Abstract
Human T-lymphotropic virus type 1 (HTLV-1) is a RNA virus belonging to Retroviridae family and is associated with the development of various diseases, including adult T-cell leukemia/lymphoma (ATLL) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Aside from HAM/TSP, HTLV-1 has been implicated in the development of several disorders that mimic auto-inflammation. T-cell migration is important topic in the context of HTLV-1 associated diseases progression. The primary objective of this case–control study was to assess the relationship between increased mRNA expression in virus migration following HTLV-1 infection. PBMCs from 20 asymptomatic patients and 20 healthy subjects were analyzed using real-time PCR to measure mRNA expression of LFA1, MLCK, RAC1, RAPL, ROCK1, VAV1 and CXCR4. Also, mRNA expression of Tax and HBZ were evaluated. Mean expression of Tax and HBZ in ACs (asymptomatic carriers) was 0.7218 and 0.6517 respectively. The results revealed a noteworthy upregulation of these genes involved in T-cell migration among ACs patients in comparison to healthy individuals. Considering the pivotal role of gene expression alterations associated with the progression into two major diseases (ATLL or HAM/TSP), analyzing the expression of these genes in the ACs group can offer probable potential diagnostic markers and aid in monitoring the condition of ACs.
Similar content being viewed by others
Introduction
Human T-cell lymphotropic virus-1 (HTLV-1) is a retrovirus that can cause various diseases, including HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP), adult T-cell leukemia/lymphoma (ATLL). Tax and HBZ are integral proteins in HTLV-1 infection and the progression of associated cancers. Tax plays a crucial role in viral replication and evading the immune system by manipulating various cellular signaling pathways. It facilitates the proliferation of T-cells, induces genomic instability, and hampers immune responses, ultimately leading to the transformation of infected cells. Conversely, HBZ, known as the basic leucine zipper protein, contributes to viral persistence and immune evasion. It supports cell survival, modulates the expression of host genes, and regulating various cellular processes [1, 2].
Cell migration can play a role in the spread of infectious agents, including HTLV-1. Infected T cells can also migrate to other tissues, such as the central nervous system, where they can cause inflammation and damage [3, 4]. In HTLV-1 infected individuals, the virus can induce changes in the expression of genes involved in cell migration and adhesion [5,6,7]. Cell motility plays a crucial role in tumor invasion and metastasis, and involves the reorganization of the cellular skeleton. Cellular actin is a primary mechanism involved in cell motility [8]. Infected T cells can cross the BBB by a process known as transmigration [9, 10]. Once inside the Central Nervous System (CNS), infected T cells can cause inflammation and damage to the neurons and other cells of the CNS. This can lead to the development of neurological complications, such as HAM/TSP.
Understanding the role of cell migration in HTLV-1 infection is important for investigation about new therapies and strategies to prevent the spread of the virus. Following this research, the migration pathway’s different genes and their interactions were identified. As a consequence of the analysis, the RT-qPCR method was employed to evaluate seven crucial genes, LFA1, MLCK, RAC1, RAPL, ROCK1, VAV1 and CXCR4 following HTLV-1 infection and their interaction in ACs carriers compared to healthy individuals.
Material and methods
Study population
A total of 40 participants were enrolled, with an equal ratio of 20 asymptomatic carriers (ACs) patients and 20 healthy subjects. Among the ACs patients, there were 16 males and 4 females, while the healthy subjects included 16 males and 4 females as well. In ACs subjects, mean age for male was 48.56 ± 5.81 with a minimum of 38 and maximum of 58. In healthy subjects, mean age for female was 48 ± 5.22 with a minimum of 43 and maximum of 53.
Sample collection
6 mL of blood samples were isolated in EDTA anticoagulant sterile tubes. The selection criteria for the participants in the research encompassed individuals with asymptomatic patients with PCR confirmation and healthy individuals who had not taken any medications, did not have a history of autoimmune diseases or any ongoing infectious illnesses such as HIV, HCV, and HBV. The study was approved by ethics committee of Alborz University of Medical Sciences, Alborz, Iran (Ethics Code: IR.ABZUMS.REC.1400.029), and each participant provided signed consent.
ELISA assessment
This ELISA test utilized the DIA. PRO kit (HTLV I, II Ab version ULTRA, DIA.PRO, Italy) to detect antibodies against HTLV-1 virus in the samples.
