Abstract
Background
Polycyclic aromatic hydrocarbon (PAH)-rich substances like cigarette smoke and PM2.5 induce aryl hydrocarbon receptor (AHR)-mediated aryl hydrocarbon receptor repressor (AHRR) methylation. AHRR cg05575921 and coagulation factor II (thrombin) receptor-like 3 (F2RL3) cg03636183 methylation patterns are well-established biomarkers for smoking. Even though AHRR cg05575921 methylation has recently been associated with PM2.5, the interaction between smoking and PM2.5 on AHRR methylation is yet to be fully explored. We evaluated AHRR and F2RL3 CpG sites to identify potential significant markers in relation to PM2.5 and smoking in Taiwanese adults.
Methods
DNA methylation and smoking data of 948 participants aged 30–70 years were obtained from the Taiwan Biobank Database (2008–2015), while PM2.5 data were obtained from the Air Quality Monitoring Database (2006–2011).
Results
Smoking and PM2.5 were independently associated with hypomethylation (lower levels) of AHRR cg05575921, AHRR cg23576855, F2RL3 cg03636183, and F2LR3 cg21911711 after multiple-comparison correction (Bonferroni P < 0.00028409). Cg05575921 was the most hypomethylated AHRR CpG site, while cg03636183 was the most hypomethylated F2RL3 CpG site. Overall, cg05575921 was the most hypomethylated CpG site: β = − 0.03909, P < 0.0001; − 0.17536, P < 0.0001 for former and current smoking, respectively (P-trendsmoking < 0.0001) and − 0.00141, P < 0.0001 for PM2.5. After adjusting for F2RL3 cg03636183, smoking and PM2.5 remained significantly associated with cg05575921 hypomethylation: β − 0.02221, P < 0.0001; − 0.11578, P < 0.0001 for former and current smoking, respectively (P-trendsmoking < 0.0001) and − 0.0070, P = 0.0120 for PM2.5. After stratification by sex, smoking and PM2.5 remained associated (P < 0.05) with cg05575921 hypomethylation in both men (β = − 0.04274, − 0.17700, and − 0.00163 for former smoking, current smoking, and PM2.5, respectively) and women (β = − 0.01937, − 0.17255, and − 0.00105 for former smoking, current smoking, and PM2.5, respectively). After stratification by residential area, former and current smoking remained associated (P < 0.05) with cg05575921 hypomethylation: β = − 0.03918 and − 0.17536, respectively (P-trendsmoking < 0.0001). Living in the central and southern areas was also associated (P < 0.05) with cg05575921 hypomethylation: β = − 0.01356 and − 0.01970, respectively (P-trendarea < 0.0001).
Conclusion
Smoking and PM2.5 were independently associated with hypomethylation of cg05575921, cg23576855, cg03636183, and cg21911711. The most hypomethylated CpG site was cg05575921 and its association with smoking and PM2.5 was dose-dependent.
Similar content being viewed by others
Background
Aryl hydrocarbon receptor plays a key role in the metabolism of xenobiotic substances like particulate matter and cigarette smoke which are rich in polycyclic aromatic hydrocarbons and dioxin [1,2,3,4,5,6]. This gene enhances tumor development through immunosuppression, proliferation, and apoptosis resistance [1, 7, 8]. The transcription factor, AHRR, is a tumor suppressor gene [1, 9, 10]. It modulates cancer enhancing processes like inflammation and proliferation [1, 11] by competing and repressing AHR. However, its downregulation causes an upsurge in AHR activities thereby enhancing tumorigenesis [10].
DNA methylation is a remarkable and stable covalent process that occurs during early tumorigenesis [12]. This epigenetic process is reversible and thus, is a potential diagnostic tool and therapeutic target for diseases especially lung cancer [12, 13]. Lung cancer is a major cause of cancer-related mortality in Taiwan [14, 15] and the world [13, 14, 16, 17]. Air pollution and cigarette smoking are two important environmental factors that independently enhance lung cancer development and mortality [16,17,18,19,20,21,22,23,24]. In 2015, approximately 8.9 and 6·4 million global deaths were attributable to PM2.5 and smoking, respectively [25, 26]. Significant joint effects of PM2.5 and smoking on lung cancer and cardiovascular disease mortality have been reported [27,28,29].
Hypomethylation of AHRR cg05575921 and F2RL3 cg03636183 was significantly associated with PAH exposure in a dose-dependent manner [30]. Moreover, these CpG sites have been consistently associated with smoking [17, 31,32,33,34,35,36,37,38,39,40,41,42,43] and lung cancer [10, 31, 35, 38, 42] and their methylation patterns are well-established biomarkers for smoking [41, 43]. AHRR is the most smoking-upregulated gene [44] and cg05575921 is the top-ranked smoking marker [45]. Even though cg05575921 methylation is markedly associated with smoking, its relationship with PM2.5 is yet to be fully established. To date, only one study has assessed the association between AHRR cg05575921 methylation and PM2.5 in Taiwan [46].
