Abstract
Background
Vitamin D deficiency and insufficiency have been established to be strongly associated with increased overall mortality and deaths from specific aging-related diseases. Recently, an epigenetic “mortality risk score” (MS) based on whole blood DNA methylation at the 10 most prominent mortality-related cytosine-phosphate-guanine (CpG) sites has also been found to be highly related to all-cause mortality. This study aimed to explore whether vitamin D status, defined by serum 25-hydroxyvitamin D [25(OH)D] concentrations, is associated with the MS and to what extent both indicators are individually and jointly capable of predicting all-cause mortality in a general population sample of older adults.
Results
The MS was derived from the blood DNA methylation profiles measured by Illumina Human Methylation 450K Beadchip, and serum 25(OH)D concentration was measured among 1467 participants aged 50–75 of the German ESTHER cohort study. There was no association between vitamin D status and the MS at baseline, but both metrics were prominently and independently associated with all-cause mortality during a median follow-up of 15.2 years. The combination of both indicators showed the potential to be a particularly strong prognostic index for all-cause mortality. Participants with vitamin D deficiency (< 30 nmol/L) and high MS (> 5 CpG sites with aberrant methylation) had almost sixfold mortality (hazard ratio 5.79, 95% CI 3.06–10.94) compared with participants with sufficient vitamin D (≥ 50 nmol/L) and a low MS (0–1 CpG site with aberrant methylation).
Conclusions
This study suggests that vitamin D and the MS are strong independent predictors of all-cause mortality in older adults.
Similar content being viewed by others
Background
Vitamin D is a critical nutrient that is, apart from some limited supply from diet and supplement use, mainly obtained from the biosynthesis within the human body in response to the exposure of solar ultraviolet B radiation [1]. Vitamin D status is commonly measured via assessing 25-hydroxyvitamin D [25(OH)D] concentrations in serum [1]. Vitamin D deficiency and insufficiency have been shown to be strongly associated with increased overall mortality, as well as deaths from specific aging-related diseases, such as cardiovascular disease (CVD) and various forms of cancer [2,3,4]. We previously performed a meta-analysis to summarize the results of eight prospective cohort studies from European countries and the USA to investigate the prognostic association of vitamin D status and mortality [3]. Comparing bottom vs. top quintiles of 25(OH)D concentrations resulted in a risk ratio of 1.57 (95% CI 1.36–1.81) for all-cause mortality.
Recently, DNA methylation, one of the most studied and stable epigenetic modifications, has been shown to be associated with aging and aging-related health outcomes [5, 6] and recognized as the indicator for all-cause and disease-specific mortality [7]. In a recent epigenome-wide association study (EWAS) with approximately 1900 older adults followed up for 14 years and an external validation with 1727 participants, we identified 58 cytosine-phosphate-guanine (CpG) sites within 19 chromosomes that were associated with all-cause mortality [8]. We constructed a “mortality risk score” (MS) based on the 10 most robustly mortality-related loci, which was found to be a robust and informative predictor of all-cause, CVD, and cancer mortality. It is unclear, however, to what extent its association is independent of other well-established indicators of mortality risks. This study aimed to explore whether vitamin D status, defined by serum 25(OH)D concentrations, is associated with the MS and to what extent both indicators are individually and jointly capable of predicting all-cause mortality in a general population sample of older adults.
Methods
Study design and population
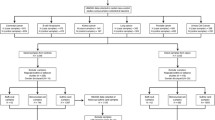
Study subjects were chosen from the ESTHER study, an ongoing statewide population-based cohort study conducted in Saarland, a state located in southwestern Germany. Details of the study design have been reported previously [8, 9]. As shown in Fig. 1, 9949 older adults (aged 50–75 years) were enrolled by their general practitioners during a routine health checkup between July 2000 and December 2002 and followed up thereafter. The cross-sectional analysis of this study is based on the data and biospecimen collected at baseline from 1467 participants (close to 100% Caucasian) who were randomly selected for the measurements of 25(OH)D concentrations and DNA methylation profiles among participants recruited consecutively at the start of the ESTHER study between July 2000 and March 2001 [10]. Participants were then regularly followed up with respect to the incidence of major chronic diseases and mortality. The ESTHER study was approved by the ethics committees of the University of Heidelberg and the state medical board of Saarland, Germany. Written informed consent was obtained from all participants.
