Abstract
Objective
A U-shaped relationship between energy cost of walking (Cw) and walking speed indicates that there is a specific speed minimizing the Cw, called economical speed (ES). It is mostly slower in older adults than young adults; however, effects of leg length on the ES have been ignored. We investigated effects of aging and exercise habituation on the normalized ES by leg length (ESnormalized). We quantified time delay of stride length and step frequency in sedentary young (SY), active young (AY), and active elderly (AE) adults in response to sinusoidal gait speed change at 30-s and 180-s periods with an amplitude of ± 0.56 m・s− 1.
Results
The ES was significantly slower in the following sequence: AE, SY, and AY, whereas ESnormalized was slower in the AE than in other young groups, with no difference between AY and SY. AE and SY showed greater step variabilities at the 180-s period, whereas AY showed relatively smaller step variabilities at both periods. Collectively, the ESnormalized slowed due to aging, not due to exercise habituation. When optimizing the appropriate SL-SF combination for sinusoidal speed changes, young and elderly adults may adopt different strategies. Exercise habituation may reduce step variabilities in young adults.
Similar content being viewed by others
Introduction
The energy cost of walking per unit distance (Cw; J·kg− 1·m− 1) presents as a U-shaped curve as a function of walking speed (s; m·s− 1) [1,2,3]. This indicates that every individual has a specific walking speed minimizing the Cw [1,2,3,4,5,6,7,8,9,10,11,12,13], referred to as economical speed (ES) [1,2,3]. A biological importance of the ES is that it is well associated with the preferred walking speed [13] in healthy populations [3,4,5,6,7,8,9,10,11,12,13].
Using some previous data, the measured ES (ESmeasured) apparently slowed in the elderly than in the young adults [1, 9,10,11,12]. Contrary findings were also presented in other studies [13,14,15]. Thus, it is controversial whether the individual ES slowed with aging. Different study settings (e.g., measured speeds), anthropometrics (height, obesity, or fitness level), or different calculation of the ESmeasured render it difficult to obtain a consensus. Of these, anthropometrics, especially in leg length, should be reconsidered because longer leg length proportionally exhibited faster ESmeasured [1]. Most of the previous studies have not normalized the ESmeasured by leg length (ESnormalized), although leg length in association with height was normally greater in the young adults than in the elderly adults [1, 9, 10, 12, 13]. Furthermore, daily exercise habituation can be a related factor. Previous studies have compared the ESmeasured between active (or non-active) young and elderly counterparts [1, 13, 15]. Conversely, there is paucity in literatures comparing the ESnormalized between sedentary and active young adults. As the physical activity level is increased, the ESmeasured is rendered faster in the elderly adults [14, 15]. Based on these previous findings, we hypothesized that the ESnormalized would be influenced by exercise habituation even in young adults. The primary objective of this study was to investigate the effects of aging and exercise habituation on the ESnormalized among active elderly (AE), active young (AY), and sedentary young (SY) adults.
When people walk at a given speed, continuous adjustment of their limbs is required to refrain from falling. This adaptation potentially contributes to minimize the Cw [16, 17], implying that the ability to adjust lower limb motions may be associated with aging and exercise habituation. Thus, sinusoidal speed changing protocol may be useful to manipulate the lower limb motions [18,19,20,21] as it requires the continuous adjustment of stride length (SL) and step frequency (SF). These continuous adjustments play a crucial role in safely performing our daily activities. Recently, we demonstrated that SL, but not SF, showed a likely delay in response to sinusoidal speed changes even in young females walking in high-heeled shoes [21]. Furthermore, peak ankle torque decreased with aging [22]. Consequently, we further hypothesized that gait-adjusting strategies may differ among the three groups. Therefore, the secondary objective of this study was to quantify the diversity of step variabilities among these three groups.
Materials and methods
We used additional data from already published paper [19] based on an entirely different perspective. Seventeen SY, 16 AY, and 16 AE participated in this study (Table 1). Thirty-three healthy university students were classified between active and sedentary groups based on a recent guideline [23]. Elderly participants, aged over 65 years, were active members of the “Walking & Climbing Association of Fukuoka City.” All participants were nonsmokers, with no history of medication use or orthopedic and cardiorespiratory diseases. This study was approved by the ethical committee at Kyusyu Sangyo University (no. 2019-0002) and was performed under consideration of the Declaration of Helsinki. All participants signed written informed consent after being informed the purpose, experimental protocols, and possible risks.