To our knowledge, no study has focused on the relationship of AHRR cg05575921 methylation with both PM2.5 and cigarette smoking. Hence, it is yet to be fully known whether PM2.5 and cigarette smoking have a joint effect on AHRR cg05575921 methylation. Therefore, the interaction between PM2.5 and smoking on AHRR cg05575921 methylation and other methylation markers for smoking merits more studies. In view of this, we evaluated AHRR and F2RL3 CpG sites to identify potential significant markers in relation to PM2.5 and smoking in Taiwanese men and women.
Results
The association of AHRR and F2LR3 CpG sites with smoking and PM2.5 is shown in Supplementary Table 1. Four CpG sites (AHRR cg05575921, AHRR cg23576855, F2RL3 cg03636183, and F2RL3 cg21911711) had significant inverse associations with smoking and PM2.5 after multiple-comparison correction (Bonferroni P value < 0.00028409). That is, AHRR cg05575921, AHRR cg23576855, F2RL3 cg03636183, and F2RL3 cg21911711 were significantly hypomethylated in relation to smoking and PM2.5. Noteworthy, cg05575921 was the most hypomethylated AHRR CpG site, while cg03636183 was the most hypomethylated F2RL3 CpG site. In general, cg05575921 was the most hypomethylated CpG site (Supplementary Table 1) and further analysis was focused on this specific CpG site.
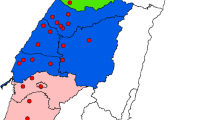
The clinical and demographic characteristics of 488 male and 460 female participants are illustrated in Table 1. The mean ± standard error (SE) AHRR cg05575921 methylation levels (beta-values) in men and women were 0.7832 ± 0.0042 and 0.8376 ± 0.0023, respectively. There were significant differences (P < 0.0001) between men and women with respect to cg05575921 methylation, smoking, alcohol drinking, and body mass index (BMI). The mean annual concentrations of PM2.5 from 2006 to 2011 were 26.53, 30.06, 36.91, and 40.68 μg/m3 for the northern, north-central, central, and southern area, respectively (Table 1).
Table 2 shows the association of smoking and PM2.5 with cg05575921 methylation in the study participants. Compared to never smoking, former and current smoking were significantly associated with lower levels of cg05575921: β = − 0.03909, P < 0.0001 and − 0.17536, P < 0.0001 for former and current smoking, respectively. Moreover, the test for linear trend was significant: P-trendsmoking < 0.0001 (Table 2). In addition to smoking, PM2.5, sex, and age were significantly associated with lower levels of cg05575921 methylation: β = − 0.00141 (P < 0.0001), − 0.01870 (P < 0.0001), and − 0.00044 (P = 0.0145) for PM2.5, male sex, and age, respectively (Table 2, model 1). Inclusion of F2RL3 cg03636183 into the model did not significantly affect the association of AHRR cg05575921 with smoking and PM2.5: β − 0.02221, P < 0.0001 and, − 0.11578, P < 0.0001 for former and current smoking, respectively (P-trendsmoking < 0.0001) and − 0.0070, P = 0.0120 for PM2.5 (Table 2, model 2). The interaction between PM2.5 and smoking on cg05575921 methylation was not significant.
After stratification by sex (Table 3), smoking and PM2.5 remained significantly associated with lower levels of cg05575921 methylation in both men: β = 0.04274, P < 0.0001 for former smoking; − 0.17700, P < 0.0001 for current smoking (P-trendsmoking < 0.0001); and − 0.00163, P = 0.0017 for PM2.5 and women: β = − 0.01937, P = 0.0417 for former smoking; − 0.17255, P < 0.0001 for current smoking (P-trendsmoking < 0.0001); and − 0.00105, P = 0.0015 for PM2.5 (Table 3).
Table 4 shows the association of smoking and living in PM2.5 areas with cg05575921 methylation. With never smoking as the reference variable, significant lower levels of AHRR cg05575921 methylation were observed in both former and current smokers: β = − 0.03918, P < 0.0001 and − 0.17536, P < 0.0001, respectively (P-trendsmoking < 0.0001). Living in areas with higher PM2.5 pollution was associated with lower levels of cg05575921 methylation: β = − 0.00267, P = 0.6230 for the north-central; − 0.01356, P = 0.0074 for the central; and − 0.01970, P < 0.0001 for the southern area. Despite the absence of an association between living in the north-central area and cg05575921 methylation, the test for trend was significant (P-trendarea < 0.0001).
After stratification by sex (Table 5), smoking and living in areas with higher PM2.5 pollution remained significantly associated with lower levels of AHRR cg05575921 methylation in both men: β = − 0.01996, P = 0.0374 for former smoking; − 0.17221, P < 0.0001 for current smoking (P-trendsmoking < 0.0001); − 0.00577, P = 0.3174 for the north-central; − 0.00935, P = 0.0820 for the central; and − 0.01620, P = 0.0016 for the southern area (P-trendarea = 0.0013) and women: β = − 0.04276, P < 0.0001 for former smoking; − 0.17706, P < 0.0001 for current smoking (P-trendsmoking < 0.0001); and 0.00092, P = 0.9198 for the north-central; − 0.01770, P = 0.0361 for the central; and − 0.02076, P = 0.0092 for the southern area (P-trendarea = 0.0025).
