Abstract
Objective
Neurotransmitters have been extensively studied as neural communication molecules. Genetic associations discovered, and indirect intervention studies in Humans and mammals have led to a general proposition that neurotransmitters have a role in structuring of neuronal network during development. olf413 is a Drosophila gene annotated as coding for dopamine beta-monooxygenase enzyme with a predicted function in octopaminergic pathway. The biological function of this gene is very little worked out. In this study we investigate the requirement of olf413 gene function for octopamine biogenesis and developmental patterning of embryonic nervous system.
Result
In our study we have used the newly characterized neuronal specific allele olf413SG1.1, and the gene disruption strain olf413MI02014 to dissect out the function of olf413. olf413 has an enhancer activity as depicted by reporter GFP expression, in the embryonic ventral nerve cord, peripheral nervous system and the somatic muscle bundles. Homozygous loss of function mutants show reduced levels of octopamine, and this finding supports the proposed function of the gene in octopamine biogenesis. Further, loss of function of olf413 causes embryonic lethality. FasII staining of these embryos reveal a range of phenotypes in the central and peripheral motor nerves, featuring axonal growth, pathfinding, branching and misrouting defects. Our findings are important as they implicate a key functional requirement of this gene in precise axonal patterning events, a novel developmental role imparted for an octopamine biosynthesis pathway gene in structuring of embryonic nervous system.
Similar content being viewed by others
Introduction
Neurotransmitters are classical communication molecules extensively studied for their synaptic function mediating transmission. Human and mammalian studies which associate many of the major neurotransmitters to neurodevelopmental disorders implicate neurotransmitters to have potential roles beyond synaptic communication, in neurodevelopment [1]. The enormous size and complexity of mammalian brain makes it difficult to visualize the developmental deformities at a finer resolution in terms of cellular processes and axonal tracts of neurons. Drosophila, which is capable of performing complex activities has a relatively several folds lesser number of neurons in their brain with simpler and definable circuits [2]. Further flies as model system offer the most tractable genetic toolkit which enables the analysis of genes for their functions in a context dependent manner [3,4,5,6,7,8]. Octopamine is a neurotransmitter similar to vertebrate nor adrenaline, controls aggression, sleep, appetite, feeding, courtship etc. in Drosophila, as understood through studies on mutants defective for enzyme Tyramine β-Hydroxylase [9]. Here we report an intriguing finding that a paralogous gene olf413, predicted to be involved in octopamine biogenesis is essential for developmental patterning of embryonic nervous system.
SG1.1 is a P-GAL4 enhancer trap strain isolated in our earlier screen for genes with expression in the nervous system [10]. This strain carries single P-GAL4 insertion on the third chromosome which is homozygous lethal. The strain shows enhancer activity in clusters of neurons in the suboesophageal ganglion (SOG), superior protocerebrum, central brain (CB) region and ventral ganglion (VG) of the adult brain [11, 12]. The expression of the reporter gene in the pupal brain in a temporally regulated cyclical pattern prompted us to characterize the native gene at the site of P-GAL4 insertion in SG1.1 strain. Here we report the molecular mapping of the P-GAL4 insertion to 1.9 bp upstream of the transcription start site of the identified native gene olf413, as annotated in Berkeley Drosophila Genome Project (BDGP) [13]. The reporter gene expression comparisons and complementation test with a null allele of olf413 confirmed SG1.1 P-GAL4 insertion as an allele of olf413. Gene olf413, annotated as CG12673 has been predicted to code for a protein paralogous to Tβh having a role as an enzyme in octopamine biogenesis [14]. Biological function of olf413 has been very little worked out, except in a few genome-wide association (GWA) screens, food preference and motor behavior analysis [15,16,17,18,19]. This study shows that olf413 mutants have decreased octopamine levels and the loss of function mutant embryos show severe disruptions in motor nerves of ventral nerve cord (VNC) and their peripheral motor axon projections. Our observations discretely demonstrate in vivo, the critical requirement of olf413 function in establishing precisely patterned neuronal network during embryonic development.
Methods
Drosophila stocks
The following fly stocks were used: Oregon-K (Drosophila Stock Centre, University of Mysore), UAS-GFP on III chromosome (National Centre for Biological Sciences, Bengaluru), SG1.1/TM3Sb (our lab), Gene disruption strain olf413MI02014/TM3Sb (#77717, Bloomington Drosophila Stock Centre (BDSC)) [20].
Molecular localization of P-GAL4 insertion
Inverse PCR was carried out using T7 and T3 primers on self-ligated Sac1 and Pst1 digests of SG1.1 genomic DNA. The genomic fragment flanking the P- insertion was sequenced. The flanking genomic sequence mapping was done using Basic Local Alignment Search Tool (BLAST) search against Drosophila melanogaster genomic sequence to obtain the precise position of P-GAL4 insertion.
Lethality test
To decipher the exact stage of lethality in homozygous SG1.1 strain, embryos were collected on 2% sucrose agar medium at 22 ℃ overnight. 1000 embryos were transferred to 20 vials containing normal wheat cream agar medium with 50 embryos in each vial. The number of live and dead individuals were scored and recorded at each stage (larva, pupa and adult).