The participants visited our laboratory twice. On their first visit, they underwent a familiarization session on a treadmill (TKK3080, Takei Scientific Instruments Co. Ltd., Niigata, Japan) at several speeds without grasping handrails of the treadmill during walking. Subsequently, we determined their individual preferred walking speed [13]. Following a 10–15 min seated rest period, they performed the ESmeasured determination protocol at 6–7 different gait speeds. These speeds were incrementally set at 0.44-0.67-0.89-1.11-1.33-1.56 m·s− 1 (elderly women), -1.67 m·s− 1 (elderly men), -1.78 m·s− 1 (young women), and − 2.00 m·s− 1 (young men) [1]. Each speed was maintained for 4-min. Oxygen uptake (VO2) and carbon dioxide (VCO2) were continuously measured using a breath-by-breath technique (AE310-S, Minato Medical Science, Osaka, Japan). To calculate the Cw, an average VO2 and VCO2 for the final 2-min at each speed was used [24].
A U-shaped relationship between Cw values and gait speeds was approximated with a quadratic Eqs. [1,2,3]:
where the coefficients a, b, and c are determined by the least squares methods. The ESmeasured, at which the U-shaped Cw-s relationship becomes minimal, can be obtained when the Cw’ (s) is zero [1,2,3]. Thus, the ESmeasured was calculated by a following equation:
The ESnormalized was calculated on the “dynamic similarity” theory [25], providing that geometrically similar legged locomotion will walk similarly at the same Froude number defined as:
where g is the gravitational acceleration (9.81 m·s− 2). Energetically optimal gait speed can be obtained when the Froude number is 0.25 [25]. Thus, the ESnormalized was calculated as follows:
One week later, biomechanical measurements were conducted on the second visit. To capture motion data, eight high-speed cameras (Kestrel300, MAC3D System, Rohnert Park, CA, USA) were set with a sampling rate of 100 Hz [20]. The participants walked at their preferred walking speed in young adults or 90% preferred walking speed in elderly adults for 30-s, thereafter, the treadmill speed was sinusoidally controlled at 30-s and 180-s periods with an amplitude of ± 0.56 m·s− 1 (± 2 km·h− 1) in a randomized order with 5-min interval. The motion data were used to determine the time delay (TD) of the SL and SF against sinusoidal speed change. The SL and SF were approximated using the following equation:
where A, ω, and t represent amplitude, angular frequency, and time (msec), respectively.
Values are mean ± standard deviation. One-way analysis of variance (ANOVA) was used for comparisons in physical characteristics, ESmeasured, and ESnormalized among three groups, respectively. Two-way (3 groups × 2 sinusoidal periods) repeated measures ANOVA was used for comparisons of the TD of SL (TDSL) and SF (TDSF). When F values were significant, Ryan’s post-hoc test, which can be used regardless of data distribution [26], was used for further analyses. The statistical significance was set at p < 0.05.
Results
Height and leg length in the AY group were greater than the other groups with no differences in body weight and body mass index among the groups (Table 1).
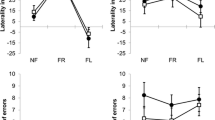
The ESmeasured was fastest in the AY group (1.361 ± 0.058 m·s− 1), followed by the SY group (1.304 ± 0.068 m·s− 1) and AE group (1.250 ± 0.061 m·s− 1), with significant differences among the groups (F = 11.781, p < 0.001, Fig. 1A). The ESnormalized was significantly slower in the AE group (0.433 ± 0.021) compared to that in the SY group (0.448 ± 0.018; t = 2.303, p = 0.026) and AY group (0.458 ± 0.018; t = 3.658, p < 0.001), with no significant difference between SY and AY (t = 1.410, p = 0.165) (Fig. 1B).
Comparisons of measured (A) and normalized (B) economical speed (ES) between sedentary young (SY; white bars), active young (AY; gray bars), and active elderly (AE; black bars) adults, respectively. Values are mean ± standard deviation. *p < 0.05 between AE and SY and between AE and AY for both ESmeasured and ESnormalized. **p < 0.05 between SY and AY for ESmeasured.