The association of F2RL3 cg03636183 (another smoking-related CpG site) with PM2.5 and smoking was similar to that observed for AHRR cg05575921. That is, PM2.5 and smoking were significantly associated with hypomethylation of F2RL3 cg03636183 (Supplementary Tables 2, 3, 4 and 5).
Discussion
In the current study, smoking and PM2.5 were significantly associated with hypomethylation of AHRR cg05575921, AHRR cg23576855, F2RL3 cg03636183, and F2LR3 cg21911711 after multiple-comparison correction (Bonferroni correction). Cg05575921 was the most hypomethylated AHRR CpG site, while cg03636183 was the most hypomethylated F2RL3 CpG site. Noteworthy, cg05575921 was the overall top significant CpG site and its relation with smoking and PM2.5 was dose-dependent. Adjusting for F2RL3 cg03636183 did not affect the association of smoking and PM2.5 with cg05575921. However, there was no significant interaction between smoking and PM2.5 on cg05575921 methylation. To the best of our knowledge, no study has assessed both the independent and joint association of smoking and PM2.5 with AHRR cg05575921 methylation.
Cigarette smoke contains toxic components capable of directly altering the epigenetic profiles of white blood cells in the bloodstream [39]. For instance, PAH in cigarette smoke induces AHR-mediated AHRR expression and methylation [1, 2, 5, 47, 48]. In light of this, we focused on the association of smoking and PM2.5 with a specific AHRR methylation marker, cg05575921. Our focus on this particular CpG site was because it has recently been associated with PM2.5 exposure in non-smokers [46]. Besides, it has consistently shown dose-response relationships with smoking [17, 31,32,33,34,35,36,37,38,39,40, 49] and has also shown high sensitivity and specificity in predicting smoking status [33, 50, 51]. Moreover, it has been the most significant hypomethylated smoking-related CpG site in many epigenome-wide association studies (EWAS) [45]. Findings from the current study are in concordance with those stated above.
In addition to being a predictor of smoking habits [52], AHRR cg05575921 methylation from whole blood or saliva DNA is a validated predictor of smoking-related morbidity and mortality [35, 49]. For instance, AHRR cg05575921 hypomethylation has been associated with enhanced carcinogenesis especially in the lungs [10, 31, 35, 38]. The mechanism underlying the relationship between AHRR cg05575921 methylation and lung cancer is unclear. However, cigarette smoke-induced AHRR hypomethylation and the associated increase in the expression of the AHRR gene could compromise the metabolizing ability of the body. This impairs the removal of deleterious environmental pollutants thereby increasing the risk of cancer [53]. Moreover, it is believed that cigarette smoke triggers vascular endothelial dysfunction by disrupting the structure of the airway epithelial barrier [54, 55]. This eases the entry, deposition, and retention of particles into the arterial wall [56, 57]. Cigarette smoke may also impair the clearance of fine particles in the alveoli thereby aggravating subsequent deleterious effects [56, 57].
Toxic components adsorbed on the surface of PM2.5 like PAHs have been associated with DNA methylation changes [58, 59]. Like PM2.5, cigarette smoke also contains PAHs which are capable of inducing AHR-mediated AHRR methylation [1, 2, 5, 47, 48]. Since both cigarette smoke and PM2.5 are inhalable carcinogens rich in PAH, PM2.5-induced AHRR methylation might also explain the pathophysiological mechanism of lung cancer. While the relationship of AHRR cg05575921 methylation with smoking is well-documented [17, 31,32,33,34,35,36,37,38,39,40, 49], PM2.5-related AHRR cg05575921 methylation on the other hand still deserves more attention. It has been suggested that air pollution could be the driving force behind the AHRR cg05575921 hypomethylation-associated impaired lung function in never smokers [60]. In our previous study, PM2.5 was significantly associated with AHRR cg05575921 hypomethylation in non-smoking Taiwanese adults [46]. In the current study, PM2.5 was also inversely associated with AHRR cg05575921 methylation in both smoking and non-smoking Taiwanese adults.
In the current study, PM2.5 and smoking were also significantly associated with hypomethylation of F2RL3 cg03636183, another prominent smoking-related methylation site. The smoking-related F2RL3 cg03636183 hypomethylation observed in this study is in line with findings from previous studies [31, 41, 42, 45, 61,62,63]. Note should be taken that AHRR cg05575921 remained the top significant hypomethylated CpG site in the current study. Moreover, its association with smoking and PM2.5 remained significant even after adjusting for F2RL3 cg03636183.
Significant interactions between smoking and PM2.5 on lung cancer and cardiovascular mortality [27,28,29] have been reported. However, we found no significant interactions between smoking and PM2.5 on cg05575921 AHRR methylation. The smoking-related cg05575921 methylation levels were lower compared to the PM2.5-related levels. Despite this, the association of PM2.5 with cg05575921 methylation cannot be neglected because exposure to air pollution is involuntary and affects everyone, unlike smoking which is individual behavior that can be modified [64]. To our knowledge, there are no available publications on the interaction of PM2.5 with smoking on cg05575921 methylation. In view of this, we recommend further research in this area.