To check embryonic lethality, embryos collected were incubated at 25 ℃ incubator for nearly 48–50 h. The number of unhatched and hatched embryos were tabulated. The unhatched embryos of genotype SG1.1-GAL4 and olf413MI02014, which had completed embryogenesis and were with visible mouth hook were imaged in halocarbon oil under Bright field microscope (ZeissAXIO Imager A2).
Complementation test
Virgin females from SG1.1-GAL4/TM3Sb were crossed to olf413MI02014/TM3Sb males, and the F1 progeny embryos were collected and grown as explained in lethality test. The number of F1 adult flies eclosed from 50 × 20 replicates were counted and recorded.
Analysis of reporter gene activity
UAS-GFP; SG1.1/TM3Sb stock was generated and used for analysis of GFP expression. olf413MI02014-GAL4 virgin females were crossed with UAS-GFP (on III chromosome) males, to obtain olf413MI02014-GAL4/UAS-GFP individuals. The embryos collected were dechorionated in 50% sodium hypochlorite solution, rinsed with water, mounted in halocarbon oil and the GFP expression was documented using confocal laser scanning microscope (Zeiss LSM 710). The Z sections were taken at every 1 µm interval. The 3D projections of Z-Stacks were obtained.
Staged larval and pupal brains were dissected in phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde (PF) fixative in PBS, for 20–30 min at room temperature. The fixed brains were washed in PBS and 0.05% PBTx and mounted in vectashield for imaging.
Immunohistochemistry
15–18 h old embryos selected from an overnight collection of Oregon-K, SG1.1/TM3Sb and olf413MI02014 stocks were anaysed. The embryos were dechorionated and fixed in 3.7% formaldehyde in PBS with equal volume of n-heptane (1:1) solution for 20 min, devitenalized by 2–3 methanol washes, followed by washes with absolute alcohol. The embryos were rehydrated with grades of alcohol and 0.1% PBTx (1:2, 1:1, 0.1%PBTx) prior to Antibody staining and processed further. Third instar larval brains were dissected in PBS, fixed in 4% PF for 25 min at room temperature, washed with 0.05% PBTx and processed for antibody staining. Antibody staining for both embryos and larval brains was carried out as described in Rohith and Shyamala [21], with minor changes. Primary antibodies used were mouse anti-Fasciclin II (1:10, 1D4, DSHB) for embryos, mouse anti-Repo (DSHB; 1:10) and mouse anti-Elav (DSHB; 1:10) for larval brains. Secondary antibodies used were Goat Anti-Mouse IgG CF™ 488A (1:200; Sigma Aldrich), Goat Alexa fluor 647 anti-mouse IgG (Invitrogen; 1:500). The embryos and brains were mounted in vectashield and imaged with confocal laser scanning microscope (Zeiss LSM 710).
Octopamine quantification
LC–MS/MS analysis was carried out for control (Oregon-K) and mutant (olf413MI02014 homozygous survivors). 10 heads of just eclosed males and females in equal ratio, were homogenised with 50 μl PBS and 50 μl of 0.1% Formic acid in Acetonitrile (ACN), and centrifuged at 10,000 rpm for 10 min at 5 °C. The supernatant was collected, and stored at − 20 °C until analysis. Octopamine levels were measured with HPLC—Shimadzhu LC Prominence 20AT, and Mass Spectrophotometer—AB Sciex, 4000 equipped with a C18, 50*4.6 mm, 4-micron column. The graph represents a mean of three independent experimental replicates for each group.
Statistics
The statistical analysis was performed using SPSS software (Version 22). The octopamine quantity is presented as mean ± SEM. One-way ANOVA and Tukey's post-hoc honestly significant difference test were applied to compare between the control and mutant groups.
Results
SG1.1 P-GAL4 insertion is an allele of olf413
Precise mapping of P-GAL4 insertion in SG1.1 strain was done by Inverse PCR (Methods for details). Two independent restriction digests of SG1.1 genomic DNA using SacI and PstI, were processed for inverse PCR with T7 and T3 primers (Methods for details). The BLAST search of flanking genomic DNA maps P-GAL4 insertion in this strain to 22,134,168th bp position on the left arm of the 3rd chromosome (79C). This insertion site is 1.9 kb upstream of the transcriptional start site of the annotated gene [13] CG12673 identified as olf413 (Fig. 1). There are no other annotated protein coding transcripts up to a distance of about 53,370 bases from the site of P-insertion. There is an annotated long non coding RNA—lncRNA-CR45236 at a distance of 12.23 kb upstream of the insertion site whose molecular and biological function are not known. The reporter gene expression pattern as depicted by the expression of UAS-GFP, driven by SG1.1-GAL4 was documented. The embryonic expression pattern of GFP (Fig. 2A) matches with that of the mRNA in situ hybridization pattern for CG12673 [22].