Proviral load
We used Ficoll density gradient medium (Cedarlane, Hornsby, ON, Canada) to separate peripheral blood mononuclear cells (PBMCs) from blood samples treated with EDTA and then extracted DNA from PBMCs with a blood mini kit (Qiagen, Germany). All DNA standards and samples were amplified in duplex. After that, we used a commercial Real-time-based absolute quantification kit (HTLV-1 RG; Novin Gene, Karaj, Iran) to perform a real-time PCR (Q 6000 machine, Qiagen, Germany) and measure the PVL of HTLV-I in PBMCs. We computed the normalized HTLV-I PVL values as the ratio HTLV-1 DNA copies/albumin DNA copies/2 × 104, and expressed them as the number of HTLV-1 proviruses/104 PBMCs.
RNA extraction and cDNA synthesis
RNA extraction was conducted by RNJia Kit (ROJE, Iran) as per the manufacturer’s instructions to purify total RNA. Subsequently, RNA elution was treated with RNase-free DNase (Qiagen, Germany), and cDNA was synthesized with 5 µl of the extracted RNA using the RT-ROSET Kit (ROJE, Iran) as outlined by the manufacturer.
Quantitative real-time PCR
Infection was authenticated by PCR on Tax, HBZ of the virus, as well as the mRNA expression of LFA1, MLCK, RAC1, RAPL, ROCK1, VAV1 and CXCR4 via a Real-time qPCR. The sequences of the PCR primers from 5′ to 3′ are shown in Table 1. Primer sequences for Tax, HBZ and RPLP0 were used according to previous studies [11]. The quantitative Real-time PCR assay was executed as per the manufacturer’s instructions. To determine the expression index, the relative copy number of the mRNA of interest was divided by the relative mRNA copies number of RPLP0, yielding the normalized value of the expression for each gene.
Statistical analysis
The statistical software GraphPad-Prism employed to analyze the data obtained and assess the significance of the relationship between the results. The collected data were analyzed using nonparametric Mann–Whitney and Spearman’s correlation tests. In this study, P-value lower than 0.05 was deemed to be significant.
Results
Proviral load
The mean ± SD of the PVL in AC individuals was 71.15 ± 35.46 copies in PBMC. The median PVL was 63 copies/PBMC with a minimum of 38 and maximum of 183 copy/ml.
Gene expression assessment
This preliminary aims to highlight the significance of mRNA expression associated with T-cell migration and HTLV-1 to shed light on its probable relevance in advancing our understanding of fundamental biological processes for this virus.
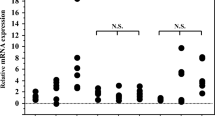
In this investigation, the average expression of LFA1 mRNA was found to be 0.82 ± 0.13 in ACs patients and 0.35 ± 0.05 in healthy group. The expression of the LFA1 gene was higher in the ACs patients in comparison to healthy group and this was statistically significant (95% CI, P < 0.0001) (Fig. 1a).
The average expression of MLCK mRNA was found to be 1.1 ± 0.14 in ACs patients and 0.30 ± 0.13 in healthy group which was significant (95% CI, P < 0.0001). (Fig. 1b).
The mean expression of RAC1 mRNA in the group of ACs and healthy group were 1.16 ± 0.20 and 0.26 ± 0.04 respectively (P < 0.0001, CI 95%) (Fig. 1c).
A significant difference has been seen in RAPL mRNA expression across two group (P < 0.0001, CI 95%). The mean expression of RAB3GAP2 (ACs 1.09 ± 0.21; healthy group 0.5082 ± 0.2417) was pairwise compared by the spearman test (Fig. 1d).
The mean ROCK1 mRNA expression in the ACs patients and healthy subjects was reported as 1.64 ± 0.14 and 0.47 ± 0.06 respectively (P < 0.0001, CI 95%) (Fig. 1e).
The average expression of VAV1 mRNA was found to be 1.13 ± 0.23 in ACs patients and 0.48 ± 0.15 in healthy group which was significant (P < 0.0001, CI 95%) and the mean expression of CXCR4 mRNA in the group of ACs and healthy group were 0.22 ± 0.03 and 0.14 ± 0.02 respectively (P < 0.0001, CI 95%) (Fig. 1f, g).
Also, comparison and correlation of difference expression among targeted genes and correlation is show in Fig. 2 and Table 2 respectively.