There were no significant main effects of group (F = 1.657, p = 0.202), period (F = 0.058, p = 0.811), or interaction effects (F = 2.253, p = 0.117) in the TDSL (Fig. 2A). There was a significant interaction effect in the TDSF (F = 3.889, p = 0.028, Fig. 2B), along with main effects of group (F = 3.914, p = 0.037) and period (F = 11.926, p = 0.001) (Fig. 2B). A simple main effect of period showed that the TDSF in the SY and AY groups at the 30-s period were significantly lower than those at the 180-s period, respectively (F = 7.821, p = 0.001 in the SY and F = 11.819, p = 0.001 in the AY). A post-hoc test further revealed that the TDSF in the AE group at the 180-s period was significantly lesser than in the SY group (t = 3.094, p = 0.004) and AY (t = 3.497, p < 0.001) (Fig. 2B).
Time delay of stride length (TDSL) and step frequency (TDSF) at 30-s and 180-s period among SY, AY, and AE groups. *significantly greater TDSF in the AE than in the AY and SY at the 180-s period. #significant difference in the TDSF between 30-s and 180-s within the same groups (within SY or AY, respectively). Two participants (AE = 1 and AY = 1) were excluded due to a data unavailability at the 180-s period
Discussion
On average, a relatively smaller difference was observed in the ESnormalized (~ 3.9%; Fig. 1B) than the ESmeasured (~ 6.2%; Fig. 1A) between AE and the other two groups, suggesting that leg length plays a key role in determining the individual ES. Additionally, no significant difference was observed between SY and AY in the ESnormalized (Fig. 1B), indicating that exercise habituation did not affect the ESnormalized, at least, in young adults. Thus, the first hypothesis was rejected. An age-related upward shift of the Cw-s curve is normally accompanied with a leftward shift of that curve, resulting in a slower ESmeasured [1, 9, 12]. It has been considered by a longer thigh muscle co-activation period in a gait cycle [9, 11, 27,28,29], which would be a trade-off between gait stability and energy expenditure during walking in the elderly adults. It was noteworthy noting that the Cw-s curve and/or Cw at some selected gait speeds was lower in elderly distance runners than in elderly habitual walkers [13,14,15, 30]. Remarkably, compared to healthy young adults, neither leftward nor upward shifts of the U-shaped curve were observed in elderly runners [14] and cyclists [15] compared to healthy young adults. These aerobic exercises can mitigate age-related upward and leftward shifts in the Cw-s curve [14, 15], thereby potentially avoiding a decline in the ES. Some considerations are still necessary because cycling is not a bipedal locomotion. Running exercise requires much faster optimization of the SL-SF combinations compared to walking. Notably, cycling also requires quick steering to maintain two-wheeled posture. Thus, such a postural adjustment ability during cycling may extend to gait stability because quick optimization of the SL-SF combinations can reduce Cw [16, 17]. Both previous studies and our current findings suggest that habitual exercise may be able to mitigate age-related deterioration of the individual ES.
Step width of the AE in our original study was not different from that of younger counterparts [20]. Instead, elderly adults normally present a shorter SL compared to the young adults [31]. In that case, a faster SF is necessary because gait speed should correspond to the product of the SL and SF. During sinusoidal speed changing condition, quick optimization of the SL and SF is continuously required to catch up with the speed change. In support of our second hypothesis, we observed that the variability of TDSL in the SY group (Fig. 2A) and TDSF in the AE group (Fig. 2B) was notably high at the 180-s period. Variability in physiological responses during exercise, such as heart rate variability, typically reflects exercise tolerance [32]. Therefore, these greater variabilities may indicate a greater locomotive flexibility to optimize SL-SF combinations in response to passive gait speed changes. However, excessive gait variability is associated with an increase in fall risks [33] and Cw [16, 17]. Thus, these greater variabilities of TDSL in the SY group (Fig. 2A) and TDSF in the AE group (Fig. 2B) at the 180-s period suggest that strategies for optimizing appropriate SL-SF combinations against sinusoidal speed changes differ between SY and AE. That is, SY adopted by manipulating SF (in particular, by preceding the SF), whereas AE adopted by manipulating SL. In contrast, AY exhibited relatively smaller variabilities in TDSL and TDSF at both periods (Fig. 2A and B), indicating that exercise habituation tends to reduce step variabilities in young adults.
Limitations
Technological limitations should be stated. Two comparative studies presented phase shift in degree [18, 19], equivalent to the TDSL and TDSF in the present study. Surprisingly, both TDSF and TDSL in healthy young adults showed negative values over 4-s in SF and 1.5-s in SL even at 60-s sinusoidal speed changing protocol [18, 19]. However, the trend of our results completely different from those of our previous studies (Fig. 2). This could be attributed to the different calculation techniques. Both previous studies used interpolated 1-s data for calculating the phase shift of the SF and SL. That is, TDSL and TDSF values with a sampling frequency of 1.0 Hz were treated based on the discrete Fourier transform. If SF and/or SL variabilities occurred above 0.5 Hz that corresponded to the Nyquist folding frequency of the original sampling frequency (1.0 Hz), those variabilities could contaminate low-frequency spectrums, so-called “aliasing” [34].