The relatively large sample size in addition to the adjusting for secondhand smoke exposure constitute the strengths of this study. However, the study is limited in that there was no available data to evaluate the functional correlation between mRNA gene expression and AHRR cg05575921 methylation. Moreover, all eligible Taiwan Biobank participants are individuals who have no personal history of cancer. Therefore, our results may not truly depict the pattern of cg05575921 methylation in individuals with cancer. Furthermore, since validated tools for individual exposure estimates are not available, the actual concentrations of PM2.5 in individuals could not be determined. The use of nearby monitoring stations to estimate exposure to PM2.5 might have resulted in exposure classification error. Smoking was self-reported and the possibility of recall bias cannot be ruled out. However, our findings were consistent with several previous findings on smoking and cg05575921 methylation [17, 31,32,33,34,35,36,37,38,39,40, 49].
Conclusion
Smoking and PM2.5 were independently associated with lower levels of AHRR cg05575921, AHRR cg23576855, F2RL3 cg03636183, and F2RL3 cg21911711 after multiple-comparison correction. The most hypomethylated CpG site was cg05575921 and its association with smoking and PM2.5 was in a dose-dependent manner. Even though both smoking and PM2.5 were inversely associated with cg05575921 methylation, the smoking-related methylation levels were relatively low. Despite this, PM2.5-related methylation cannot be ignored because exposure to air pollution is involuntary and could affect everyone while smoking is a modifiable individual behavior. Our findings suggest that smoking and PM2.5 may independently but not jointly affect AHRR cg05575921 methylation. Considering the remarkable harmful impact of PM2.5, smoking, and cg05575921 hypomethylation on cardiovascular and pulmonary health, measures should be put in place to reduce PM2.5 pollution and individuals should be sensitized on the harmful effects of smoking.
Methods
Study participants and data sources
A total of 948 individuals (488 men and 460 women) were included in the current study. Genomic and demographic data including DNA methylation, smoking, residence, exercise, age, BMI, alcohol drinking, and secondhand smoke were obtained from the Taiwan Biobank Database (2008–2015), while PM2.5 data were obtained from the Air Quality Monitoring Database (2006–2011).
The Taiwan Biobank project is a community-based cohort study aimed at collecting genetic and lifestyle data, alongside tracking the health of more than 200,000 ethnic Taiwanese adults for at least 10 years [65, 66]. This national health resource contains 29 recruitment centers that are distributed all over the island [65]. Only Taiwanese adults aged 30–70 years who have no personal history of cancer are eligible for recruitment [65]. Relevant guidelines and regulations are followed during the recruitment process. All eligible participants signed a letter of consent before the data collection process.
Long-term and well-maintained air quality monitoring systems are a major basis of effective air quality protection and control. Air quality monitoring in Taiwan began in 1993 and is run by the Taiwan Air Quality Monitoring Network (TAQMN) of the Environmental Protection Administration (EPA) [67, 68]. More information about this TAQMN has been described elsewhere [69]. Currently, there are about 77 fully automated air quality monitoring stations distributed throughout Taiwan which provide daily levels of air pollutants [68, 70]. The TAQMN began providing regular daily mean measures of PM2.5 since 2006 [70].
DNA Methylation determination
DNA methylation experiments and analyses were performed by the Health GeneTech Corporation in Taiwan. In brief, sodium bisulfite treatment of DNA extracted from whole blood was done using the EZ DNA Methylation Kit (Zymo Research, CA, USA). Methylation levels were measured using the Illumina Infinium MethylationEPIC BeadChip (Illumina Inc., San Diego, CA, USA) which detects over 850,000 CpG sites within the human genome [71, 72]. Methylation levels were represented by β values which range from 0 (no methylation) to 1 (full methylation). To ensure good quality control, probes with poor detection (P > 0.05) and bead count < 3 were excluded, while background signals were subtracted. In addition, dye-bias across batches was corrected by normalization and outliers were removed using the median absolute deviation method. Finally, cell-type heterogeneity was corrected using the Reference-Free Adjustment for Cell-Type composition (ReFACTor) method [73].
Exposure and covariate assessment
We estimated exposure to PM2.5 using participants’ residential addresses which were grouped into 4 (northern, north-central, central, and southern area) as previously described [22, 46]. Annual average concentrations of PM2.5 from 2006 to 2011 were used in the final analysis. Smoking habits were self-reported and participants were classified as current, former, or non-smokers. Covariates included exercise, sex, age, BMI, alcohol drinking habits (current, former and non-drinking), and secondhand smoke exposure. Smoking habits and covariates were defined using the same criteria described by Tantoh and colleagues [46].
Statistical analysis
Data management and statistical analyses were performed using the SAS 9.3 software (SAS Institute, Cary, NC). Continuous data were analyzed using the t test and expressed as mean ± standard error (SE), while categorical data were analyzed using the chi-square test and expressed as percentages (%). Multivariate linear regression models were used to determine the association of smoking and PM2.5 with AHRR and F2RL3 CpG sites. The most significant Bonferroni-corrected CpG site was used for further analysis. Adjustments were made for covariates including exercise, sex, age, BMI, alcohol drinking, and secondhand smoke exposure.