The mean expression for Tax was 0.72 ± 0.22 with a maximum of 0.40 and minimum of 0.42 For HBZ, mean expression was 0.65 ± 0.21, with maximum expression of 1.09 and minimum of 0.42.
Discussion
Previous studies have explored cellular protein interactions following HTLV-1 infection, but there has been limited investigation into the specific topic of interest. Our study provides evidence for increased mRNA expression of LFA1, MLCK, RAC1, RAPL, ROCK1, VAV1, and CXCR4 in HTLV-1 infection and highlights the potential role of these host factors in promoting migration and disease progression.
LFA-1 is primarily expressed on leukocytes, including T cells, B cells, and natural killer cells, and it plays a crucial role in leukocyte migration and adhesion. LFA-1 also mediates the formation of immunological synapses between T cells and antigen-presenting cells, which are essential for T cell activation and immune responses [12, 13]. In cancer cells, LFA-1 has been shown to promote tumor cell migration and invasion, contributing to cancer metastasis [14, 15].
In our study, this increase in LFA1 was seen in ACs patients compared to the healthy group.
Myosin Light Chain Kinase (MLCK) is a kinase enzyme that regulates the contraction of actin-myosin fibers in the cytoskeleton of cells. It also plays a critical role in cell migration by regulating the formation and retraction of actin-rich protrusions, such as lamellipodia and filopodia [16, 17]. In the review article by Bhat et al., and Lin et al., it was pointed out that by increasing the expression of MLCK, we subsequently see an increase in IL1B, which itself can cause inflammation, especially in the BBB [18, 19]. It was mentioned in the study of Kim et alLoss of MLCK leads to disruption of cell–cell adhesion and invasive behavior via elevated expression of EGFR and ERK/JNK signaling [20].
As mentioned in various studies and in line with the results of our studies, with the increase in the expression of MLCK and subsequently various injuries and an increase in the level of migration.
RAC1 has been reported to mediate microtubule stabilization through phosphorylation and the inhibition of the microtubule-destabilizing protein stathmin in HTLV-1 infection and our study also reports its elevation following HTLV-1 infection [21].
RAPL is a signaling protein that is involved in various cellular processes, including cell migration and adhesion. RAPL interacts with Rap1 and enhances its activation, which leads to the recruitment of integrins to the cell surface and the formation of focal adhesions [22]. In addition, RAPL’s interaction with CXCR4 enhances cell migration in response to CXCL12, a chemokine that is involved in cell migration and homing [23, 24].
In our study, we also see an increase in the expression of RAPL in the ACs group compared to the healthy group, which helps to increase the virus migration capacity. This could facilitate viral dissemination, immune evasion, and the development of HTLV-1-associated diseases.
VAV1 also plays an important role in cell migration. It activates Rho family small GTPases, including RhoA, Rac1, and Cdc42, which are key regulators of cytoskeletal dynamics and actin polymerization [25, 26]. VAV1 also regulates the activation of downstream signaling pathways, including MAPK and PI3K, which are involved in cell migration and invasion [27, 28]. Our study reported an elevated levels of VAV1 following HTLV-1 infection.
ROCK1 is a serine/threonine kinase phosphorylates and activates several downstream targets, including myosin light chain (MLC) and LIM kinase (LIMK), which regulate actin-myosin contractility and actin dynamics, respectively [29, 30]. ROCK1-mediated phosphorylation of MLC leads to increased actomyosin contractility, resulting in cell contraction and retraction of the trailing edge of migrating cells [31,32,33].
Various studies have pointed out the role of ROCK1 and its increased expression in increasing the capacity of migration and metastasis in various types of cancers such as lung cancer [31, 34, 35].
Also, various studies have pointed out the relationship between the increase in CXCR4 expression following HTLV-1 infection and the increase in migration capacity. In our study, CXCR4 expression was increased in ACs compared to the healthy group. The increased expression of CXCR4 enhances the infectivity of HTLV and may contribute to the dissemination of the virus within the host, leading to the development of HTLV-associated pathologies [36, 37].
Conclusion
Our study has demonstrated the critical role of infected T cell migration in the progression of HTLV-1-associated diseases. Also, this study shed light on the specific factors that can influence the migration of infected T cells, including the expression levels of adhesion molecules and chemokine receptors. This knowledge presents potential therapeutic targets that may be harnessed for the development of new treatment strategies aimed at modulating and controlling the migration process.
