al. Cerebrospinal fluid tau/α-synuclein ratio in Parkinson’s disease and degenerative dementias. Mov Disord. 2011;26:1428–35.
Hansson O, Hall S, Ohrfelt A, Zetterberg H, Blennow K, Minthon L, et al. Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimers Res Ther. 2014;6:25.
Yeo JM, Green A, Pal S. The diagnostic utility of cerebrospinal fluid α-synuclein analysis in dementia with Lewy bodies—a systematic review and meta-analysis. Parkinsonism Relat Disord. 2013;19:851–8.
Majbour NK, Vaikath NN, Eusebi P, Chiasserini D, Ardah M, Varghese S, et al. Longitudinal changes in CSF α-synuclein species reflect Parkinson’s disease progression. Mov Disord. 2016;31:1535–42.
Parnetti L, Chiasserini D, Persichetti E, Eusebi P, Varghese S, Qureshi MM, et al. Cerebrospinal fluid lysosomal enzymes and α-synuclein in Parkinson’s disease. Mov Disord. 2014;29:1019–27.
Hall S, Öhrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol. 2012;69:1445–52.
Vranová HP, Hényková E, Kaiserová M, Menšíková K, Vaštík M, Mareš J, et al. Tau protein, β-amyloid1–42 and clusterin CSF levels in the differential diagnosis of parkinsonian syndrome with dementia. J Neurol Sci. 2014;343:120–4.
Struyfs H, Niemantsverdriet E, Goossens J, Fransen E, Martin JJ, De Deyn PP, et al. Cerebrospinal fluid P-tau181P: biomarker for improved differential dementia diagnosis. Front Neurol. 2015;6:138.
Murphy EJ, Owada Y, Kitanaka N, Kondo H, Glatz JF. Brain arachidonic acid incorporation is decreased in heart fatty acid binding protein gene-ablated mice. Biochemistry. 2005;44:6350–60.
Veerkamp JH, Zimmerman AW. Fatty acid-binding proteins of nervous tissue. J Mol Neurosci. 2001;16:133–42.
Shioda N, Yamamoto Y, Watanabe M, Binas B, Owada Y, Fukunaga K. Heart-type fatty acid binding protein regulates dopamine D2 receptor function in mouse brain. J Neurosci. 2010;30:3146–55.
Guillaume E, Zimmermann C, Burkhard PR, Hochstrasser DF, Sanchez JC. A potential cerebrospinal fluid and plasmatic marker for the diagnosis of Creutzfeldt-Jakob disease. Proteomics. 2003;3:1495–9.
Mollenhauer B, Steinacker P, Bahn E, Bibl M, Brechlin P, Schlossmacher MG, et al. Serum heart-type fatty acid-binding protein and cerebrospinal fluid tau: marker candidates for dementia with Lewy bodies. Neurodegener Dis. 2007;4:366–75.
Guo LH, Alexopoulos P, Perneczky R. Heart-type fatty acid binding protein and vascular endothelial growth factor: cerebrospinal fluid biomarker candidates for Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 2013;263:553–60.
Bjerke M, Zetterberg H, Edman Å, Blennow K, Wallin A, Andreasson U. Cerebrospinal fluid matrix metalloproteinases and tissue inhibitor of metalloproteinases in combination with subcortical and cortical biomarkers in vascular dementia and Alzheimer’s disease. J Alzheimers Dis. 2011;27:665–76.
Harari O, Cruchaga C, Kauwe JSK, Ainscough BJ, Bales K, Pickering EH, et al. Phosphorylated tau-Aβ42 ratio as a continuous trait for biomarker discovery for early-stage Alzheimer’s disease in multiplex immunoassay panels of cerebrospinal fluid. Biol Psychiatry. 2014;75:723–31.
Chiasserini D, Parnetti L, Andreasson U, Zetterberg H, Giannandrea D, Calabresi P, et al. CSF levels of heart fatty acid binding protein are altered during early phases of Alzheimer’s disease. J Alzheimers Dis. 2010;22:1281–8.
Bjerke M, Kern S, Blennow K, Zetterberg H, Waern M, Börjesson-Hanson A, et al. Cerebrospinal fluid fatty acid-binding protein 3 is related to dementia development in a population-based sample of older adult women followed for 8 years. J Alzheimers Dis. 2015;49:733–41.
Shioda N, Yabuki Y, Kobayashi Y, Onozato M, Owada Y, Fukunaga K. FABP3 protein promotes α-synuclein oligomerization associated with 1-methyl-1,2,3,6-tetrahydropiridine-induced neurotoxicity. J Biol Chem. 2014;289:18957–65.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack Jr CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:263–9.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4.
Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18:467–86.
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72.
Teunissen CE, Petzold A, Bennett JL, Berven FS, Brundin L, Comabella M, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73:1914–22.
Parnetti L, Chiasserini D, Eusebi P, Giannandrea D, Bellomo G, De Carlo C, et al. Performance of Aβ1–40, Aβ1–42, total tau, and phosphorylated tau as predictors of dementia in a cohort of patients with mild cognitive impairment. J Alzheimer’s Dis. 2012;29:229–38.
Chiasserini D, Biscetti L, Farotti L, Eusebi P, Salvadori N, Lisetti V, et al. Performance evaluation of an automated ELISA system for Alzheimer’s disease detection in clinical routine. J Alzheimers Dis. 2016;54:55–67.
Stoops E, Majbour N, Mauroo K, Demeyer L, Lashuel H, Trojanowski JQ, et al. Performance evaluation of new absorbance-based ELISAs for measuring different α-synuclein (A-SYN) species in CSF and plasma [abstract]. Alzheimer’s Dement. 2016;12(7 Suppl):677–8.
R Core Development Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. p. 409. Available from: http://www.r-project.org/. Accessed 2 Feb 2017.
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29.
Schoonenboom NSM, Reesink FE, Verwey NA, Kester MI, Teunissen CE, van de Ven PM. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78:47–54.
Bibl M, Esselmann H, Lewczuk P, Trenkwalder C, Otto M, Kornhuber J, et al. Combined analysis of CSF tau, Aβ42, Aβ1–42% and Aβ1–40ox% in Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease dementia. Int J Alzheimers Dis. 2010;2010:761571.
Parnetti L, Lanari A, Amici S, Gallai V, Vanmechelen E, Hulstaert F. CSF phosphorylated tau is a possible marker for discriminating Alzheimer’s disease from dementia with Lewy bodies. Neurol Sci. 2001;22:77–8.
Koopman K, Le Bastard N, Martin JJ, Nagels G, De Deyn PP, Engelborghs S. Improved discrimination of autopsy-confirmed Alzheimer’s disease (AD) from non-AD dementias using CSF P-tau181P. Neurochem Int. 2009;55:214–8.
Tang W, Huang Q, Yao YY, Wang Y, Le Wu Y, Wang ZY. Does CSF p-tau181 help to discriminate Alzheimer’s disease from other dementias and mild cognitive impairment? A meta-analysis of the literature. J Neural Transm. 2014;121:1541–53.
Compta Y, Martí MJ, Ibarretxe-Bilbao N, Junqué C, Valldeoriola F, Muñoz E, et al. Cerebrospinal tau, phospho-tau, and β-amyloid and neuropsychological functions in Parkinson’s disease. Mov Disord. 2009;24:2203–10.
Slaets S, Vanmechelen E, Le Bastard N, Decraemer H, Vandijck M, Martin JJ, et al. Increased CSF α-synuclein levels in Alzheimer’s disease: correlation with tau levels. Alzheimer’s Dement. 2014;10(5 Suppl):S290–8.
Llorens F, Schmitz M, Varges D, Kruse N, Gotzmann N, Gmitterová K, et al. Cerebrospinal α-synuclein in α-synuclein aggregation disorders: tau/α-synuclein ratio as potential biomarker for dementia with Lewy bodies. J Neurol. 2016;263:2271–7.
Parnetti L, Cicognola C, Eusebi P, Chiasserini D. Value of cerebrospinal fluid α-synuclein species as biomarker in Parkinson’s diagnosis and prognosis. Biomark Med. 2016;10:35–49.
Parnetti L, Castrioto A, Chiasserini D, Persichetti E, Tambasco N, El-Agnaf O, et al. Cerebrospinal fluid biomarkers in Parkinson disease. Nat Rev Neurol. 2013;9:131–40.
Kruse N, Persson S, Alcolea D, Bahl JMC, Baldeiras I, Capello E, et al. Validation of a quantitative cerebrospinal fluid α-synuclein assay in a European-wide interlaboratory study. Neurobiol Aging. 2015;36:2587–96.
Öhrfelt A, Grognet P, Andreasen N, Wallin A, Vanmechelen E, Blennow K, et al. Cerebrospinal fluid α-synuclein in neurodegenerative disorders—a marker of synapse loss? Neurosci Lett. 2009;450:332–5.
Toledo JB, Korff A, Shaw LM, Trojanowski JQ, Zhang J. CSF α-synuclein improves diagnostic and prognostic performance of CSF tau and Aβ in Alzheimer’s disease. Acta Neuropathol. 2013;126:683–97.