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AE:
-
active elderly
- ANOVA:
-
analysis of variance
- AY:
-
active young
- C w :
-
energy cost of walking
- ESmeasured :
-
measured economical speed
- ESnormalized :
-
normalized economical speed
- s :
-
speed
- SD:
-
standard deviation
- SF:
-
step frequency
- SL:
-
stride length
- SY:
-
sedentary young
- TD:
-
time delay
- TDSF :
-
time delay of step frequency
- TDSL :
-
time delay of stride length
- VO2 :
-
oxygen uptake
- VCO2 :
-
carbon dioxide output
References
Horiuchi M, Endo J, Horiuchi Y, Abe D. Comparisons of energy cost and economical walking speed at various gradients in healthy, active younger and older adults. J Exerc Sci Fit. 2015;13(2):79–85.
Abe D, Fukuoka Y, Horiuchi M. Muscle activities during walking and running at energetically optimal transition speed under normobaric hypoxia on gradient slopes. PLoS ONE. 2015;10(9):e0138154.
Abe D, Sakata S, Motoyama K, Toyota N, Nishizono H, Horiuchi M. Economical and preferred walking speed using body weight support apparatus with a spring-like characteristics. BMC Sports Sci Med Rehabil. 2021;13(1):107.
Wezenberg D, van der Woude LH, Faber WX, de Haan A, Houdijk H. Relation between aerobic capacity and walking ability in older adults with a lower-limb amputation. Arch Phys Med Rehabil. 2013;94(9):1714–20.
Browning RC, Kram R. Energetic cost and preferred speed of walking in obese vs. normal weight women. Obes Res. 2005;13(5):891–9.
Wall-Scheffler CM, Myers MJ. Reproductive costs for everyone: how female loads impact human mobility strategies. J Hum Evol. 2013;64(5):448–56.
DeJaeger D, Willems PA, Heglund NC. The energy cost of walking in children. Pflügers Arch. 2001;441(4):538–43.
Bastien GJ, Willems PA, Schepens B, Heglund NC. Effect of load and speed on the energetic cost of human walking. Eur J Appl Physiol. 2005;94(1–2):76–83.
Pincheira PA, Stenroth L, Avela J, Cronin NJ. Individual leg muscle contributions to the cost of walking: Effects of age and walking speed. J Aging Phys Act. 2017;25(2):295–304.
Malatesta D, Simar D, Dauvilliers Y, Candau R, Borrani F, Prefaut C, et al. Energy cost of walking and gait instability in healthy 65- and 80-yr-olds. J Appl Physiol. 2003;95(6):2248–56.
Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol. 2006;186(2):127–39.
Ortega JD, Farley CT. Individual limb work does not explain the greater metabolic cost of walking in elderly adults. J Appl Physiol. 2007;102(6):2266–73.
Martin PE, Rothstein DE, Larish DD. Effects of age and physical activity status on the speed-aerobic demand relationship of walking. J Appl Physiol. 1992;73(1):200–6.
Ortega JD, Beck ON, Roby JM, Turney AL, Kram R. Running for exercise mitigates age-related deterioration of walking economy. PLoS ONE. 2014;9(11):e113471.
Aslan DH, Collette JM, Ortega JD. Bicycling exercise helps maintain a youthful metabolic cost of walking in older adults. J Aging Phys Act. 2021;29(1):36–42.
Selinger JC, O’Connor SM, Wong JD, Donelan JM. Humans can continuously optimize energetic cost during walking. Curr Biol. 2015;25(18):2452–6.
Rock CG, Marmelat V, Yentes JM, Siu KC, Takahashi KZ. Interaction between step-to-step variability and metabolic cost of transport during human walking. J Exp Biol. 2018;221:jeb181834.
Fukuoka Y, Iihoshi M, Nazunin JT, Abe D, Fukuba Y. Dynamic characteristics of ventilatory and gas exchange during sinusoidal walking in humans. PLoS ONE. 2017;12(1):e0168517.