Mapping of insertion site of P-GAL4 element in olf413SG1.1 allele of olf413 gene. The figure depicts the P-GAL4 insertion site in olf413SG1.1 and the gene disruption strain, olf413MI02014 on a 5’ to 3’ directed olf413 gene span. The rectangular boxes represent the exons. The thin lines indicate the introns. The numbers along each intron represents the length of the respective intron. SG1.1 P-GAL4 element is inserted at 1922 bp upstream of the transcriptional start site of olf413 (red star). The insertion site of Trojan-GAL4, and the position of start codon (ATG) and Stop codon in olf413 are marked on the basis of BDGP genome data [13, 20]
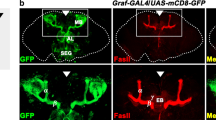
Enhancer activity pattern in olf413SG1.1 enhancer trap strain and olf413MI02014 as depicted by reporter GFP expression. UAS-GFP strain was crossed with the two olf413 strains and the reporter GFP expression was analysed. A Embryo (stage 16) of olf413SG1.1 shows an expression in the ventral nerve cord region, in ventral (VC), lateral (LC) and dorsal (DC) neuronal clusters of nervous system. B and C Stage 12 and stage 17 embryos of olf413SG1.1 respectively showing the strong expression of GFP in the developing ventral nerve cord (VNC). D Stage 16 embryo of olf413MI02014-GAL4/UAS-GFP showing expression pattern similar to olf413SG1.1. E and F Stage 12 and stage 17 embryos of olf413MI02014 respectively with a very prominent reporter expression in the somatic muscle precursors (MPC) and Somatic muscle bundles (SM). G and H 72 h after larval hatched (ALH) (late 3rd instar larva) staged brain of olf413SG1.1 and olf413MI02014 strains respectively shows the reporter gene expression in clusters of neurons in central brain, suboesophageal ganglion (SOG) and Kenyon cells (KC). Ventral ganglion has the expression in centrointermedial neurons (CIMN) and the dorsolateral neurons (DLN) of individual segmental neuromeres. I and J Frontal view of adult brain (101 h APF) of olf413SG1.1 and olf413MI02014 respectively showing lobular plate region. The vertical system neurons of the optomotor responsive system [25] (ORS) shows strong enhanced activity in both the strains. All embryos have anterior to the left and the posterior to the right. K–M Brains at 72 h ALH, stained with Anti-Elav antibody (L, Red) marked with olf413SG1.1 GFP (K, green). M merged image showing that olf413SG1.1 cells are Elav positive. N–P Brains at 72 h ALH stained with Anti- Repo antibody (O, Red) and marked with olf413SG1.1 GFP (N, green). P merged image showing that olf413SG1.1 cells do not overlap with Repo positive cells. Q Represents the results of LC–MS/MS analysis for octopamine quantification. The bar graphs represent the mean ± SEM of octopamine levels (n = 3) quantified in head samples for homozygous olf413MI02014 with Oregon-K as control. A, B, D, E, F are lateral views, C is ventral view. Scale bar in Fig A-J represents 50 μm and in (K–P) represents 10 µm
SG1.1 strain has an enhancer activity which covers a subset of expression domains of olf413 in embryonic and adult nervous system
Lee et al. have generated a gene disruption library of strains which have gene specific T2A-GAL4 insertion in one of the introns of the target gene, thus will be expressed under the endogenous promoter of the gene in question. The transgenic construct also has a polyadenylation signal at 3’end of the GAL4 coding sequence which will arrest transcription following the GAL4 sequence. Thus it will code for a truncated protein for the target gene, and acts as a null/severe loss of function allele for the gene [20]. olf413MI02014 is a gene disruption strain from this library which has the T2A-GAL4 insertion in the second intron after the translational start site for CG12673, the annotated gene for olf413. olf413MI02014 and SG1.1-GAL4 strains were crossed to UAS-GFP and the GAL4 driven reporter GFP expression was analyzed and compared. The reporter gene expression driven by SG1.1-GAL4 is seen in the ventral (VC), lateral (LC) and dorsal clusters (DC) of neurons of the peripheral nervous system (PNS) in stage 16 embryo [23] (Fig. 2A). A similar pattern of enhancer activity is seen in olf413MI02014/UAS-GFP embryos (Fig. 2D). The two strains share an overall similarity in the pattern of reporter GFP expression in the larval brain (Fig. 2G and H). Clusters of neurons in the central brain, suboesophageal (SOG) and the ventral ganglion are strongly marked while Mushroom body neurons, the kenyon cells (KCs) show faint expression. Segmentally reiterated pairs of neurons along the neuromeres of the VG, which localize to the centrointermedial (CIMN) and the dorsolateral (DLN) compartments of the fasciclin II (FasII) landmark system [24], express reporter GFP. It is seen that the SG1.1 enhancer activity is restricted to fewer number of neurons as compared to that seen in case of olf413MI02014 in the respective domains of expression. Figure 2I and J show the lobula plate region of the adult brains of SG1.1/UAS-GFP and olf413MI02014/UAS-GFP individuals respectively. The dendritic arborization of the vertical system neurons (VS1, VS2, VS3) of the optomotor responsive system (ORS) [25] are strongly marked by the reporter GFP