Abstract
Introduction
The aim of this study was to investigate the association between visual hallucinations in dementia with Lewy bodies (DLB) and brain perfusion using single-photon emission computed tomography.
Methods
We retrospectively included 66 patients with DLB, 36 of whom were having visual hallucinations (DLB-hallu) and 30 of whom were not (DLB-c). We assessed visual hallucination severity on a 3-point scale of increasing severity: illusions, simple visual hallucinations and complex visual hallucinations. We performed voxel-level comparisons between the two groups and assessed correlations between perfusion and visual hallucinations severity.
Results
We found a significant decrease in perfusion in the left anterior cingulate cortex, the left orbitofrontal cortex and the left cuneus in the DLB-hallu group compared with the DLB-c group. We also found a significant correlation between decreased bilateral anterior cingulate cortex, left orbitofrontal cortex, right parahippocampal gyrus, right inferior temporal cortex and left cuneus perfusion with the severity of hallucinations.
Conclusions
Visual hallucinations seem to be associated with the impairment of anterior and posterior regions (secondary visual areas, orbitofrontal cortex and anterior cingulate cortex) involved in a top-down and bottom-up mechanism, respectively. Furthermore, involvement of the bilateral anterior cingulate cortex and right parahippocampal gyrus seems to lead to more complex hallucinations.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
In people older than 65 years of age, dementia with Lewy bodies (DLB) is the second most common cause of neurodegenerative dementia after Alzheimer disease (AD) [1,2]. Visual hallucinations (VHs) are one of the commonest features of DLB, present in 54% to 70% of patients [3]. It most frequently consists of simple visual illusions wherein objects are distorted or deformed, even though more complex hallucinations may also occur.
Different hypotheses have been proposed to explain the occurrence of VHs in DLB. One of the main hypotheses is that VHs could be consecutive to visuospatial, visuoperceptual or attention deficits, which are more frequent in DLB with VHs than in either DLB without VHs or AD [4]. These visual deficits of central origin are coherent with the specific occipital hypoperfusion in DLB [5]. Another hypothesis is a dysregulation of the gating and filtering of external perception and internal image production [6]. The developers of the Perception and Attention Deficit model proposed that VHs are caused by a combination of impaired attentional binding (top-down) and perceptual processes (bottom-up) [7].
To support theses hypotheses, some study researchers have investigated the relationship between VH and cerebral perfusion or metabolism [8-15] or atrophy by using magnetic resonance imaging (MRI) [16] in patients with neurodegenerative disease. Howard et al. [8] found a decreased reaction of the visual cortex to a visual stimulus during hallucinations in a patient with DLB. Nagahama et al. [10] studied 100 patients with DLB patients with or without hallucinations using single-photon emission computed tomography (SPECT). Those authors demonstrated the involvement of both posterior (bilateral occipital and parietal cortices) and less significantly anterior regions (bilateral middle frontal gyri and bilateral posterior cingulate gyri) in the group with hallucinations. In another study, Pernezcky et al. [13] suggested the involvement of hypometabolism in both visual associative areas (right temporo-occipital cortex) and the prefrontal region (right middle frontal gyrus). Moreover, the involvement of the anterior region was demonstrated in a volumetric study in which Sanchez-Castaneda et al. [16] showed right inferior frontal gyrus atrophy in patients with DLB with VHs. The authors supposed that the prefrontal region is involved in insight into and consciousness of the hallucinations.
The aim of our study was to investigate the neural basis of VHs in DLB and, moreover, the qualitative intensity of VH, which has never previously been investigated in DLB, to the best of our knowledge. Working with the hypothesis that a deficit both in visual treatment of information and in executive control could contribute to VH, we posited that the group of DLB patients with VH would have greater hypoperfusion in posterior regions (that is, occipital and parietotemporal cortices) and the anterior region (that is, prefrontal cortex), respectively, as compared with the group without VH.
Methods
Ethics statement
Our study did not need ethical approval or the patients’ written consent according to French legislation, because it was a retrospective study and SPECT was performed during the patients’ follow-up.