Fujita M, Kamibayashi K, Aoki T, Horiuchi M, Fukuoka Y. Influence of step frequency on the dynamic characteristics of ventilation and gas exchange during sinusoidal walking in humans. Front Physiol. 2022;13:820666.
Abe D, Motoyama K, Tashiro T, Saito A, Horiuchi M. Effects of exercise habituation and aging on the intersegmental coordination of lower limbs during walking with sinusoidal speed change. J Physiol Anthropol. 2022;41(1):24.
Abe D, Tashiro T, Motoyama K, Horiuchi M, Saito A. Gait variability of interlimb coordination in high-heeled shoes with detachable base sockets under conditions of sinusoidal speed change. Gait Posture. 2023;106:85–90.
Franz JR, Thelen DG. Imaging and simulation of Achilles tendon dynamics: implications for walking performance in the elderly. J Biomech. 2016;49(9):1403–10.
World Health Organization. WHO guidelines on physical activity and sedentary behaviour. 2020. https://www.who.int/publications/i/item/9789240015128.
Brouwer E. On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (oxygen intake and carbonic acid output) and urine-N. Acta Physiol Pharmacol Neerl. 1957;6:795–802.
Alexander RM, Jayes AS. A dynamic similarity hypothesis for the gaits of quadrupedal mammals. J Zool. 1983;201:135–52.
Ryan TH. Significance tests for multiple comparison of proportions, variances, and other statistics. Psychol Bull. 1960;57:318–32.
Peterson DS, Martin PE. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture. 2010;31(3):355–9.
Hortobagyi T, Finch A, Solnik S, Rider P, DeVita P. Association between muscle activation and metabolic cost of walking in young and old adults. J Gerontol A Biol Sci Med Sci. 2011;66(5):541–7.
Franz JR, Kram R. How does age affect leg muscle activity/coactivity during uphill and downhill walking? Gait Posture. 2013;37(3):378–84.
Beck ON, Grabowski AM, Ortega JD. Neither total muscle activation nor co-activation explains the youthful walking economy of older runners. Gait Posture. 2018;65:163–4.
Shkuratova N, Morris ME, Huxham F. Effects of age on balance control during walking. Arch Phys Med Rehabil. 2004;85(4):582–8.
Yamamoto Y, Hughson RL, Nakamura Y. Autonomic nervous system responses to exercise in relation to ventilatory threshold. Chest. 1992;101(Suppl):206S–10S.
Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehabil. 2005;2:21.
Mitchell DP, Netravali AN. Reconstruction filters in computer-graphics. ACM SIGGRAPH ‘88: Proc 15th Ann Conf CG Interactive Tech. 1988;22(4):221–8.https://dl https://doi.org/10.1145/54852.378514
Acknowledgements
We specially thank Mr. Akinobu Sakamoto, Mr. Tomokazu Iwatani, Mr. Hiromichi Ikegami, Mr. Takeshi Saito, Mr. Masaru Hashimura, and Mr. Shizuo Takatoh (Takei Scientific Instruments Co., Ltd.) for customizing the treadmill to control the gait speed sinusoidally. We also thank Enago for English editing.
Funding
This study was financially supported by Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JP19K11541 and JP22K11517 to DA, JP20K19623 to KM, JP21K17613 to AS, and JP18K11012 to MH). This study was also supported by Grant-in-Aid for Kyushu Sangyo University (KSU) Scientific Research and Encouragement of Scientists (K035124 and R035027 to DA). JP19K11541, JP22K11517 and K035124 to DA were used for equipment and software installations. R035027 to DA was used for paying participants’ reward and purchasing most of consumable supplies. JP20K19623 to KM and JP21K17613 to AS were used for the initial data analysis. JP18K11012 to MH was used for English editing service.
Author information
Authors and Affiliations
Contributions
All authors designed the original study settings on consensus. DA, KM, MH, and TT performed measurements. DA, TT, MH, AS, and KM analyzed and interpreted the data. MH and DA prepared figure presentations and drafted the first manuscript. DA and AS revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was approved by the ethical committee at Kyusyu Sangyo University (no. 2019-0002) and was carried out under consideration of the Declaration of Helsinki. All participants provided written informed consent after being informed the purpose, experimental protocols, and possible risks.
Consent for publication
Not appreciable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Horiuchi, M., Saito, A., Motoyama, K. et al. Normalized economical speed is influenced by aging and not by exercise habituation. BMC Res Notes 16, 254 (2023). https://doi.org/10.1186/s13104-023-06545-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-023-06545-2