Vitamin D measurements
Blood samples were taken during the health checkup and stored at − 80 °C until further processing. As previously described [11], the automated Diasorin–Liaison analyzer (Diasorin, Inc.) was used to measure total serum 25(OH)D concentrations in women from baseline serum samples in the central laboratory of the University Clinic of Heidelberg in 2006 within the framework of a project on women’s health. Additional funding was obtained in 2009 to measure total serum 25(OH)D concentrations in men as well. As the Diasorin–Liaison method used for women was no longer available at that time, the automated IDS-iSYS analyzer (Immunodiagnostic Systems, GmbH) was used instead. Both assays with their within- and between-assay coefficients and lower limits of detection have been comprehensively described elsewhere [12, 13]. Both immunoassays were standardized retrospectively to measurements by liquid chromatography-tandem mass spectrometry (LC-MS/MS), the gold standard method [14], in a random subsample of 97 males and 97 females as previously described [12, 13]. Vitamin D status defined by total serum 25(OH)D concentrations were classified into three categories with the following criteria: deficiency, < 30 nmol/L; insufficiency, 30 to < 50 nmol/L; and sufficiency, ≥ 50 nmol/L.
DNA methylation data
DNA from whole blood samples was collected using a salting out procedure [15]. DNA methylation profiles were determined by the Illumina Human Methylation 450K Beadchip (Illumina, San Diego, CA, USA). As previously described [10], samples were analyzed following the manufacturer’s instruction at the Genomics and Proteomics Core Facility of the German Cancer Research Center, Heidelberg, Germany. Illumina’s GenomeStudio® (version 2011.1; Illumina Inc.) was employed to extract DNA methylation signals from the scanned arrays (Module version 1.9.0; Illumina Inc.). The methylation level of a specific CpG site was quantified as a β value ranging from 0 (no methylation) to 1 (full methylation). According to the manufacturer’s protocol, no background correction was done and data were normalized to internal controls provided by the manufacturer. All controls were checked for inconsistencies in each measured plate. Probes with a detection p value > 0.05 were excluded from analysis. Illumina normalization and preprocessing methods implemented in Illumina’s GenomeStudio® were utilized.
As described by Zhang et al., 10 CpG sites (cg01612140, cg05575921, cg06126421, cg08362785, cg10321156, cg14975410, cg19572487, cg23665802, cg24704287, and cg25983901) were selected from the whole epigenome data to build the MS [8]. Values within the fourth quartile of cg08362785 and first quartile of the other nine loci were defined to reflect aberrant methylation for each CpG site, and the ordinal MS was determined as the cumulative number of aberrantly methylated CpG sites (0–10). The participants were further classified into three risk levels: low: MS = 0–1, moderate: MS = 2–5, and high: MS > 5.
Covariate and outcome assessment
Information on socio-demographic characteristics, lifestyle factors, dietary habits, and health status at baseline was obtained by standardized self-administered questionnaires [16]. Participants were asked about their past and present smoking behaviors and were then categorized into current, former, and never smokers. Information on BMI and systolic blood pressure was extracted from a standardized form filled by the general practitioners during the health checkups. Serum total cholesterol and C-reactive protein (CRP) were measured with Backman Synchon LX and turbudimetry, respectively [11]. Prevalent CVD at baseline was defined by either physician-reported coronary heart disease or a self-reported history of a major cardiovascular event, such as myocardial infarction, stroke, pulmonary embolism, or revascularization of coronary arteries. Prevalent diabetes was defined by physician diagnosis or the use of glucose-lowering drugs. Prevalent cancer [ICD-10 C00-C99 except non-melanoma skin cancer (C44)] was determined by self-report or record linkage with data from the Saarland Cancer Registry (in German) [17].