Methodology
We conducted a retrospective study of patients diagnosed with DLB by three neurologist experts in dementia in the Memory Clinic of the Department of Neurology, University Hospital of Strasbourg, France, between 2006 and 2010. To be included, the patients had to have a probable or possible DLB diagnosis as defined by McKeith’s 2005 criteria [17], and a SPECT scan had to have been performed during the patients’ follow-up. SPECT is included in routine diagnostic workups of these patients and is performed for all patients to help in making the diagnosis. To distinguish DLB from Parkinson disease associated with dementia, we excluded patients in whom cognitive impairment had occurred more than 2 years after they were diagnosed with the extrapyramidal syndrome.
Inclusion and exclusion criteria
We studied 100 patients’ records, and a total of 66 patients were included in the study. Nineteen patients were excluded because they had not undergone a SPECT scan; twelve patients were excluded because the diagnosis was uncertain (AD for two patients, trisomy 21 for three patients, epilepsy with memory deficit for two patients, vascular dementia for two patients, metabolic encephalopathy for one patient, Parkinson disease for one patient and no diagnosis for one patient); two patients were excluded because they had only auditory hallucinations; and one patient was excluded because he did not speak French. We also excluded patients with clinical features that could be explained by another cause, patients whose clinical records were incomplete and patients with hallucinations of a nonvisual type (for example, auditory, somatosensory). However, we included patients with another type of hallucination (n = 6) if they were associated with VHs.
Patient records
Patients’ records were analyzed for the following items: sex, age, family history, personal history of depression, presence and severity of an extrapyramidal syndrome (tremor, extrapyramidal rigidity or akinesia), existence and type of hallucinations, presence of motor or cognitive fluctuations, Mini Mental State Examination (MMSE) and neuropsychological assessment, presence of a psychiatric disease or a sleep disorder evocative of a rapid eye movement sleep disorder, results of other investigations (cerebrospinal fluid biomarkers, including tau, phosphorylated tau, Aβ1–42 (Innogenetics, Ghent, Belgium), brain [123I]FP-CIT SPECT, brain MRI, electroencephalography) and patients’ medications at the time of the SPECT. By using the Unified Parkinson’s Disease Rating Scale III score [18], akinesia, rigidity and tremor were rated from 0 to 4 (0 = no symptoms to 4 = serious impairment).
Assessments
Hallucinations were assessed by neurology experts. The patients were asked the following question: “Have you ever seen things that do not exist?” Different types of tests were applied in different patients during follow-up to evaluate cognitive function. The most frequently used tests were the Free and Cued Selective Reminding Test (FCSRT) [19] for episodic memory, the Frontal Assessment Battery (FAB) [20], Trail Making Test (TMT) A and B [21] and formal and semantic lexical evocation [22] for executive function; and a digit-span test for attention and working memory and the Rey-Osterrieth Complex Figure Test [23] for visuoconstructive function.
Single-photon emission computed tomography
Image acquisition
A SPECT scan was obtained by a nuclear medicine physician for every patient during follow-up. The procedure used was as follows. The patients received an injection of 740 MBq of [99mTc]ethyl cysteinate dimer (Neurolite; Lantheus Medical Imaging, North Billerica, MA, USA) (eight patients received 740 MBq of [99mTc]exametazime Ceretec; GE Healthcare, Little Chalfont, UK). The image acquisition began 15 minutes after injection with a dual-head gamma camera (Siemens Medical Imaging, Hoffman Estates, IL, USA) equipped with a fan beam collimator specially manufactured for the study of the brain. Patients were imaged while in the supine position. The heads of the gamma camera were 15 cm away from the center of rotation. The height of the table was 20 cm. Image acquisition included 32 tomographic projections of 50 seconds each. The acquisition matrix was 128 × 128 pixels with zoom set at 1.23. The acquisition window was focused on the energy of the 99mTc isotope photopeak (that is, 140 keV) with a window width of 15%.