Availability of data and materials
The data that support the findings of this study are available from Taiwan Biobank but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Taiwan Biobank.
Abbreviations
- PAH:
-
Polycyclic aromatic hydrocarbon
- PM2.5 :
-
Particulate matter < 2.5 microns in aerodynamic diameter
- AHR:
-
Aryl hydrocarbon receptor
- AHRR:
-
Aryl hydrocarbon receptor repressor
- F2RL3:
-
Coagulation factor II (thrombin) receptor-like 3
- DNA:
-
Deoxyribonucleic acid
- SE:
-
Standard error
- β :
-
Regression coefficient
- TAQMN:
-
Taiwan Air Quality Monitoring Network
- EPA:
-
Environmental Protection Administration
- CpG:
-
Cytosine-phosphate-guanine
- BMI:
-
Body mass index
- MOST:
-
Ministry of Science and Technology
- n :
-
Sample size
References
Vogel CF, Haarmann-Stemmann T. The aryl hydrocarbon receptor repressor–more than a simple feedback inhibitor of AhR signaling: clues for its role in inflammation and cancer. Curr Opin Toxicol. 2017;2:109–19.
Andrysík Z, Vondráček J, Marvanová S, Ciganek M, Neča J, Pěnčíková K, Mahadevan B, Topinka J, Baird WM, Kozubík A. Activation of the aryl hydrocarbon receptor is the major toxic mode of action of an organic extract of a reference urban dust particulate matter mixture: the role of polycyclic aromatic hydrocarbons. Mutat Res. 2011;714:53–62.
van der Zee SC, Fischer PH, Hoek G. Air pollution in perspective: health risks of air pollution expressed in equivalent numbers of passively smoked cigarettes. Environ Res. 2016;148:475–83.
Harlid S, Xu Z, Panduri V, Sandler DP, Taylor JA. CpG sites associated with cigarette smoking: analysis of epigenome-wide data from the sister study. Environ Health Perspect. 2014;122:673–8.
Zhu X, Li J, Deng S, Yu K, Liu X, Deng Q, Sun H, Zhang X, He M, Guo H. Genome-wide analysis of DNA methylation and cigarette smoking in a Chinese population. Environ Health Perspect. 2016;124:966.
Köhle C, Bock KW. Coordinate regulation of phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem Pharmacol. 2007;73:1853–62.
Bekki K, Vogel H, Li W, Ito T, Sweeney C, Haarmann-Stemmann T, Matsumura F, Vogel CF. The aryl hydrocarbon receptor (AhR) mediates resistance to apoptosis induced in breast cancer cells. Pestic Biochem Physiol. 2015;120:5–13.
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197.
Neidhart M. DNA methylation and complex human disease: Academic Press; 2015.
Zudaire E, Cuesta N, Murty V, Woodson K, Adams L, Gonzalez N, Martínez A, Narayan G, Kirsch I, Franklin W. The aryl hydrocarbon receptor repressor is a putative tumor suppressor gene in multiple human cancers. J Clin Invest. 2008;118:640–50.
Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–91.
Diaz-Lagares A, Mendez-Gonzalez J, Hervas D, Saigi M, Pajares MJ, Garcia D, Crujerias AB, Pio R, Montuenga LM, Zulueta J. A novel epigenetic signature for early diagnosis in lung cancer. Clin Cancer Res. 2016;22:3361–71.
Battram T, Richmond R, Baglietto L, Haycock P, Perduca V, Bojesen S, Gaunt T, Hemani G, Guida F, Torres RC. Appraising the causal relevance of DNA methylation for risk of lung cancer. bioRxiv. 2019;287888.
Weng C-F, Chen L-J, Lin C-W, Chen H-M, Lee HH-C, Ling T-Y, Hsiao F-Y. Association between the risk of lung cancer and influenza: a population-based nested case-control study. Int J Infect Dis. 2019;88:8–13.
Wang B-Y, Huang J-Y, Cheng C-Y, Lin C-H, Ko J-L, Liaw Y-P. Lung cancer and prognosis in Taiwan: a population-based cancer registry. J Thorac Oncol. 2013;8:1128–35.
Pallis AG, Syrigos KN. Lung cancer in never smokers: disease characteristics and risk factors. Crit Rev Oncol Hematol. 2013;88:494–503.
Pikor LA, Ramnarine VR, Lam S, Lam WL. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer. 2013;82:179–89.
Chiu H-F, Cheng M-H, Tsai S-S, Wu T-N, Kuo H-W, Yang C-Y. Outdoor air pollution and female lung cancer in Taiwan. Inhal Toxicol. 2006;18:1025–31.
Chen G, Wan X, Yang G, Zou X. Traffic-related air pollution and lung cancer: a meta-analysis. Thorac Cancer. 2015;6:307–18.