Deaths of follow-up until the end of 2015 were retrieved by record linkage with population registries in Saarland. Participants who moved out of Saarland were considered as censored at the date last known to be alive. Information on the causes of death was obtained from death certificates provided by local public health offices and was coded with ICD-10 codes.
Statistical analysis
First, major socio-demographic characteristics, lifestyle factors, and the MS at baseline of 1467 participants, overall and stratified by vitamin D status, were summarized by descriptive statistics, and differences among subsets were tested for statistical significance by Kruskal-Wallis test (continuous variables) and chi-square test (categorical variables).
We then examined the associations of vitamin D status and MS (risk levels) with all-cause mortality using three multivariate Cox regression models which increasingly adjusted for potential covariates, including age (years), sex (male/ female), smoking status (current/former/never smoking), alcohol consumption (g/day), BMI class [kg/m2; underweight (< 18.5, < 1% of the study population) or normal weight (18.5 to < 25), overweight (25 to < 30), obese (≥ 30)], physical activity [inactive (< 1 h of physical activity/week), medium or high (≥ 2 h of light or ≥ 2 h of vigorous physical activity/week), low (other)], the prevalence of CVD (yes/no), diabetes (yes/no) and cancer (yes/no), systolic blood pressure (mmHg), CRP (mg/L), total cholesterol (mg/dL), intake of vitamin supplements (yes/no), fish consumption (≤ 1 time/week/> 1 time/week), and the season of blood draw (spring: March–May; summer: June–August; autumn: September–November; winter: December–February). Leukocyte distribution estimated by Houseman’s algorithm was additionally controlled for in the models in which the MS was involved [18]. The dose-response curves of continuous 25(OH)D concentrations and ordinal MS with all-cause mortality were evaluated by restricted cubic spline regression using the SAS macro from Desquilbet et al. [19]. All models for dose-response analyses were adjusted for the covariates described above. The 25th, 50th, and 75th percentiles of the MS and the 30, 50, and 70 nmol/L of serum 25(OH)D concentrations were selected as knots for each marker, respectively. In addition to models including either vitamin D status or MS (risk levels) as the predictor, we further evaluated their independent associations with all-cause mortality in a model containing both predictors.
Finally, we assessed the joint associations of both indicators with all-cause mortality. Survival was first compared by the health status defined by the combination of both indicators using direct-adjusted survival curves (adjusted for age and sex), followed by Cox regression analyses with multivariable adjustment. In addition, adjusted survival curves were constructed and Cox models were run to assess the associations of MS with mortality within subgroups of participants defined by vitamin D status and of vitamin D status with mortality within subgroups of participants defined by the MS (risk levels). To avoid overadjustment, only covariates related to either of the two predictors (p value < 0.2 in bivariable analyses) were included in the models assessing joint associations.
All analyses were performed by SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). For all statistical analyses, a p value less than 0.05 in two-sided tests was considered as statistically significant.
Results
Participant characteristics
Characteristics of all 1467 participants and the subsets based on vitamin D status are shown in Table 1. For the total population, the average age was about 62 years. More than half of the participants were smokers (current or former smokers). The majority of participants were overweight or obese, reported no or low amounts of alcohol consumption, and no or only low physical activity. More than half of the participants had a MS > 1, and about 46% had a MS of 2–5. The mean standardized serum 25(OH)D concentration was 52.1 nmol/L, with 15.2 and 43.4% of participants meeting the criteria for vitamin D deficiency (< 30 nmol/L) and insufficiency (30 to < 50 nmol/L), respectively. During a median follow-up time of 15.2 years, 365 (24.9%), 128 (8.9%), and 134 (9.3%) participants died from any cause, CVD, or cancer, respectively.
Participants with sufficient vitamin D (≥ 50 nmol/L) included larger proportions of males, former smokers, consumers of low amounts of alcohol, were less often obese and more often reported medium or high physical activity than those with vitamin D deficiency and insufficiency. By contrast, no differences in the prevalence of major diseases and in the distribution of the MS were observed among participants with vitamin D deficiency, insufficiency, or sufficiency.