Image processing
For image processing, we used SPM8 software (Statistical Parametric Mapping; Wellcome Department of Imaging Neuroscience, University College London [24]) running on MATLAB R2010a (MathWorks, Natick, MA, USA). SPECT images of each patient were spatially normalized to the Montreal Neurological Institute space. Intensities were linearly scaled using the average perfusion of the central regions of cerebellum because these areas are almost preserved in patients with DLB. Finally, images were smoothed with a Gaussian kernel of 12 mm.
Statistical analysis
Patients were divided into two groups: a group of patients with DLB who were having VHs (DLB-hallu) and a control group of patients with DLB who were not having VHs (DLB-c). There were 36 patients in the DLB-hallu group and 30 patients in the DLB-c group.
We used the voxel-based statistical framework provided in SPM8 to compare the images of the two groups. We performed voxel-level comparison of perfusion of the DLB-hallu group with that of the DLB-c group using a two-sample t-test with age and type of tracer as covariates. Statistical maps were thresholded with P < 0.001 with a minimum cluster size of 25 voxels.
A second one-tailed analysis was then undertaken to investigate the putative correlation between perfusion intensity and the severity score of hallucinations while still considering age and type of radiotracer as covariates. We also chose a threshold of P < 0.001 and a minimum cluster size of 25 voxels.
These analyses were conducted without correction for multiple testing. Statistical maps were analyzed with Xjview [25], which allowed us to identify the brain regions associated with the detected clusters.
To compare the general characteristics of the two groups, we used a χ2 test for qualitative data and a Student’s t-test for quantitative data. We used a Kruskal-Wallis test to compare the three subgroups according to the type of hallucination. A difference was considered significant at P < 0.05.
Results
The DLB-hallu and DLB-c groups were comparable with regard to age, sex and MMSE score (see Table 1). In terms of clinical symptoms, there was no significant difference between the two groups for the primary criteria for DLB. However, fluctuations seemed to be more frequent in the DLB-hallu group, but this result did not reach statistical significance (P = 0.07, 95% confidence interval: 0.059, 1.2351).
With regard to cognitive performance, there were no significant differences between the two groups (all P > 0.05) (see Table 2). The DLB-c group performed worse than the DLB-hallu group on the FCSRT, and the DLB-hallu group performed worse than the DLB-c group on the TMT A, but without a significant difference.
The DLB-hallu group was more often treated with neuroleptics: seven by clozapine, two by olanzapine, two by tiapride, one by cyamemazine and one by aripiprazole.
Within the DLB-hallu group, we divided patients into three subgroups according to the type of hallucination. Group 1 (n = 8) included patients with visual illusions (for example, feeling of movement or deformation of an object). Group 2 (n = 9) consisted of patients with simple hallucinations with vision of an isolated entity (for example, a person or an animal). Group 3 (n = 6) comprised patients who had complex hallucinations with the vision of scenes (for example, several persons). For 13 patients, we had insufficient information on the type of hallucinations they were having. We did not find any significant difference between the three subgroups according to the type of hallucination on the basis of clinical data (age, fluctuations, extrapyramidal syndrome) or on the basis of neuropsychological tests, except for direct digit span test (4 in the group 2 versus 5.75 in the group 3, P = 0.033).
Cerebral perfusion
DLB-hallu versus DLB-c
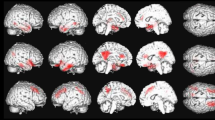
Analyses revealed significant hypoperfusion (P < 0.001) in three brain regions in the DLB-hallu group compared with the DLB-c group: the left anterior cingulate cortex (ACC) within the limbic regions (Brodmann area (BA) 32), the left orbitofrontal cortex (BA 11 and 47) and the left cuneus within the occipital cortex (BA 18) (Figure 1 and Table 3). To evaluate the influence of neuroleptics on these results, we performed supplementary analyses comparing DLB-hallu group and DLB-c group after exclusion of patients who were taking neuroleptics. The results were similar to those of the previous analysis, with additional significant hypoperfusion in the left fusiform gyrus (P < 0.001) in the DLB-hallu group compared with the DLB-c group.