Availability of data and materials
Data will be made available on reasonable request from corresponding authors. No datasets were generated or analysed during the current study.
References
Grassmann R, Aboud M, Jeang K-T. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene. 2005;24(39):5976–85.
Cook LB, Elemans M, Rowan AG, Asquith B. HTLV-1: persistence and pathogenesis. Virology. 2013;435(1):131–40.
Thomsen AR, Nansen A, Madsen AN, Bartholdy C, Christensen JP. Regulation of T cell migration during viral infection: role of adhesion molecules and chemokines. Immunol Lett. 2003;85(2):119–27.
Trepat X, Chen Z, Jacobson K. Cell migration. Compr Physiol. 2012;2(4):2369.
Hieshima K, Nagakubo D, Nakayama T, Shirakawa A-K, Jin Z, Yoshie O. Tax-inducible production of CC chemokine ligand 22 by human T cell leukemia virus type 1 (HTLV-1)-infected T cells promotes preferential transmission of HTLV-1 to CCR4-expressing CD4+ T cells. J Immunol. 2008;180(2):931–9.
Mothes W, Sherer NM, Jin J, Zhong P. Virus cell-to-cell transmission. J Virol. 2010;84(17):8360–8.
Poon B. Virus–host interactions of human retroviruses. Los Angeles: University of California; 1997.
Kedrin D, van Rheenen J, Hernandez L, Condeelis J, Segall JE. Cell motility and cytoskeletal regulation in invasion and metastasis. J Mammary Gland Biol Neoplasia. 2007;12:143–52.
Man S, Ubogu EE, Ransohoff RM. Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain Pathol. 2007;17(2):243–50.
Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol. 2012;33(12):579–89.
Shayeghpour A, Forghani-Ramandi M-M, Solouki S, Hosseini A, Hosseini P, Khodayar S, et al. Identification of novel miRNAs potentially involved in the pathogenesis of adult T-cell leukemia/lymphoma using WGCNA followed by RT-qPCR test of hub genes. Infect Agents Cancer. 2023;18(1):1–11.
Reina M, Espel E. Role of LFA-1 and ICAM-1 in cancer. Cancers. 2017;9(11):153.
Walling BL, Kim M. LFA-1 in T cell migration and differentiation. Front Immunol. 2018;9:952.
Shen W, Zhang X, Du R, Fan Y, Luo D, Bao Y, et al. ICAM3 mediates tumor metastasis via a LFA-1-ICAM3-ERM dependent manner. Biochim Biophys Acta (BBA) Mol Basis Dis. 2018;1864(8):2566–78.
Goodman SL, Picard M. Integrins as therapeutic targets. Trends Pharmacol Sci. 2012;33(7):405–12.
Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol. 1995;130(3):613–27.
Kohama K, Ye L-H, Hayakawa K, Okagaki T. Myosin light chain kinase: an actin-binding protein that regulates an ATP-dependent interaction with myosin. Trends Pharmacol Sci. 1996;17(8):284–7.
Bhat AA, Uppada S, Achkar IW, Hashem S, Yadav SK, Shanmugakonar M, et al. Tight junction proteins and signaling pathways in cancer and inflammation: a functional crosstalk. Front Physiol. 2019;9:1942.
Lin C-Y, Zu C-H, Yang C-C, Tsai P-J, Shyu J-F, Chen C-P, et al. IL-1β-induced mesenchymal stem cell migration involves MLCK activation via PKC signaling. Cell Transplant. 2015;24(10):2011–28.
Kim D, Helfman DM. Loss of MLCK leads to disruption of cell–cell adhesion and invasive behavior of breast epithelial cells via increased expression of EGFR and ERK/JNK signaling. Oncogene. 2016;35(34):4495–508.
Nejmeddine M, Barnard AL, Tanaka Y, Taylor GP, Bangham CR. Human T-lymphotropic virus, type 1, tax protein triggers microtubule reorientation in the virological synapse. J Biol Chem. 2005;280(33):29653–60.
Zhang Y-L, Wang R-C, Cheng K, Ring BZ, Su L. Roles of Rap1 signaling in tumor cell migration and invasion. Cancer Biol Med. 2017;14(1):90.