Individual associations of vitamin D status and the mortality risk score with all-cause mortality
Table 2 shows the associations of vitamin D status and MS (risk levels) with all-cause mortality among 1467 participant during a median follow-up time of 15.2 years. Significant associations were observed between each indicator and mortality, which persisted after controlling for multiple covariates. Vitamin D insufficiency and deficiency were associated with about 1.5- [hazard ratio (HR) 1.46; 95% CI 1.11–1.93] and 2.0 (HR 1.99; 95% CI 1.38–2.87)-fold mortality compared to the vitamin D sufficiency subgroup after adjustment of multiple covariates (model 3). The moderate and high MS risk levels were associated with about 1.9- (HR 1.87; 95% CI 1.37–2.54) and 3.4 (HR 3.42; 95% CI 2.32–5.04)-fold mortality compared to the low MS risk level. Additional mutual adjustment for both indicators did not alter the patterns in any relevant manner with essentially unchanged HRs.
Figure 2 further depicts the dose-response relationships of 25(OH)D concentrations and ordinal MS with all-cause mortality after controlling for all potential covariates. Mortality strongly increased for 25(OH)D concentrations < 40 nmol/L and steadily increased with increasing MS.
Joint associations of vitamin D status and mortality risk score with all-cause mortality
Figure 3 presents the direct-adjusted survival curves for the joint association of vitamin D status and the MS (risk levels) with all-cause mortality during a median follow-up time of 15.2 years. People in the highest risk group with vitamin D deficiency and high MS risk level had a substantially higher overall death rate than all other groups. After adjustment for the selected covariates based on the results of bivariate associations (Additional file 1: Table S1), a clearly increasing mortality risk (i.e., HR) was observed with both vitamin D deficiency and a high MS (Table 3). In particular, people in the highest risk group of both factors had about 5.8-fold mortality compared to the people in the reference group that had sufficient vitamin D and a low MS (HR 5.79; 95% CI 3.06–10.94).
Age- and sex-adjusted survival curves for the joint associations of vitamin D status and mortality risk score (risk levels) with all-cause mortality (log-rank p value < 0.0001). Legend: Each curve represents the subgroup defined by the combination of vitamin D status and mortality risk score (risk levels)
We further stratified the total population by each indicator and assessed the association of the other indicator with all-cause mortality. As demonstrated in Tables 4 and 5 and Additional file 2: Figure S1, the identified patterns in subgroup-specific analyses are in line with the findings for the total study population. However, the association of a high MS with all-cause mortality was particularly strong among participants with vitamin D deficiency (HR 6.90; 95% CI 2.83–16.83). Additional sex-specific analysis yielded similar patterns for both sexes (data not shown).
Discussion
In this study with 1467 older adults recruited in a population-based cohort with both measurements of serum 25(OH)D concentrations and DNA methylation profiles at baseline, vitamin D status and an epigenetic-based MS strongly and independently predicted mortality from any causes. In particular, the combination of both indicators demonstrated the potential to be a robust prognostic marker for all-cause mortality with an approximately sixfold mortality among those with vitamin D deficiency and a high MS compared to participants with sufficient vitamin D and a low MS.
Previous studies suggested that there might be a reciprocal relationship between vitamin D and epigenetic changes: epigenetic alterations regulate the expression of vitamin D receptor genes and vitamin D could influence epigenetic events [20]. Previous studies have also suggested epigenetic effects of vitamin D on histone modification, another form of epigenetic regulation [20, 21]. Although a potential link between severe vitamin D deficiency and DNA methylation has been reported in African American adolescents [22], no such link was observed in a previous analysis of the ESTHER study [23]. In addition, Chavez et al. using the Illumina 450K array did not find significant alterations in DNA methylation profiles when they exposed human blood cells to vitamin D for up to 120 h [24]. In our study, vitamin D deficiency was not related to the methylation of the sites included in the MS. Among the 10 CpG sites of the MS, four loci are smoking-related, including cg05575921 (AHRR), cg06126421 (6p21.33), cg19572487 (RARA), and cg01612140 (6q14.1) [25]. Even though vitamin D sufficiency may have a protective effect against the damaging effects of smoking on lung function [26], we did not observe any association of the MS and vitamin D status. Along the same lines, although loci cg08362785 and cg23665802 are mapped to genes MKL1 and MIR19A that are associated with the risks of lung and breast cancer [27, 28], vitamin D, which was found to be related to the incidence and mortality of both cancers [29], did not show any relation with either locus nor the MS.