expression. Besides this shared enhancer activity, there are expression patterns which are distinct to the two strains. We see that the embryonic ventral nerve cord in SG1.1-GAL4 is marked by reporter expression from stage 12, which becomes pronounced by stage 17 (Fig. 2B, C). The endogenous enhancer activity as in olf413MI02014, is seen in the developing somatic muscles. Reporter expression is seen in myoblast clusters of stage 12 embryo, and the dorsal and lateral somatic muscle (SM) bundles of late 17 stage embryo (Fig. 2E, F). This mesodermal specific enhancer activity is not seen in SG1.1-GAL4 strain. The adult and larval expression patterns of SG1.1-GAL4 enhancer trap strain recorded during our earlier screening had shown reporter expression restricted to the brain and ventral ganglion of the adult and the larva. Adult and the larval muscles, including other tissues do not show reporter expression [10, 11]. Further to confirm the neural or glial identity of these cells, we have here stained the SG1.1 GAL4-GFP larval brains with Anti-Elav (neuronal) and Anti-Repo (glial) marker antibodies. Figure 2K–P depicts the results. We can clearly see that all SG1.1-GFP expressing cells stain positive with anti-Elav antibody (Fig. 2K–M), whereas none of the GFP expressing cells co-localize with anti-Repo antibody (Fig. 2N–P). Thus, demonstrating clearly that, the P-GAL4 insertion in SG1.1 marks a neuronal specific enhancer of olf413.
SG1.1-GAL4 insertion fails to complement lethality in-trans with olf413 MI02014
Prompted by the fact that, P-GAL4 insertion localizes at 1.9 kb upstream of olf413 transcription start site, and the local enhancer activity pattern maps to a subset of endogenous enhancer activity domains of olf413, we subjected the two strains for complementation in-trans test. As mentioned earlier, SG1.1-GAL4 insertion strain is homozygous lethal at embryonic stage. We checked the gene disruption strain olf413MI02014 and found that it also shows lethality at embryonic stage with very few escapers (3–4%). The results of the complementation test (Methods) are presented in Table 1. The transheterozygotes SG1.1-GAL4/olf413MI02014 showed a partial lethality of about 36.4%. This failure in complementation further confirmed that SG1.1-GAL4 strain is a new allele of olf413. And here onwards we denote the strain as olf413SG1.1- a new allele of olf413.
olf413 loss of function mutants show reduced levels of octopamine
Based on the annotated protein product and the identified functional domains, the function of olf413 gene product is predicted as dopamine beta-monooxygenase, an enzyme in octopamine/ norepinephrine biogenesis. In this context, we wanted to check if octopamine levels are affected in the loss of function mutants of olf413. The head samples from olf413MI02014 homozygous survivor adults were subjected to LC–MS/MS analysis for quantification of octopamine. Three sample runs for each trial set were carried out for the control and the mutant (Methods for details). The results are presented as bar graphs in Fig. 2Q. We find that, in all the trials, the mutant heads consistently showed reduced quantity of octopamine compared to that of the head samples from the control. The results strongly substantiate the predicted function of olf413 in octopamine biosynthesis.
olf413 loss of function results in severe axonal growth, pathfinding and connectivity defects in embryonic nervous system
The homozygous embryos of olf413SG1.1 as well as, that of olf413MI02014 when observed, revealed that they die at very late stage of embryonic development, with the mouth hook and trachea formed (Fig. 3A and B). Intriguingly, some of these embryos showed wriggling movements within the chorion and made attempts to hatch out, but failed to move out of the chorion. This observation was strongly indicative of a probable problem with the neuromuscular coordination and disability. Fasciclin II antibodies provide an effective tool to identify motor axon defects. We stained the late staged (16–17 stage) homozygous embryos with Anti-FasII antibody. The results are presented in Fig. 3C–S. Figure 3C and D represent wild type ventral nerve cord and peripheral motor nerves pattern. The olf413SG1.1 homozygous embryos showed grades of deformities in the longitudinal tracts of the ventral nerve cord and the peripheral motor nerves (Fig. 3E–I). Most severe phenotypes with an occurrence of 40% had highly disorganized ventral nerve cord and the peripheral motor projections (Fig. 3E). Embryos with moderate phenotypes had breaks in the VNC, axonal growth defects, disordered and misrouted axons evading segmental boundaries (Fig. 3F and G). The milder phenotypes presented frequent midline crossing of the longitudinal tracts (Fig. 3H). The enlarged views highlight these defects in detail (Fig. 3E’–H’). The inter segmental (ISN) and segmental (SN) motor projection neurons showed defects like fusion of ISN and SN nerves into single tract, misrouting of axons to cross the segmental boundaries. Excessive and abnormal branching, premature termination of growth before they reach their respective target muscles were commonly seen among motor axon projections (Fig. 3I).