Laufer JM, Legler DF. Beyond migration—chemokines in lymphocyte priming, differentiation, and modulating effector functions. J Leukoc Biol. 2018;104(2):301–12.
Kinashi T, Katagiri K. Regulation of lymphocyte adhesion and migration by the small GTPase Rap1 and its effector molecule. RAPL Immunol Lett. 2004;93(1):1–5.
Liu BP, Burridge K. Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not β1 integrins. Mol Cell Biol. 2000;20(19):7160–9.
Valderrama F, Thevapala S, Ridley AJ. Radixin regulates cell migration and cell–cell adhesion through Rac1. J Cell Sci. 2012;125(14):3310–9.
Reynolds LF, Smyth LA, Norton T, Freshney N, Downward J, Kioussis D, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of phospholipase C-γ1 via phosphoinositide 3-kinase-dependent and-independent pathways. J Exp Med. 2002;195(9):1103–14.
Shah VB, Ozment-Skelton TR, Williams DL, Keshvara L. Vav1 and PI3K are required for phagocytosis of β-glucan and subsequent superoxide generation by microglia. Mol Immunol. 2009;46(8–9):1845–53.
Montalvo J, Spencer C, Hackathorn A, Masterjohn K, Perkins A, Doty C, et al. ROCK1 & 2 perform overlapping and unique roles in angiogenesis and angiosarcoma tumor progression. Curr Mol Med. 2013;13(1):205–19.
Gagliardi PA, di Blasio L, Primo L. PDK1: a signaling hub for cell migration and tumor invasion. Biochim Biophys Acta (BBA) Rev Cancer. 2015;1856(2):178–88.
Hu C, Zhou H, Liu Y, Huang J, Liu W, Zhang Q, et al. ROCK1 promotes migration and invasion of non-small-cell lung cancer cells through the PTEN/PI3K/FAK pathway. Int J Oncol. 2019;55(4):833–44.
Zhang R, Li G, Zhang Q, Tang Q, Huang J, Hu C, et al. Hirsutine induces mPTP-dependent apoptosis through ROCK1/PTEN/PI3K/GSK3β pathway in human lung cancer cells. Cell Death Dis. 2018;9(6):598.
Li G-B, Cheng Q, Liu L, Zhou T, Shan C-Y, Hu X-Y, et al. Mitochondrial translocation of cofilin is required for allyl isothiocyanate-mediated cell death via ROCK1/PTEN/PI3K signaling pathway. Cell Commun Signaling. 2013;11(1):1–13.
Zhao Y, Lv M, Lin H, Hong Y, Yang F, Sun Y, et al. ROCK1 induces ERK nuclear translocation in PDGF-BB-stimulated migration of rat vascular smooth muscle cells. IUBMB Life. 2012;64(2):194–202.
Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu P-Y, et al. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Investig. 2008;118(5):1632–44.
Moriuchi M, Moriuchi H, Fauci AS. HTLV-I Tax activation of the cxcr4 promoter by association with nuclear respiratory factor 1. JAIDS J Acquir Immune Defic Syndr. 1999;20(4):A77.
Moriuchi M, Moriuchi H, Fauci AS. Short communication HTLV type I Tax activation of the CXCR4 promoter by association with nuclear respiratory factor 1. AIDS Res Hum Retrovir. 1999;15(9):821–7.
Acknowledgements
We are grateful to all of those with whom we have had the pleasure to work during this and other related projects especially oncology departments and staff of Alborz blood transfusion center.
Funding
This project was financially supported by Alborz University of Medical Sciences, Iran. (Grant number: 4269).
Author information
Authors and Affiliations
Contributions
A.L, A.B, M.N: methodology, investigation, validation, writing original draft. G.M and A.R: investigation. MH.Y: supervision, validation. SH.M and Z.S: supervision, funding, review and editing, investigation.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committees of Medical Sciences Research at Alborz University of Medical Sciences, Iran. Also, Informed consent letter was provided for each participant included in the study. All procedures were performed in accordance with relevant guidelines.
Consent for publication
Informed consent for publication of identifiable information/images journal was obtained from all study participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Letafati, A., Bahavar, A., Norouzi, M. et al. Effects of HTLV-1 on leukocyte trafficking and migration in ACs compared to healthy individuals. BMC Res Notes 17, 222 (2024). https://doi.org/10.1186/s13104-024-06887-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-024-06887-5