Comparison of patients with dementia with Lewy bodies with versus without hallucinations. A comparison of patients with dementia with Lewy bodies (DLB) with versus without hallucinations (P < 0.001, including age and type of radiotracer as nuisance covariates and a minimum cluster size of 25 voxels) revealed significant hypoperfusion in the left anterior cingulate gyrus (Brodmann area (BA) 32), the left orbitofrontal cortex (BA 11/47) and the left cuneus (BA 19).
Severity of hallucinations
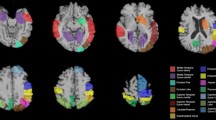
In correlation analysis of cerebral perfusion regarding the severity of hallucinations in the patients with DLB, we found significant hypoperfusion in the bilateral ACC (BA 32), the right parahippocampal gyrus, the right inferior temporal gyrus (BA 20), the left orbitofrontal cortex (BA 11 and 47) and the left cuneus (BA 18) (Figure 2 and Table 3).
Correlation analyses between cerebral hypoperfusion and severity of hallucinations in patients with dementia with Lewy bodies. Correlation analyses between cerebral hypoperfusion and the severity of the hallucinations in the patients with dementia with Lewy bodies revealed the involvement of the bilateral anterior cingulate cortex (Brodmann area (BA) 32), the left orbitofrontal cortex (BA 11/47), the right parahippocampal gyrus (BA 20) and the left cuneus (BA 18) (P < 0.001, including age and type of radiotracer as nuisance covariates and a minimum cluster size of 25 voxels).
Discussion
In this work, we studied two groups of patients with DLB, one with and the other without VHs, who were broadly comparable for general and clinical characteristics. We found three regions with hypoperfusion in patients with DLB and VH. The first was posterior and consisted of the occipital cortex (cuneus), which is involved in visual information processing. The second corresponded to the ACC, which is involved in control process and error detection. The third corresponded to the orbitofrontal cortex, which is involved in inhibitory control and has a network lateralized primarily to the left.
In our study, hypoperfusion in the cuneus (BA 18) seemed to be associated with the presence of hallucinations. This secondary visual area, BA 18, is involved in the recognition and extraction of object features (shape, color, position in space, movement). Dysfunction of BA 18 causes errors in visual processing, with occurrence of object distortions that explain visual illusions particularly well. Patients with DLB report visual illusions more frequently than true hallucinations [26]. The localized relative dysfunction of this visual area, which we found in the present study, is consistent with previous data reported in the literature [9,10,12,15,27]. It confirms the original hypothesis that the occipital dysfunction specific to DLB [28,29] might be involved in the occurrence of VHs. The implication of visual areas was previously confirmed by other studies [10,12,16], in association with the parietal cortex [10] or the right temporo-occipital junction [12]. In previous work by our group involving patients with AD chosen from the Alzheimer’s Disease Neuroimaging Initiative database, we showed atrophy of the occipital cortex in patients with VHs. In our study, the left occipital region seemed predominant. Nagahama et al. [10] also found a predominance of left occipital involvement. This lateralization suggests that impairment of visual perception is crucial in VHs, for which the dominant pathway is the left occipitotemporal cortex, whereas visuospatial function preferentially follows the right occipitoparietal pathway [30,31].
In addition to these posterior aspects, we found hypoperfusion in the left ACC (BA 32) and the left orbitofrontal cortex (BA 11 and 47), suggesting that involvement of the occipital cortex alone is not sufficient to cause hallucinations. The ACC is a part of the limbic lobe, which is activated in tasks involving attention on the Stroop test and go/no-go tasks and is supposed to play an important role in attention, motivation, executive function and error detection [32-34]. The ACC, aside from the insula, is also known to contain the neurons called von Economo neurons [35], which would be implicated in intuitive decision making. Thus, in a complex situation where a quick decision is needed, these cells would be crucial to detect errors and make the correct decision. The functional deficit of the ACC in patients with DLB could lead to difficulty in error detection and correct decision making. The orbitofrontal cortex is well known for this role in inhibitory control and decision making [36]. Dysfunction of this area could prevent the patient from inhibiting the production of internal images.