Associations between both vitamin D deficiency and the MS, and all-cause mortality have been identified and validated by previous studies among older adults [3, 7, 8, 30]. To our best knowledge, this study is the first investigation which further demonstrated that both markers are not only capable of independently predicting the death from any cause over 15 years of follow-up, but also can be integrated together to be an extraordinarily strong indicator for mortality.
In particular, the combination of vitamin D and the MS appears to be a better predictor of all-cause mortality than any previously suggested biomarkers of mortality risk [31]. The strength of the association is the more remarkable, as most of the deaths in our study with long-term follow-up occurred many years after baseline measurements of vitamin D and MS, whereas the strength of prediction for most biomarkers is typically attenuated with increasing length of follow-up [32, 33]. Even stronger associations of both biomarkers and their combinations with mortality were seen when the follow-up was restricted to 5 or 10 years, even though confidence intervals became wider due to the lower numbers of deaths (data not shown).
One of the major strengths of this study is the availability of a broad range of covariates adjusted for in addition to epigenome-wide methylation data in a population-based cohort that was comprehensively followed up with high completeness over 15 years. We also acknowledge several limitations in the interpretation of results. First, the overall number of deaths for the survival analyses was limited, which caused rather wide confidence intervals for some of the HR estimates. Furthermore, shifts of leukocyte distribution might affect the associations of DNA methylation in whole blood samples [34]. Hence, we adjusted for leukocyte distribution by the Houseman algorithm to restrict potential confounding from differential blood counts to the greatest possible extent [18]. In addition, 25(OH)D concentrations were originally measured by two different methods. Nevertheless, standardization by the gold standard LC-MS/MS method, which yielded results that were highly correlated with original measurements [13], should ensure comparability and validity of the vitamin D status included in our analysis. Finally, due to the heterogeneity of populations with respect to food fortification, use of dietary supplements, UV radiation levels, and susceptibility of the epigenome in response to external exposure, further studies are needed to evaluate to what extent our findings can be generalized to other populations: in particular younger individuals and non-Caucasians.
Conclusions
In summary, our results contribute to a rapidly growing body of literature investigating the prediction of age-related morbidity and long-term mortality by various markers identified from environmental, genetic, and epigenetic research. We showed that the application of both vitamin D status and the MS based on DNA methylation signatures yielded a robust, independent predictor for all-cause mortality, suggesting that vitamin D status and DNA methylation signatures in combination may be most useful in risk stratification as potential intermediate biomarkers. Further studies are warranted to elucidate the underlying pathophysiological mechanisms and potential clinical applications of the combination of both indicators in routine medical practice and intervention research aimed at reducing mortality.
Abbreviations
- 25(OH)D:
-
25-Hydroxyvitamin D
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CpG:
-
Cytosine-phosphate-guanine
- HR:
-
Hazard ratio
- MS:
-
Mortality risk score
References
Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–8. https://doi.org/10.1016/j.annepidem.2007.12.001.
Chowdhury R, Kunutsor S, Vitezova A, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903. https://doi.org/10.1136/bmj.g1903.
Schöttker B, Jorde R, Peasey A, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656. https://doi.org/10.1136/bmj.g3656.
Tomson J, Emberson J, Hill M, et al. Vitamin D and risk of death from vascular and non-vascular causes in the Whitehall study and meta-analyses of 12,000 deaths. Eur Heart J. 2013;34(18):1365–74. https://doi.org/10.1093/eurheartj/ehs426.
Mikeska T, Craig JM. DNA methylation biomarkers: cancer and beyond. Genes. 2014;5(3):821–64. https://doi.org/10.3390/genes5030821.