Tomczak A, Miller AB, Weichenthal SA, To T, Wall C, van Donkelaar A, Martin RV, Crouse DL, Villeneuve PJ. Long-term exposure to fine particulate matter air pollution and the risk of lung cancer among participants of the Canadian National Breast Screening Study. Int J Cancer. 2016;139:1958–66.
Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, Hoffmann B, Fischer P, Nieuwenhuijsen MJ, Brunekreef B. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013;14:813–22.
Lo W-C, Shie R-H, Chan C-C, Lin H-H. Burden of disease attributable to ambient fine particulate matter exposure in Taiwan. J Formos Med Assoc. 2017;116:32–40.
Soberanes S, Gonzalez A, Urich D, Chiarella SE, Radigan KA, Osornio-Vargas A, Joseph J, Kalyanaraman B, Ridge KM, Chandel NS. Particulate matter air pollution induces hypermethylation of the p16 promoter via a mitochondrial ROS-JNK-DNMT1 pathway. Sci Rep. 2012;2:275.
Belinsky SA, Nikula KJ, Palmisano WA, Michels R, Saccomanno G, Gabrielson E, Baylin SB, Herman JG. Aberrant methylation of p16INK4a is an early event in lung cancer and a potential biomarker for early diagnosis. Proc Natl Acad Sci. 1998;95:11891–6.
Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA, Apte JS, Brauer M, Cohen A, Weichenthal S. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci. 2018;115:9592–7.
Reitsma MB, Fullman N, Ng M, Salama JS, Abajobir A, Abate KH, Abbafati C, Abera SF, Abraham B, Abyu GY. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389:1885–906.
Lin H, Zhang X, Feng N, Wang R, Zhang W, Deng X, Wang Y, Yu X, Ye X, Li L. LncRNA LCPAT1 mediates smoking/particulate matter 2.5-induced cell autophagy and epithelial-mesenchymal transition in lung cancer cells via RCC2. Cell Physiol Biochem. 2018;47:1244–58.
Turner MC, Cohen A, Jerrett M, Gapstur SM, Diver WR, Pope CA III, Krewski D, Beckerman BS, Samet JM. Interactions between cigarette smoking and fine particulate matter in the Risk of Lung Cancer Mortality in Cancer Prevention Study II. Am J Epidemiol. 2014;180:1145–9.
Turner MC, Cohen A, Burnett RT, Jerrett M, Diver WR, Gapstur SM, Krewski D, Samet JM, Pope CA III. Interactions between cigarette smoking and ambient PM2. 5 for cardiovascular mortality. Environ Res. 2017;154:304–10.
Alhamdow A, Lindh C, Hagberg J, Graff P, Westberg H, Krais AM, Albin M, Gustavsson P, Tinnerberg H, Broberg K. DNA methylation of the cancer-related genes F2RL3 and AHRR is associated with occupational exposure to polycyclic aromatic hydrocarbons. Carcinogenesis. 2018;39:869–78.
Fasanelli F, Baglietto L, Ponzi E, Guida F, Campanella G, Johansson M, Grankvist K, Johansson M, Assumma MB, Naccarati A. Hypomethylation of smoking-related genes is associated with future lung cancer in four prospective cohorts. Nat Commun. 2015;6:10192.
Ambatipudi S, Cuenin C, Hernandez-Vargas H, Ghantous A, Le Calvez-Kelm F, Kaaks R, Barrdahl M, Boeing H, Aleksandrova K, Trichopoulou A. Tobacco smoking-associated genome-wide DNA methylation changes in the EPIC study. Epigenomics. 2016;8:599–618.
Philibert R, Hollenbeck N, Andersen E, Osborn T, Gerrard M, Gibbons FX, Wang K. A quantitative epigenetic approach for the assessment of cigarette consumption. Front Psychol. 2015;6:656.
Reynolds LM, Wan M, Ding J, Taylor JR, Lohman K, Su D, Bennett BD, Porter DK, Gimple R, Pittman GS. DNA methylation of the aryl hydrocarbon receptor repressor associations with cigarette smoking and subclinical atherosclerosis CLINICAL PERSPECTIVE. Circulation. 2015;8:707–16.
Bojesen SE, Timpson N, Relton C, Smith GD, Nordestgaard BG. AHRR (cg05575921) hypomethylation marks smoking behaviour, morbidity and mortality. Thorax. 2017;72:646–53.
Teschendorff AE, Yang Z, Wong A, Pipinikas CP, Jiao Y, Jones A, Anjum S, Hardy R, Salvesen HB, Thirlwell C. Correlation of smoking-associated DNA methylation changes in buccal cells with DNA methylation changes in epithelial cancer. JAMA Oncol. 2015;1:476–85.
Joehanes R, Just AC, Marioni RE, Pilling LC, Reynolds LM, Mandaviya PR, Guan W, Xu T, Elks CE, Aslibekyan S. Epigenetic signatures of cigarette smoking. Circulation. 2016;9:436–47.