Central and peripheral motor neuron axon guidance defects in mutant olf413SG1.1, olf413MI02014 and trans-heterozygotes olf413SG1.1/olf413MI02014 embryos. A and B The bright field images of lethal embryos of olf413SG1.1 and olf413MI02014 homozygotes respectively showing the mouth hook (MH) and trachea (TR) completely formed (green arrows). C–S Embryos at stage 16–17 stained with Anti-Fasciclin II antibody (mAB-1D4) to label the axonal tracts of both ventral nerve cord and peripheral nerves. C and D are the Oregon-K embryos of stage 16–17, showing the wild type VNC and the peripheral motor nerves (PMN) respectively. The inter-segmental nerve (ISN) and segmental nerve (SN) are marked with white arrows. E–I Represent the VNC and the PMN of olf413SG1.1 homozygotes. E Most severe VNC and PNS deformities. F and G Moderate phenotype with axonal growth defect in VNC, disrupted and misrouted PMN. H Midline crossing of longitudinal tracts. E’–H’ Enlarged view of marked region in (E–H). I Lateral view of peripheral projection nerves showing different growth and pathfinding defects. J–N Represent the VNC and PMN of olf413MI02014 homozygotes. J–M Most severe VNC and PNS deformities. J’–M’ Enlarged view of marked region in (J–M). N Lateral view of peripheral projection nerves showing severe growth and pathfinding defects. O–S Represent the VNC and PMN of olf413SG1.1/olf413MI02014 transheterozygotes. O Most severe VNC and PNS deformities. P and Q moderate growth and pathfinding defects. R Mid line crossing of longitudinal tracts. O’–R’ Enlarged view of marked region in (O–R). S Lateral view of peripheral projection nerves showing different growth and pathfinding defects. In all figures, arrows indicate breaks in VNC (red), axonal thinning and growth defect (purple), disordered or misrouted axons evading segmental boundaries (blue), and midline crossing of the longitudinal tracts (yellow). The peripheral motor projection defects are indicated by arrowheads- premature termination of growth, asterisks- fusion or fasciculation of ISN and SN into one nerve, rhomboid- excessive or abnormal branching of axons, star- misrouting of axons extending across the segmental boundaries, where the ISN of one segment fasciculate with the nearby ISN segment, and white arrow- de-fasciculation or branching at the ends of ISN. In the images showing VNC, the embryos are positioned with anterior to the top, whereas for the embryos showing PMN, they are positioned with anterior to the left. Scale bar represents 50 μm
The VNC and peripheral motor projection axon phenotypes seen in homozygous embryos of olf413MI02014 strain were relatively much more severe (Fig. 3J–N). This was expected as loss of function of olf413 in this case is both in the neurons as well as in the somatic muscles. Highly disordered and messed up VNC with stunted and misrouted projection nerves featured the phenotypes with an occurrence of 56.2% (Fig. 3J–M). Detailed view of these defects are seen in Fig. 3J’–M’. Multiple defects found in peripheral motor projection nerves were comparable to that seen in olf413SG1.1 homozygotes (Fig. 3N). In summary, the phenotype observed demonstrates that the function of olf413 is critically required for proper axonal growth, guidance, branching and target connectivity in both the VNC and peripheral motor projections during embryonic development. The transheterozygote olf413SG1.1/olf413MI02014 embryos stained with Anti-FasII antibodies showed similar range of longitudinal tract and motor axon pathfinding defects (Fig. 3O–R). The frequency of occurrence of the most severe phenotypes was 46.15%. Figure 3O’–R’ show enlarged views of the VNC defects. Figure 3S depicts various peripheral motor projection defects observed. All the axonal tract defects seen in the olf413SG1.1 and olf413MI02014 homozygotes were also seen in the transheterozygotes, though represented in lesser number of embryos. These results imply that the two strains fail to complement each other with respect to the axonal tract defects as well.
Discussion
SG1.1, a P-GAL4 strain was studied in detail for its molecular localization, enhancer activity pattern and genetic complementation with a putative candidate native gene olf413. Our experiments demonstrated that SG1.1-GAL4 strain is a neuronal specific allele of olf413 (CG12673). olf413 has been annotated as a protein coding gene with predicted copper type II ascorbate-dependent monooxygenase domain, tyramine/dopamine beta- hydroxylase signature domains [26]. The biological functions of olf413 have been little studied. It has been identified as an associated gene in a few GWA analysis studies carried out for psychostimulant drug preferences [15] and dietary dependent reduction in life span and starvation resistance [16, 17]. Recently studies have shown food preference, feeding and motor activity defects in olf413 mutant adults [18, 19].