The following hypothesis on the occurrence of hallucinations in DLB can therefore be proposed. Secondary visual areas are deficient and send false data to the entire cortex (ascending or bottom-up phenomenon). The patient cannot recognize this information as abnormal, because the ACC and the orbitofrontal cortex are also impaired and the VH seems real (descending or top-down phenomenon). The presence of hallucinations requires both a lesion of visual areas and one of control regions such as the ACC and orbitofrontal cortex. Previous studies have implicated the cingulate gyrus in the genesis of hallucinations [37]. It has been found to be the case for the ACC in patients with AD and for the posterior cingulate cortex in patients with DLB [10,11]. Menthis et al. found a significant hypometabolism in orbitofrontal and cingulate areas bilaterally in patients with AD who had delusional misidentification syndromes [38].
As in the study by Nagahama et al. [10], the left cingulum was implicated in our study, whereas the right hemisphere has been observed to be more particularly affected in most studies. Nevertheless, we noted bilateral involvement of the ACC when we took the severity of the hallucinations into account. Severity was defined here not by the frequency of hallucinations, but by their type. A hallucination was considered mild if it was an illusion and severe if it was a complex scene. On the basis of our results, we suggest that the severity of VH depends on the extent to which the system of error detection within the ACC is impaired, with unilateral dysfunction being sufficient for visual illusions, whereas bilateral involvement would be necessary for the vision of complex and aberrant scenes. The severity of hallucinations also correlated with the inferior temporal cortex and parahippocampal hypoperfusion. These results are consistent with previously published data. Harding et al. [39] found an association between hallucinations and high densities of Lewy bodies in the parahippocampal and inferior temporal cortices. In a recent study, Megevand et al. [40] showed that VHs could be evoked by direct electrical stimulation of the parahippocampal area. Our results support the role of the parahippocampal gyrus in the perception of visual scenes [41] because its hypoperfusion seems necessary for the occurrence of complex VHs, but not for visual illusions.
Our study has some limitations. The diagnosis of DLB was based on clinical features, and we did not have access to the postmortem examinations to confirm these diagnoses. However, the McKeith criteria for DLB have very good specificity (98%) [42]. Exploration of hallucinations is difficult because they are transient and short, and SPECT is often performed outside the hallucinatory period. Functional imaging studies to show which areas are involved during hallucinations would be interesting, but they are difficult to achieve because hallucinations are not predictable and require considerable cooperation on the part of the patient, which is not easy to obtain in patients with DLB. A higher proportion of patients with hallucinations were on neuroleptics, and these drugs could have influenced the SPECT findings. Handley et al. showed frontal hypoperfusion secondary to neuroleptics (haloperidol and aripiprazole) in healthy volunteers, but anterior cingulate perfusion was increased after neuroleptic treatment [43]. The same results were found by Pardo et al. [44]. The relative hypoperfusion found in the ACC is possibly minimized by neuroleptics.
Conclusions
Overall, our study, together with previous studies, suggests that the occurrence of VHs in DLB requires the dysfunction of both the anterior and posterior regions, which are involved in top-down and bottom-up mechanisms, respectively. VHs seem to be related to impairment of secondary visual areas involved in visual perception and impairment of the ACC and orbitofrontal cortex involved in control processes and error detection. Well-formed hallucinations with complex scenes seem to be related specifically to impairment of bilateral ACC and parahippocampal gyrus involved in the perception of visual scenes.
Abbreviations
- ACC:
-
Anterior cingulate cortex
- AChEI:
-
Acetylcholinesterase inhibitor
- AD:
-
Alzheimer disease
- BA:
-
Brodmann area
- CFR:
-
Cued free recall
- CTR:
-
Cued total recall
- DLB:
-
Dementia with Lewy bodies
- DLB-c:
-
Control group of patients with dementia with Lewy bodies who were not having visual hallucinations
- DLB-hallu:
-
Study group of patients with dementia with Lewy bodies who were having visual hallucinations
- FAB:
-
Frontal Assessment Battery
- FCSRT:
-
Free and Cued Selective Reminding Test
- FR:
-
Free recall
- IR:
-
Immediate recall
- MMSE:
-
Mini Mental State Examination
- MNI:
-
Montreal Neurological Institute
- MRI:
-
Magnetic resonance imaging
- SPECT:
-
Single-photon emission computed tomography
- SPM:
-
Statistical Parametric Mapping
- TMT:
-
Trail Making Test
- TR:
-
Total recall
- VH:
-
Visual hallucination
References
Buracchio T, Arvanitakis Z, Gorbien M. Dementia with Lewy bodies: current concepts. Dement Geriatr Cogn Disord. 2005;20:306–20.
McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, et al. Dementia with Lewy bodies. Lancet Neurol. 2004;3:19–28.
Aarsland D, Ballard C, Larsen JP, McKeith I. A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson’s disease with and without dementia. Int J Geriatr Psychiatry. 2001;16:528–36.
Mori E, Shimomura T, Fujimori M, Hirono N, Imamura T, Hashimoto M, et al. Visuoperceptual impairment in dementia with Lewy bodies. Arch Neurol. 2000;57:489–93.
Lobotesis K, Fenwick JD, Phipps A, Ryman A, Swann A, Ballard C, et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology. 2001;56:643–9.
Diederich NJ, Goetz CG, Stebbins GT. Repeated visual hallucinations in Parkinson’s disease as disturbed external/internal perceptions: focused review and a new integrative model. Mov Disord. 2005;20:130–40.
Collerton D, Perry E, McKeith I. Why people see things that are not there: a novel Perception and Attention Deficit model for recurrent complex visual hallucinations. Behav Brain Sci. 2005;28:737–94.
Howard R, David A, Woodruff P, Mellers I, Wright J, Brammer M, et al. Seeing visual hallucinations with functional magnetic resonance imaging. Dement Geriatr Cogn Disord. 1997;8:73–7.
Imamura T, Ishii K, Hirono N, Hashimoto M, Tanimukai S, Kazuai H, et al. Visual hallucinations and regional cerebral metabolism in dementia with Lewy bodies (DLB). Neuroreport. 1999;10:1903–7.
Nagahama Y, Okina T, Suzuki N, Matsuda M. Neural correlates of psychotic symptoms in dementia with Lewy bodies. Brain. 2010;133:557–67.
O’Brien JT, Firbank MJ, Mosimann UP, Burn DJ, McKeith IG. Change in perfusion, hallucinations and fluctuations in consciousness in dementia with Lewy bodies. Psychiatry Res. 2005;139:79–88.
Perneczky R, Drzezga A, Boecker H, Forstl H, Kurz A, Haussermann P. Cerebral metabolic dysfunction in patients with dementia with Lewy bodies and visual hallucinations. Dement Geriatr Cogn Disord. 2008;25:531–8.
Perneczky R, Drzezga A, Boecker H, Wagenpfeil S, Forstl H, Kurz A, et al. Right prefrontal hypometabolism predicts delusions in dementia with Lewy bodies. Neurobiol Aging. 2009;30:1420–9.
Sato T, Hanyu H, Hirao K, Shimizu S, Kanetaka H, Iwamoto T. Deep gray matter hyperperfusion with occipital hypoperfusion in dementia with Lewy bodies. Eur J Neurol. 2007;14:1299–301.
Taylor JP, Firbank MJ, He J, Barnett N, Pearce S, Livingstone A, et al. Visual cortex in dementia with Lewy bodies: magnetic resonance imaging study. Br J Psychiatry. 2012;200:491–8.
Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, Campdelacreu J, Gascon J, Falcon C, et al. Frontal and associative visual areas related to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease with dementia. Mov Disord. 2010;25:615–22.
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72.
Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18:738–50.
Frasson P, Ghiretti R, Catricala E, Pomati S, Marcone A, Parisi L, et al. Free and Cued Selective Reminding Test: an Italian normative study. Neurol Sci. 2011;32:1057–62.
Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–6.
Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–14.
Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y. Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol Belg. 1990;90:207–17.
Osterrieth PA. Test of copying a complex figure; contribution to the study of perception and memory. Arch Psychol. 1944;30:206–356.
Statistical Parametric Mapping (SPM). http://www.fil.ion.ucl.ac.uk/spm/. Accessed 26 January 2015.
xjView, a viewing program for SPM. http://www.alivelearn.net/xjview8/. Accessed 26 January 2015.