Baglietto L, Ponzi E, Haycock P, Hodge A, Bianca Assumma M, Jung CH, Chung J, Fasanelli F, Guida F, Campanella G. DNA methylation changes measured in pre-diagnostic peripheral blood samples are associated with smoking and lung cancer risk. Int J Cancer. 2017;140:50–61.
Shenker NS, Polidoro S, van Veldhoven K, Sacerdote C, Ricceri F, Birrell MA, Belvisi MG, Brown R, Vineis P, Flanagan JM. Epigenome-wide association study in the European Prospective Investigation into Cancer and Nutrition (EPIC-Turin) identifies novel genetic loci associated with smoking. Hum Mol Genet. 2012;22:843–51.
Monick MM, Beach SR, Plume J, Sears R, Gerrard M, Brody GH, Philibert RA. Coordinated changes in AHRR methylation in lymphoblasts and pulmonary macrophages from smokers. Am J Med Genet B Neuropsychiatr Genet. 2012;159:141–51.
Gao X, Jia M, Zhang Y, Breitling LP, Brenner H. DNA methylation changes of whole blood cells in response to active smoking exposure in adults: a systematic review of DNA methylation studies. Clin Epigenetics. 2015;7:113.
Zhang Y, Elgizouli M, Schöttker B, Holleczek B, Nieters A, Brenner H. Smoking-associated DNA methylation markers predict lung cancer incidence. Clin Epigenetics. 2016;8:127.
Lee D-H, Hwang S-H, Lim MK, Oh J-K, Song DY, Yun EH, Park EY. Performance of urine cotinine and hypomethylation of AHRR and F2RL3 as biomarkers for smoking exposure in a population-based cohort. PLoS One. 2017;12.
Bossé Y, Postma DS, Sin DD, Lamontagne M, Couture C, Gaudreault N, Joubert P, Wong V, Elliott M, van den Berge M. Molecular signature of smoking in human lung tissues. Cancer Res. 2012;72:3753–63.
Zeilinger S, Kühnel B, Klopp N, Baurecht H, Kleinschmidt A, Gieger C, Weidinger S, Lattka E, Adamski J, Peters A. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS One. 2013;8.
Tantoh DM, Lee K-J, Nfor ON, Liaw Y-C, Lin C, Chu H-W, Chen P-H, Hsu S-Y, Liu W-H, Ho C-C. Methylation at cg05575921 of a smoking-related gene (AHRR) in non-smoking Taiwanese adults residing in areas with different PM 2.5 concentrations. Clin Epigenetics. 2019;11:69.
van Voorhis M, Knopp S, Julliard W, Fechner JH, Zhang X, Schauer JJ, Mezrich JD. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS One. 2013;8:e82545.
Haarmann-Stemmann T, Abel J. The arylhydrocarbon receptor repressor (AhRR): structure, expression, and function. Biol Chem. 2006;387:1195–9.
Zhang Y, Schöttker B, Florath I, Stock C, Butterbach K, Holleczek B, Mons U, Brenner H. Smoking-associated DNA methylation biomarkers and their predictive value for all-cause and cardiovascular mortality. Environ Health Perspect. 2015;124:67–74.
Bauer M. Cell-type-specific disturbance of DNA methylation pattern: a chance to get more benefit from and to minimize cohorts for epigenome-wide association studies. Int J Epidemiol. 2018.
Bauer M, Fink B, Thürmann L, Eszlinger M, Herberth G, Lehmann I. Tobacco smoking differently influences cell types of the innate and adaptive immune system—indications from CpG site methylation. Clin Epigenetics. 2016;8:83.
Philibert R, Dogan M, Beach SR, Mills JA, Long JD. AHRR methylation predicts smoking status and smoking intensity in both saliva and blood DNA. Am J Med Genet B Neuropsychiatr Genet. 2019.
Lee KW, Pausova Z. Cigarette smoking and DNA methylation. Front Genet. 2013;4:132.
Sharman J, Cockcroft J, Coombes J. Cardiovascular implications of exposure to traffic air pollution during exercise. Qjm. 2004;97:637–43.
Maresh JG, Xu H, Jiang N, Gairola CG, Shohet RV. Tobacco smoke dysregulates endothelial vasoregulatory transcripts in vivo. Physiol Genomics. 2005;21:308–13.
Möller WB, Martin Kohlhäufl, Karl Häussinger, Willi Stahlhofen, Joachim Heyder, Winfried. Human alveolar long-term clearance of ferromagnetic iron oxide microparticles in healthy and diseased subjects. Exp Lung Res 2001; 27:547-568.
Wong C-M, Ou C-Q, Lee N-W, Chan K-P, Thach T-Q, Chau Y-K, Ho S-Y, Hedley AJ, Lam T-H. Short-term effects of particulate air pollution on male smokers and never-smokers. Epidemiology. 2007;18:593–8.
Martin EM, Fry RC. Environmental influences on the epigenome: exposure-associated DNA methylation in human populations. Annu Rev Public Health. 2018;39:309–33.
Wright RO, Schwartz J, Wright RJ, Bollati V, Tarantini L, Park SK, Hu H, Sparrow D, Vokonas P, Baccarelli A. Biomarkers of lead exposure and DNA methylation within retrotransposons. Environ Health Perspect. 2010;118:790–5.