Hedman AK, Mendelson MM, Marioni RE, et al. Epigenetic patterns in blood associated with lipid traits predict incident coronary heart disease events and are enriched for results from genome-wide association studies. Circ Cardiovasc Genet. 2017;10(1) https://doi.org/10.1161/CIRCGENETICS.116.001487.
Zhang Y, Schöttker B, Florath I, et al. Smoking-associated DNA methylation biomarkers and their predictive value for all-cause and cardiovascular mortality. Environ Health Perspect. 2016;124(1):67–74. https://doi.org/10.1289/ehp.1409020.
Zhang Y, Wilson R, Heiss J, et al. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat Commun. 2017;8:14617. https://doi.org/10.1038/ncomms14617.
Schöttker B, Haug U, Schomburg L, et al. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr. 2013;97(4):782–93. https://doi.org/10.3945/ajcn.112.047712.
Gao X, Gao X, Zhang Y, Breitling LP, Schöttker B, Brenner H. Associations of self-reported smoking, cotinine levels and epigenetic smoking indicators with oxidative stress among older adults: a population-based study. Eur J Epidemiol. 2017;32(5):443–56. https://doi.org/10.1007/s10654-017-0248-9.
Brenner H, Jansen L, Saum KU, Holleczek B, Schöttker B. Vitamin D supplementation trials aimed at reducing mortality have much higher power when focusing on people with low serum 25-hydroxyvitamin D concentrations. J Nutr. 2017;147(7):1325–33. https://doi.org/10.3945/jn.117.250191.
Perna L, Haug U, Schottker B, et al. Public health implications of standardized 25-hydroxyvitamin D levels: a decrease in the prevalence of vitamin D deficiency among older women in Germany. Prev Med. 2012;55(3):228–32. https://doi.org/10.1016/j.ypmed.2012.06.010.
Schöttker B, Jansen EH, Haug U, Schomburg L, Kohrle J, Brenner H. Standardization of misleading immunoassay based 25-hydroxyvitamin D levels with liquid chromatography tandem-mass spectrometry in a large cohort study. PLoS One. 2012;7(11):e48774. https://doi.org/10.1371/journal.pone.0048774.
Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87(4):1087S–91S.
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215.
Gao X, Zhang Y, Saum KU, Schottker B, Breitling LP, Brenner H. Tobacco smoking and smoking-related DNA methylation are associated with the development of frailty among older adults. Epigenetics: official journal of the DNA Methylation Society 2017;12(2):149–156. doi:https://doi.org/10.1080/15592294.2016.1271855.
Caramaschi D, Sharp GC, Nohr EA, et al. Exploring a causal role of DNA methylation in the relationship between maternal vitamin B12 during pregnancy and child’s IQ at age 8, cognitive performance and educational attainment: a two-step Mendelian randomization study. Hum Mol Genet. 2017;26(15):3001–13. https://doi.org/10.1093/hmg/ddx164.
Houseman EA, Accomando WP, Koestler DC, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC bioinformatics. 2012;13:86. https://doi.org/10.1186/1471-2105-13-86.
Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–57. https://doi.org/10.1002/sim.3841.
Fetahu IS, Hobaus J, Kallay E. Vitamin D and the epigenome. Front Physiol. 2014;5:164. https://doi.org/10.3389/fphys.2014.00164.
Karlic H, Varga F. Impact of vitamin D metabolism on clinical epigenetics. Clin Epigenetics. 2011;2(1):55–61. https://doi.org/10.1007/s13148-011-0021-y.
Zhu H, Wang X, Shi H, et al. A genome-wide methylation study of severe vitamin D deficiency in African American adolescents. J Pediatr. 2013;162(5):1004–9 e1. https://doi.org/10.1016/j.jpeds.2012.10.059.
Florath I, Schottker B, Butterbach K, Bewerunge-Hudler M, Brenner H. Epigenome-wide search for association of serum 25-hydroxyvitamin D concentration with leukocyte DNA methylation in a large cohort of older men. Epigenomics. 2016;8(4):487–99. https://doi.org/10.2217/epi.16.4.