In the present study, we have used the gene disruption strain olf413MI02014 in conjunction with olf413SG1.1 to describe for the first time, the detailed expression pattern and role of the gene olf413 in embryonic development and octopamine biogenesis. Our finding that the homozygous mutants show a decreased level of octopamine, strongly agrees with the predicted function of the gene in octopamine biogenesis. Analysis of lethal homozygous (olf413SG1.1, olf413MI02014) and transheterozygote (olf413SG1.1/olf413MI02014) by Anti-FasII antibody staining has revealed extremely severe to mild deformities in the embryonic ventral nerve cord and peripheral motor projection nerves. Relatively more distorted VNC and peripheral axonal phenotypes in olf413MI02014 embryos indicate that, the neuronal, as well as the muscle specific expression of the gene are required, and function in synergy with each other to facilitate axon growth and guidance to establish precise patterning of the neuronal tracts during embryonic development.
By DRSC integrative ortholog prediction tool (DIOPT) reports [27], olf413 has been identified as a paralogue of TβH gene which codes for Tyramine β Hydroxylase, a key enzyme in octopamine biosynthesis pathway [28, 29]. Octopamine being a neurotransmitter and neuromodulator [30, 31] TβH null mutants which suffer octopamine deficits have been assayed for various behavioural phenotypes. TβH function has been implied in regulating aggression [32], courtship [33], sleep behavior [34], learning and memory [35], dietary response [36, 37] larval locomotion [38] and Tau pathogenicity in flies [39]. Nevertheless, so far in vivo embryonic expression and embryonic mutant phenotypes imparting a developmental role, have not been demonstrated for TβH. TβH null flies survive till adulthood with normal morphology but exhibit ovulation defect [14, 40]. While orthologous genes typically perform equivalent functions, paralogues in general evolve through subfunctionalization and subsequent neofunctionalization [41]. Here we have shown that olf413, a paralogue of TβH, has been deployed to perform a distinct role in the development of embryonic nervous system. Developmental role for neurotransmitters has long been implicated in Humans and other mammalian systems [1]. Studies have been carried out through pharmacological interventions using receptor blockers and antagonists in mammalian models for major neurotransmitters like serotonin [42,43,44,45], norepinephrine [46], dopamine [47], GABA [48, 49] and acetylcholine [50]. Genetic studies in Humans include classical chromosomal aberrations associations, and genome-wide association studies (GWAS) relating neurotransmitters to neurodevelopmental diseases [51, 52]. The probable structural connectivity disturbances in these studies have been suggested based on the behavioral and cognitive impairments and altered electrical recordings observed in the subjects. A few in vitro cell culture studies have shown that neurotransmitters administered in culture modulate axon growth and branching. [53, 54]. These studies show indirectly the non-synaptic, developmental roles of neurotransmitters in mammalian brain. The relative simplicity of the cellular content and the neuronal circuitry in Drosophila nervous system has allowed us to demonstrate in this study for the first time, the functional requirement of an octopamine biosynthesis pathway gene for precise axonal growth, and patterning during embryonic development at a finer resolution in vivo.
Limitations
We have demonstrated the critical requirement of olf413 for embryonic nervous system development and biosynthesis of neurotransmitter octopamine. But further qPCR and immunohistochemical quantification experiments are needed to be carried out to demonstrate if the olf413 transcript and protein levels are affected in the mutants studied.
Availability of data and materials
Data available on request from the corresponding author.
Abbreviations
- SOG:
-
Suboesophageal ganglion
- CB:
-
Central brain
- VG:
-
Ventral ganglion
- BDGP:
-
Berkeley Drosophila Genome Project
- TβH:
-
Tyramine β hydroxylase
- GWA:
-
Genome-wide association
- VNC:
-
Ventral nerve cord
- BDSC:
-
Bloomington Drosophila Stock Centre
- BLAST:
-
Basic Local Alignment Search Tool
- VC:
-
Ventral clusters
- LC:
-
Lateral clusters
- DC:
-
Dorsal clusters
- PNS:
-
Peripheral nervous system
- KCs:
-
Kenyon cells
- CIMN:
-
Centrointermedial neurons
- DLN:
-
Dorsolateral neurons
- VS:
-
Vertical system neurons
- ORS:
-
Optomotor responsive system
- SM:
-
Somatic muscle
- MPC:
-
Muscle precursor cells
- FasII:
-
Fasciclin II
- ISN:
-
Inter segmental neurons
- SN:
-
Segmental neurons
- MH:
-
Mouth hook
- TR:
-
Trachea
- DIOPT:
-
DRSC integrative ortholog prediction tool
- DSHB:
-
Developmental Studies Hybridoma Bank
References
Kolk SM, Rakic P. Development of prefrontal cortex. Neuropsychopharmacology. 2022;47(1):41–57.
Kasture AS, Hummel T, Sucic S, Freissmuth M. Big lessons from tiny flies: Drosophila melanogaster as a model to explore dysfunction of dopaminergic and serotonergic neurotransmitter systems. Int J Mol Sci. 2018;19(6):1788.
O’Kane CJ, Gehring WJ. Detection in situ of genomic regulatory elements in Drosophila. Proc Natl Acad Sci USA. 1987;84(24):9123–7.
Wilson C, Pearson RK, Bellen HJ, O’Kane CJ, Grossniklaus U, Gehring WJ. P-element-mediated enhancer detection: an efficient method for isolating and characterizing developmentally regulated genes in Drosophila. Genes Dev. 1989;3(9):1301–13.
Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–15.
Kennerdell JR, Carthew RW. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell. 1998;95:1017–26.
Perkins LA, Holderbaum L, Tao R, Hu Y, Sopko R, McCall K, et al. The transgenic RNAi project at Harvard Medical School: resources and validation. Genetics. 2015;201(3):843–52.
Bassett AR, Tibbit C, Ponting CP, Liu JL. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 2013;4:220–8.
Tyramine beta hydroxylase, InteractiveFly: GeneBrief, Society for Developmental Biology. https://www.sdbonline.org/sites/fly/genebrief/tyraminebetahyd.htm. Accessed 10 Nov 2023.
Shyamala BV, Chopra A. Drosophila melanogaster chemosensory and muscle development: Identification and properties of a novel allele of scalloped and of a new locus, SG18.1, in a Gal4 enhancer trap screen. J Genet. 1999;78:87.
Venkatesh CR, Shyamala BV. Developmentally regulated expression of reporter gene in adult brain specific GAL4 enhancer traps of Drosophila melanogaster. J Genet. 2010;20:1–6.
Venkatesh CR, Shyamala BV. GAL4 enhancer trap strains with reporter gene expression during the development of adult brain in Drosophila melanogaster. J Genet. 2010;89(4):e38-42.
Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287(5461):2185–95.
Monastirioti M, Linn CE, White K. Characterization of Drosophila tyramine β-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16:3900–11.
Highfill CA, Baker BM, Stevens SD, Anholt RRH, Mackay TFC. Genetics of cocaine and methamphetamine consumption and preference in Drosophila melanogaster. PLoS Genet. 2019;15(5): e1007834.
Huang W, Campbell T, Carbone MA, Jones WE, Unselt D, Anholt RRH, Mackay TFC. Context-dependent genetic architecture of Drosophila life span. PLoS Biol. 2020;18(3): e3000645.
Patel SP, Talbert ME. Identification of genetic modifiers of lifespan on a high sugar diet in the Drosophila Genetic Reference Panel. Heliyon. 2021;7(6): e07153.
Ramya R, Shyamala BV. olf413 gene controls taste recognition, preference and feeding activity in Drosophila melanogaster. Annu Res Rev Biol. 2023;38(5):24–31.
Ramya R, Shyamala BV. olf413, a putative octopamine biosynthesis pathway gene is required for negative geotactic motor function in Drosophila melanogaster. J Genet. 2023;102:52.
Lee PT, Zirin J, Kanca O, Lin WW, Schulze KL, Li-Kroeger D, et al. A gene-specific T2A-GAL4 library for Drosophila. Elife. 2018;7: e35574.
Rohith BN, Shyamala BV. Scalloped a member of the Hippo tumor suppressor pathway controls mushroom body size in Drosophila brain by non-canonical regulation of neuroblast proliferation. Dev Biol. 2017;432(2):203–14.
Fisher B, Weiszmann R, Frise E, Hammonds A, Tomancak P, Beaton A. et al. BDGP insitu homepage. Patterns of gene expression in Drosophila embryogenesis. 2012. https://flybase.org/reports/FBrf0219073.html. Accessed 10 Nov 2023.
Campos-Ortega JA, Hartenstein V. Stages of Drosophila embryogenesis. In: Campos-Ortega JA, Hartenstein V, editors. The embryonic development of Drosophila melanogaster. Berlin: Springer; 1985. p. 84.
Vömel M, Wegener C. Neuroarchitecture of aminergic systems in the larval ventral ganglion of Drosophila melanogaster. PLoS ONE. 2008;3(3): e1848.
Rajashekhar KP, Shamprasad VR. Golgi analysis of tangential neurons in the lobula plate of Drosophila melanogaster. J Biosci. 2004;29(1):93–104.
Gaudet P, Livstone MS, Lewis SE, Thomas PD. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief Bioinform. 2011;12(5):449–62.
Hu Y, Flockhart I, Vinayagam A, Bergwitz C, Berger B, Perrimon N, Mohr SE. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinform. 2011;12:357.
Livingstone MS, Tempel BL. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature. 1983;303(5912):67–70.
Wallace BG. The biosynthesis of octopamine–characterization of lobster tyramine beta-hydroxylase. J Neurochem. 1976;26(4):761–70.
Evans PD. Octopamine. In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology, biochemistry and pharmacology. Oxford: Pergamon; 1985. p. 499–530.
David JC, Coulon JF. Octopamine in invertebrates and vertebrates: a review. Prog Neurobiol. 1985;24(2):141–85.
Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL, Heisenberg M. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18(3):159–67.
Certel SJ, Savella MG, Schlegel DC, Kravitz EA. Modulation of Drosophila male behavioral choice. Proc Natl Acad Sci. 2007;104:4706–11.
Crocker A, Sehgal A. Octopamine regulates sleep in Drosophila through protein kinase A-dependent mechanisms. J Neurosci. 2008;28(38):9377–85.