Mackin RS, Insel P, Zhang J, Mohlenhoff B, Galasko D, Weiner M, et al. Cerebrospinal fluid α-synuclein and Lewy body-like symptoms in normal controls, mild cognitive impairment, and Alzheimer’s disease. J Alzheimers Dis. 2015;43:1007–16.
Majbour NK, Chiasserini D, Vaikath NN, Eusebi P, Tokuda T, van de Berg W, et al. Increased levels of CSF total but not oligomeric or phosphorylated forms of α-synuclein in patients diagnosed with probable Alzheimer’s disease. Sci Rep. 2017;7:40263.
Steinacker P, Mollenhauer B, Bibl M, Cepek L, Esselmann H, Brechlin P, et al. Heart fatty acid binding protein as a potential diagnostic marker for neurodegenerative diseases. Neurosci Lett. 2004;370:36–9.
Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–84.
Zanier ER, Longhi L, Fiorini M, Cracco L, Bersano A, Zoerle T, et al. Increased levels of CSF heart-type fatty acid-binding protein and tau protein after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2008;102:339–43.
Olsson B, Hertze J, Ohlsson M, Nägga K, Höglund K, Basun H, et al. Cerebrospinal fluid levels of heart fatty acid binding protein are elevated prodromally in Alzheimer’s disease and vascular dementia. J Alzheimers Dis. 2013;34:673–9.
Desikan RS, Thompson WK, Holland D, Hess CP, Brewer JB, Zetterberg H, et al. Heart fatty acid binding protein and Aβ-associated Alzheimer’s neurodegeneration. Mol Neurodegener. 2013;8:39.
Höglund K, Kern S, Zettergren A, Börjesson-Hansson A, Zetterberg H, Skoog I, et al. Preclinical amyloid pathology biomarker positivity: effects on tau pathology and neurodegeneration. Transl Psychiatry. 2017;7:e995.
Bäckström DC, Eriksson Domellöf M, Linder J, Olsson B, Öhrfelt A, Trupp M, et al. Cerebrospinal fluid patterns and the risk of future dementia in early, incident Parkinson disease. JAMA Neurol. 2015;72:1175–82.
Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson’s disease. Brain Pathol. 2010;20:633–9.
Ballard C, Ziabreva I, Perry R, Larsen JP, O’Brien J, McKeith I, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67:1931–4.
Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Döring F, Trenkwalder C, Schlossmacher MG. αSynuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 2011;10:230–40. A published erratum appears in Lancet Neurol. 2011;10:297.
Magdalinou NK, Paterson RW, Schott JM, Fox NC, Mummery C, Blennow K, et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 2015;86:1240–7.
Acknowledgements
We thank Cristiano Spaccatini for his excellent technical support.
Funding
This work received support from the Italian Ministry for Research and Education under PRIN project “Synaptic dysfunction in Alzheimer Disease: from new in vitro models to identification of new targets (SynAD”; grant number 2010PWNJXK), the Italian Ministry of Health (grant number GR-2013-02357757), and the European Union (EU)/European Federation of Pharmaceutical Industries and Associations (EFPIA) Innovative Medicines Initiative Joint Undertaking (European Medical Information Framework [EMIF] grant number 115372). This research was in part supported by the University Research Fund of the University of Antwerp, the Institute Born-Bunge, the Foundation for Alzheimer Research (SAO-FRA), Neurosearch Antwerp, the Research Foundation - Flanders (FWO), the Agency for Innovation by Science and Technology (IWT), the Interuniversity Attraction Poles (IAP) program of the Belgian Science Policy Office, the Flemish Government Methusalem excellence program (Belgium), and the Flanders Impulse Program on Networks for Dementia Research (VIND). This work is part of the Biomarkers for Alzheimer’s Disease and Parkinson’s Disease (BIOMARKAPD) project within the EU Joint Program for Neurodegenerative Disease Research (JPND). ADx NeuroSciences obtained funding from the Michael J. Fox Foundation to develop assays for α-synuclein.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on request.
Authors’ contributions
DC designed the study, performed the experiments, contributed to data analysis, interpreted the results, and wrote the manuscript. LB enrolled the patients, acquired the data, interpreted the results, and participated in the drafting of the manuscript. PE performed the statistical analysis, interpreted the results, and participated in the drafting of the manuscript; ES and HV performed experiments and critically revised the manuscript. NS, GF, SS, BM, SE, NdR, NT, and PC enrolled the patients, acquired the data, contributed to the interpretation of the results, and revised the manuscript. LP designed and supervised the study, contributed in the interpretation of the results, provided funding, and critically revised the manuscript. All authors read and approved the final manuscript.