Chavez Valencia RA, Martino DJ, Saffery R, Ellis JA. In vitro exposure of human blood mononuclear cells to active vitamin D does not induce substantial change to DNA methylation on a genome-scale. J Steroid Biochem Mol Biol. 2014;141:144–9. https://doi.org/10.1016/j.jsbmb.2014.01.018.
Gao X, Jia M, Zhang Y, Breitling LP, Brenner H. DNA methylation changes of whole blood cells in response to active smoking exposure in adults: a systematic review of DNA methylation studies. Clin Epigenetics 2015;7(1):113. doi:https://doi.org/10.1186/s13148-015-0148-3.
Lange NE, Sparrow D, Vokonas P, Litonjua AA. Vitamin D deficiency, smoking, and lung function in the Normative Aging Study. Am J Respir Crit Care Med. 2012;186(7):616–21. https://doi.org/10.1164/rccm.201110-1868OC.
Michailidou K, Hall P, Gonzalez-Neira A, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45(4):353–61, 61e1–2. https://doi.org/10.1038/ng.2563.
Risch A, Plass C. Lung cancer epigenetics and genetics. International journal of cancer. Journal international du cancer 2008;123(1):1–7. doi:https://doi.org/10.1002/ijc.23605.
Mondul AM, Weinstein SJ, Layne TM, Albanes D. Vitamin D and cancer risk and mortality: state of the science, gaps, and challenges. Epidemiol Rev. 2017;39(1):28–48. https://doi.org/10.1093/epirev/mxx005.
Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95(1):91–100. https://doi.org/10.3945/ajcn.111.014779.
Peto MV, De la Guardia C, Winslow K, Ho A, Fortney K, Morgen E. MortalityPredictors.org: a manually-curated database of published biomarkers of human all-cause mortality. Aging. 2017;9(8):1916–25. https://doi.org/10.18632/aging.101280.
Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31(6):737–46. https://doi.org/10.1093/eurheartj/ehp487.
Schutte AE, Conti E, Mels CM, et al. Attenuated IGF-1 predicts all-cause and cardiovascular mortality in a Black population: a five-year prospective study. Eur J Prev Cardiol. 2016;23(16):1690–9. https://doi.org/10.1177/2047487316661436.
Schwartz J, Weiss ST. Cigarette smoking and peripheral blood leukocyte differentials. Ann Epidemiol. 1994;4(3):236–42. https://doi.org/10.1016/1047-2797(94)90102-3.
Acknowledgements
The authors gratefully acknowledge contributions of DKFZ Genomics and Proteomics Core Facility in the processing of DNA samples and performing the laboratory work, Dr. Jonathan Heiss for providing the estimation of leukocyte distribution, and Ms. Chen Chen for the language assistance.
Funding
The ESTHER study was supported by the Baden-Württemberg State Ministry of Science, Research and Arts (Stuttgart, Germany), the Federal Ministry of Education and Research (Berlin, Germany), and the Federal Ministry of Family Affairs, Senior Citizens, Women and Youth (Berlin, Germany). Ben Schöttker is supported by the German Research Foundation (DFG, grant no.: SCHO 1545/3-1).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author (HB) on reasonable request.
Author information
Authors and Affiliations
Contributions
XG contributed to the drafting and other authors contributed to the critical revision of the manuscript, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ESTHER study was approved by the ethics committees of the University of Heidelberg and the state medical board of Saarland, Germany. Written informed consent was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Bivariate associations of vitamin D status and mortality risk score (risk levels) with potential confounders. Legend: Each curve represents the subgroup defined by the combination of vitamin D status and mortality risk score (risk levels). (DOCX 16 kb)
Additional file 2:
Figure S1. Age and sex-adjusted survival curves for joint associations of mortality risk score/vitamin D status with all-cause mortality within subgroups defined by vitamin D status/mortality risk score (all log-rank p values < 0.05). (PDF 428 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gao, X., Zhang, Y., Schöttker, B. et al. Vitamin D status and epigenetic-based mortality risk score: strong independent and joint prediction of all-cause mortality in a population-based cohort study. Clin Epigenet 10, 84 (2018). https://doi.org/10.1186/s13148-018-0515-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13148-018-0515-y