Hirono N, Mori E, Imamura T, Shimomura T, Hashimoto M. Neuropsychiatric features in dementia with Lewy bodies and Alzheimer’s disease. No To Shinkei. 1998;50:45–9. Japanese.
Mori T, Ikeda M, Fukuhara R, Nestor PJ, Tanabe H. Correlation of visual hallucinations with occipital rCBF changes by donepezil in DLB. Neurology. 2006;66:935–7.
Albin RL, Minoshima S, D’Amato CJ, Frey KA, Kuhl DA, Sima AA. Fluoro-deoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology. 1996;47:462–6.
Donnemiller E, Heilmann J, Wenning GK, Berger W, Decristoforo C, Moncayo R, et al. Brain perfusion scintigraphy with 99mTc-HMPAO or 99mTc-ECD and 123I-β-CIT single-photon emission tomography in dementia of the Alzheimer-type and diffuse Lewy body disease. Eur J Nucl Med. 1997;24:320–5.
Kohler S, Kapur S, Moscovitch M, Winocur G, Houle S. Dissociation of pathways for object and spatial vision: a PET study in humans. Neuroreport. 1995;6:1865–8.
Shen L, Hu X, Yacoub E, Ugurbil K. Neural correlates of visual form and visual spatial processing. Hum Brain Mapp. 1999;8:60–71.
Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–46.
Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9.
Robinson H, Calamia M, Glascher J, Bruss J, Tranel D. Neuroanatomical correlates of executive functions: a neuropsychological approach using the EXAMINER battery. J Int Neuropsychol Soc. 2014;20:52–63.
Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for von Economo neurons. Trends Cogn Sci. 2005;9:367–73.
Peters F, Perani D, Herholz K, Holthoff V, Beuthien-Baumann B, Sorbi S, et al. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;21:373–9.
Onofrj M, Taylor JP, Monaco D, Franciotti R, Anzellotti F, Bonanni L, et al. Visual hallucinations in PD and Lewy body dementias: old and new hypotheses. Behav Neurol. 2013;27:479–93.
Mentis MJ, Weinstein EA, Horwitz B, McIntosh AR, Pietrini P, Alexander GE, et al. Abnormal brain glucose metabolism in the delusional misidentification syndromes: a positron emission tomography study in Alzheimer disease. Biol Psychiatry. 1995;38:438–49.
Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002;125:391–403.
Megevand P, Groppe DM, Goldfinger MS, Hwang ST, Kingsley PB, Davidesco I, et al. Seeing scenes: topographic visual hallucinations evoked by direct electrical stimulation of the parahippocampal place area. J Neurosci. 2014;34:5399–405.
Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci. 2008;12:388–96.
Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2010;257:359–66.
Handley R, Zelaya FO, Reinders AA, Marques TR, Mehta MA, O’Gorman R, et al. Acute effects of single-dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum Brain Mapp. 2013;34:272–82.
Pardo BM, Garolera M, Ariza M, Pareto D, Salamero M, Valles V, et al. Improvement of cognitive flexibility and cingulate blood flow correlates after atypical antipsychotic treatment in drug-naive patients with first-episode schizophrenia. Psychiatry Res. 2011;194:205–11.
Acknowledgement
The authors are grateful to all neuropsychologists of the Neuropsychologic Unit of Strasbourg, for help in patients' assessments. We also thank Julien Lamy for help in translation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
VN performed the image processing. BC and NP examined patients, diagnosed DLB and participated in the design of the study. LK and MS helped to analyze patients’ records. FH and IN performed image acquisition. JPA participated in the coordination and design of the study. FB conceived of the study, participated in its design and coordination and helped to draft the manuscript. CH analyzed patients’ records, performed the statistical analysis and drafted the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Heitz, C., Noblet, V., Cretin, B. et al. Neural correlates of visual hallucinations in dementia with Lewy bodies. Alz Res Therapy 7, 6 (2015). https://doi.org/10.1186/s13195-014-0091-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-014-0091-0