Competing interests
LP has received research support from Michael J. Fox Foundation for Parkinson’s Disease and research reagents from Fujirebio and EUROIMMUN. PC has received research support from Bayer Schering Pharma AG, Biogen, Boehringer Ingelheim, Lundbeck, Sanofi-Aventis, Sigma-Tau, Ricerca Corrente Istituto Di Ricovero e Cura a Carattere Scientifico (IRCCS), and Ricerca Finalizzata IRCCS (European Community Grant REPLACES). SE was/is a consultant for and/or received research funding from Janssen, ADx NeuroSciences, Innogenetics/Fujirebio Europe, Lundbeck, Pfizer, Novartis, UCB, Roche Diagnostics, and Nutricia/Danone. BM has received independent research grants from Teva Pharmaceutical Industries, Desitin, Boehringer Ingelheim, and GE Healthcare; as well as honoraria for consultancy from Bayer Schering Pharma AG, Roche, AbbVie, Teva Pharmaceutical Industries, and Biogen and for presentations from GlaxoSmithKline, Orion Pharma, and Teva Pharmaceutical Industries; as well as travel costs from Teva Pharmaceutical Industries. BM is also a member of the executive steering committee of the Parkinson Progression Marker Initiative and principal investigator for the Systemic Synuclein Sampling Study, both of the Michael J. Fox Foundation for Parkinson’s Research, and has received grants from the Federal Ministry of Education and Research (BMBF), EU, Parkinson Fonds Deutschland, Deutsche Parkinson Vereinigung, Michael J. Fox Foundation for Parkinson’s Research, and Stifterverband für die Deutsche Wissenschaft, and has engaged in scientific collaborations with Roche, Bristol-Myers Squibb, Ely Lilly, Covance, and Biogen. HV is a cofounder of ADx NeuroSciences. ES is an employee and shareholder of ADx NeuroSciences. The other authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All the procedures involving human subjects were performed according to the Helsinki declaration. All patients and/or their legal representatives gave written informed consent before inclusion in this study, which was approved by the local ethics committee (Comitato Etico delle Aziende Sanitarie della Regione Umbria - CEAS Umbria registry number 1287/08).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1:
Diagnosis of patients with OND. The diagnosis, sex, and age (when available) of each patient with OND are reported. In the OND group, we enrolled, as control subjects, patients diagnosed with other neurological conditions without cognitive impairment who had undergone lumbar puncture for diagnostic reasons. The exclusion criteria for the OND group are reported in the main text. OND Other neurological diseases, NA Not available. (DOCX 17 kb)
Additional file 2:
Variability of ELISAs used in this study. The intra- and interassay coefficients of variation (CVs) are reported. The intra-assay CV was calculated using duplicate values of two internal controls, whereas the inter-assay CVs derive from five different runs in different plates of the same internal controls. (DOCX 14 kb)
Additional file 3:
Influence of amyloid positivity on CSF biomarker levels. The patients were divided into two groups according to Aβ1–42 CSF levels. A cutoff of 500 pg/ml was used for Aβ1–42, corresponding to the internal cutoff used in our clinic. The levels of the CSF biomarkers were compared in each diagnostic group and in the whole cohort. An increase of FABP3, t-tau, and p-tau was noted in the whole cohort. FABP3 Fatty acid binding protein 3, heart type, t-tau Total tau, p-tau Phosphorylated tau 181, α-syn α-Synuclein. (DOCX 18 kb)
Additional file 4:
Correlation matrix of the CSF biomarker panel. Correlations among CSF biomarkers were calculated according to Spearman’s correlation. Spearman’s rho and corresponding p values are reported. (DOCX 16 kb)
Additional file 5:
ROC analysis of the CSF biomarkers for the different comparisons. The diagnostic performance of each biomarker was calculated according to ROC analysis. AUC, sensitivity, and specificity, together with the 95% CI of each parameter, are included. AD Alzheimer’s disease, PD Parkinson’s disease, PDD Parkinson’s disease with dementia, DLB Dementia with Lewy bodies, OND Other neurological diseases. (DOCX 16 kb)
Additional file 6:
Spearman’s correlations adjusted with Benjamini-Hochberg correction between biomarkers and clinical parameters in the whole cohort and within each group. (DOCX 21 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chiasserini, D., Biscetti, L., Eusebi, P. et al. Differential role of CSF fatty acid binding protein 3, α-synuclein, and Alzheimer’s disease core biomarkers in Lewy body disorders and Alzheimer’s dementia. Alz Res Therapy 9, 52 (2017). https://doi.org/10.1186/s13195-017-0276-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-017-0276-4