Kodal JB, Kobylecki CJ, Vedel-Krogh S, Nordestgaard BG, Bojesen SE. AHRR hypomethylation, lung function, lung function decline and respiratory symptoms. Eur Respir J. 2018;51:1701512.
Breitling LP, Yang R, Korn B, Burwinkel B, Brenner H. Tobacco-smoking-related differential DNA methylation: 27 K discovery and replication. Am J Hum Genet. 2011;88:450–7.
Jhun MA, Smith JA, Ware EB, Kardia SL, Mosley TH Jr, Turner ST, Peyser PA, Park SK. Modeling the causal role of DNA methylation in the association between cigarette smoking and inflammation in African Americans: a 2-step epigenetic Mendelian randomization study. Am J Epidemiol. 2017;186:1149–58.
Su D, Wang X, Campbell MR, Porter DK, Pittman GS, Bennett BD, Wan M, Englert NA, Crowl CL, Gimple RN. Distinct epigenetic effects of tobacco smoking in whole blood and among leukocyte subtypes. PLoS One. 2016;11.
Cao Q, Rui G, Liang Y. Study on PM2. 5 pollution and the mortality due to lung cancer in China based on geographic weighted regression model. BMC Public Health. 2018;18:925.
Biobank T. Purpose Of Taiwan Biobank; 2015.
Fan C-T, Lin J-C, Lee C-H. Taiwan Biobank: a project aiming to aid Taiwan’s transition into a biomedical island. Pharmacogenomics. 2008;9:235–46.
Lin C-H, Wu Y-L, Lai C-H, Watson JG, Chow JC. Air quality measurements from the southern particulate matter supersite in Taiwan. Aerosol Air Qual Res. 2008;8:233–64.
Environmental Protection Administration T. Taiwan Air Quality Monitoring Network. Taiwan; 2019.
Huang H-C, Tantoh DM, Hsu S-Y, N for ON, Frank C-FL, Lung C-C, Ho C-C, Chen C-Y, Liaw Y-P. Association between coarse particulate matter (PM10-2.5) and nasopharyngeal carcinoma among Taiwanese men. J Investig Med. 2019:jim-2019-001119.
Hung L-J, Tsai S-S, Chen P-S, Yang Y-H, Liou S-H, Wu T-N, Yang C-Y. Traffic air pollution and risk of death from breast cancer in Taiwan: fine particulate matter (PM2. 5) as a proxy marker. Aerosol Air Qual Res. 2012;12:275–82.
Solomon O, MacIsaac J, Quach H, Tindula G, Kobor MS, Huen K, Meaney MJ, Eskenazi B, Barcellos LF, Holland N. Comparison of DNA methylation measured by Illumina 450 K and EPIC BeadChips in blood of newborns and 14-year-old children. Epigenetics. 2018;13:655–64.
Bojovic B, Blancher C. Epigenetic analysis on Illumina EPIC arrays. Epigenetics. 2017;28.
Rahmani E, Zaitlen N, Baran Y, Eng C, Hu D, Galanter J, Oh S, Burchard EG, Eskin E, Zou J. Sparse PCA corrects for cell type heterogeneity in epigenome-wide association studies. Nat Methods. 2016;13:443.
Funding
This work was partially funded by the Ministry of Science and Technology (MOST) (MOST 105-2627-M-040-002, 106-2627-M-040-002, 107-2627-M-040-002, 108-2621-M-040-00, 106-EPA-F-016-001, and 107-EPA-F-017-002).
Author information
Authors and Affiliations
Contributions
DMT, MCW, and YPL conceived and designed the study. DMT, MCW, CCC, PHC, YST, ONN, WYL, and YPL performed the literature search. YPL, PHC, and WYL acquired data and performed the data analysis. DMT, MCW, CCC, PHC, YST, ONN, WYL, and YPL interpreted the data. DMT and MCW wrote the manuscript. DMT, MCW, ONN, and YPL made critical revisions of the manuscript for important intellectual contents. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Chung Shan Medical University Institutional Review Board (CS2-17070).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
AHRR and F2LR3 CpG sites and their association with smoking and PM2.5. Table S2. Multiple linear regression analysis showing the association of smoking and PM2.5 with F2RL3 cg03636183 methylation in the study participants. Table S3. Multiple linear regression analysis showing the association of smoking and PM2.5 with F2RL3 cg03636183 methylation in the study participants stratified by sex. Table S4. Multiple linear regression analysis showing the association of smoking and living in PM2.5 areas with F2RL3 cg03636183 methylation in the study participants. Table S5. Multiple linear regression analysis showing the association of smoking and living in PM2.5 areas and F2RL3 cg03636183 methylation in the study participants stratified by sex.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tantoh, D.M., Wu, MC., Chuang, CC. et al. AHRR cg05575921 methylation in relation to smoking and PM2.5 exposure among Taiwanese men and women. Clin Epigenet 12, 117 (2020). https://doi.org/10.1186/s13148-020-00908-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13148-020-00908-3