Iliadi KG, Iliadi N, Boulianne GL. Drosophila mutants lacking octopamine exhibit impairment in aversive olfactory associative learning. Eur J Neurosci. 2017;46(5):2080–7.
Schutzler N, Girwert C, Hugli I, Mohana G, Roignant JY. Ryglewski S Duch C: tyramine action on motoneuron excitability and adaptable tyramine/octopamine ratios adjust Drosophila locomotion to nutritional state. Proc Natl Acad Sci USA. 2019;116(9):3805–10.
Damrau C, Toshima N, Tanimura T, Brembs B, Colomb J. Octopamine and tyramine contribute separately to the counter-regulatory response to sugar deficit in Drosophila. Front Syst Neurosci. 2018;11:100.
Fox LE, Soll DR, Wu CF. Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine β hydroxylase mutation. J Neurosci. 2006;26:1486–98.
Nangia V, O’Connell J, Chopra K, Qing Y, Reppert C, Chai CM, Bhasiin K, Colodner KJ. Genetic reduction of tyramine β hydroxylase suppresses Tau toxicity in a Drosophila model of tauopathy. Neurosci Lett. 2021;755:135937.
Monastirioti M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev Biol. 2003;264:38–49.
Koonin EV. Orthologs, paralogs, and evolutionary genomics. Annu Rev Genet. 2005;39:309–38.
Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–12.
Trowbridge S, Narboux-Neme N, Gaspar P. Genetic models of serotonin (5-HT) depletion: what do they tell us about the developmental role of 5-HT? Anat Rec. 2011;294:1615–23.
Kinast K, Peeters D, Kolk SM, Schubert D, Homberg JR. Genetic and pharmacological manipulations of the serotonergic system in early life: neurodevelopmental underpinnings of autism-related behavior. Front Cell Neurosci. 2013;7:72.
Garcia LP, Witteveen JS, Middelman A, van Hulten JA, Martens GJM, Homberg JR, et al. Perturbed developmental serotonin signaling affects prefrontal catecholaminergic innervation and cortical integrity. Mol Neurobiol. 2019;56:1405–20.
Saboory E, Ghasemi M, Mehranfard N. Norepinephrine, neurodevelopment and behavior. Neurochem Int. 2020;135:104706.
Money KM, Stanwood GD. Developmental origins of brain disorders: roles for dopamine. Front Cell Neurosci. 2013;7:260.
Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–39.
Wang DD, Kriegstein AR. Defining the role of GABA in cortical development. J Physiol. 2009;587:1873–9.
Janiesch PC, Kruger HS, Poschel B, Hanganu-Opatz IL. Cholinergic control in developing prefrontal-hippocampal networks. J Neurosci. 2011;31:17955–70.
Marin-Padilla M. Structural abnormalities of the cerebral cortex in human chromosomal aberrations: a Golgi study. Brain Res. 1972;44:625–9.
Niemi MEK, Martin HC, Rice DL, Gallone G, Gordon S, Kelemen M, et al. Common genetic variants contribute to risk of rare severe neurodevelopmental disorders. Nature. 2018;562:268–71.
Torrão AS, Britto LR. Neurotransmitter regulation of neural development: acetylcholine and nicotinic receptors. An Acad Bras Cienc. 2002;74(3):453–61.
Ojeda J, Ávila A. Early actions of neurotransmitters during cortex development and maturation of reprogrammed neurons. Front Synaptic Neurosci. 2019;21(11):33.
Acknowledgements
We thank Drosophila stock center, Mysuru; National Center for Biological Sciences, Bengaluru; Dr. Gurudata V Baraka, Center for Human Genetics, Bangalore; Bloomington Drosophila Stock Center, USA; for the fly stocks. We thank DSHB, USA for the antibodies. We are thankful to the Institute of Excellence, University of Mysore, Mysuru and National Center for Biological Sciences, Bengaluru, for providing confocal and bright field imaging facility. We acknowledge the support of Dr. B.N. Rohith in confocal microscopy.
Funding
RR was supported by funds from Institute of Excellence, Project fellowship, University of Mysore, Mysuru, Karnataka, India (UOM/IOE/Project fellow 699/2019-20 Dt: 01/01/2020). Research was supported by funds from University of Mysore CAS-SAP funds, (DV4/228/UGC-CAS-1/2015-2016).
Author information
Authors and Affiliations
Contributions
BVS conceived and designed the study. CRV carried out the molecular localization experiments and did data analysis. RR carried out the expression studies immunohistochemistry and mutant analysis experiments. BVS and RR and CRV made interpretations of the data. RR prepared the Figs. 1, 2, 3. BVS and RR prepared the manuscript with contributions from all the authors, and are involved in critically reviewing and revising the analysis and interpretations. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ramya, R., Venkatesh, C.R. & Shyamala, B.V. olf413 an octopamine biogenesis pathway gene is required for axon growth and pathfinding during embryonic nervous system development in Drosophila melanogaster. BMC Res Notes 17, 46 (2024). https://doi.org/10.1186/s13104-024-06700-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-024-06700-3