Abstract
Objectives
This study investigated the effects of levothyroxine replacement therapy on insulin resistance, lipid profile, and thyroid function in patients with untreated primary hypothyroidism. 105 patients with hypothyroidism with indication for levothyroxine replacement were enrolled in the present study. Insulin, fasting blood glucose and lipid profile were assessed at the beginning of diagnosis and three months after levothyroxine replacement. Insulin resistance was calculated by hemostasis model assessment of insulin resistance (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI).
Results
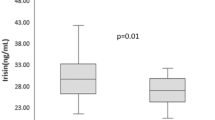
Our data revealed a significant reduction in body mass index (27.18 ± 4.27 versus 26.81 ± 4.18 kg/m2, p = 0.028), cholesterol (199.79 ± 37.61 versus 178.10 ± 32.25 mg/dl, p < 0.001), triglyceride (160.41 ± 71.86 versus 146 ± 61.11 mg/dl, p = 0.012), low density lipoprotein-cholesterol (123.54 ± 30.7 versus 107.08 ± 26.98 mg/dl, p < 0.001), fasting insulin (8.91 ± 3.92 versus 8.05 ± 2.65 mIU/l, p < 0.001), and thyroid stimulating hormone (47.47 ± 3.4 versus 2.22 ± 1.84 µIU/ml, p < 0.001) levels before and after drug intervention. However, no statistical differences were observed in HOMA-IR, QUICKI, and high density lipoprotein-cholesterol. In conclusion, in patients with untreated primary hypothyroidism, levothyroxine replacement therapy based on HOMA-IR and QUICKI did not improve insulin resistance; however, lipid profile was significantly improved following levothyroxine administration.
Trial registration
This study was registered in the Iranian Registry of Clinical Trials (IRCT) with ID number: IRCT20130610013612N10 on the date 2019-09-02.
Similar content being viewed by others

Introduction
Insulin resistance (IR) and the metabolic syndrome are complex metabolic traits defined as decreased response to insulin in tissues. Insulin is an endocrine peptide hormone which controls whole-body glucose homeostasis. It has been reported that both genetic and environmental factors are known to influence IR [1].
Thyroid hormones influence glucose homeostasis and the development of IR. They also upregulate genes such as glucose transporter type-4 (GLUT-4) and phosphoglycerate kinase that encode enzymes stimulating glycogenolysis and gluconeogenesis, thereby increasing the hepatic glucose production [2]. Recently, evidence has been shown that the sympathetic nerves can also stimulate hepatic glucose production, in addition to the direct effect of thyroid hormones [3]. Many studies have also reported the association between IR and hypothyroidism [2, 4, 5]. Hypothyroidism and hyperthyroidism constitute an IR state. However, the mechanism of each is different. So that, in hyperthyroidism, IR is predominant at the hepatic level and, in hypothyroidism, IR is at the level of peripheral tissues [6].
IR is the basis of metabolic syndrome pathophysiology, which is itself a risk factor for cardiovascular diseases (CVDs) [7]. It has been reported that subclinical hypothyroid with the thyroid stimulating hormone (TSH) levels ≥ 10 µIU/ml is associated with an increased risk of CVD and mortality [8].
The association between subclinical hypothyroidism and IR has also been shown in some previous studies [1, 5, 9]. However, other studies have not reported such relationship [10]. Mazaheri et al. [11] also compared IR in patients with Hashimoto’s hypothyroidism and hypothyroidism after thyroidectomy; however, no significant difference was observed between two groups.
Hormone replacement therapy in early hypothyroidism has also reduced IR in several studies [12, 13], although, other studies reported no significant effects in this regard [14, 15]. Thyroid hormones also play a significant role in 3-Hydroxy-3-methylglutaryl–coenzyme A (HMG-CoA) reductase induction as the main enzyme in cholesterol biosynthesis. Moreover, triiodothyronine (T3) hormone by inducing the low density lipoprotein-cholesterol (LDL-C) receptor gene plays a crucial role in LDL-C clearance from blood circulation [16, 17]. It has previously reported that total cholesterol and LDL-C levels are increased in patients with hypothyroidism [18]. Hence, hypothyroidism is a common cause of secondary dyslipidemia [19, 20]. Additionally, the activity of the lipoprotein lipase enzyme is reduced in hypothyroidism; therefore, hypothyroidism may be associated with higher triglyceride levels [21,22,23]. On the other hand, the association between hypothyroidism and ischemic heart disease has also been reported by several studies [24, 25]. Since, IR can be considered as the underlying cause in different diseases and there are controversial results regarding the association between thyroid hormone levels and IR, the present clinical trial was designed to estimate the effects of levothyroxine replacement therapy on IR, lipid profile, and thyroid function in patients with untreated primary hypothyroidism.
Main text
Materials and methods
Study setting
The present interventional clinical trial (IRCT20130610013612N10) was performed on patients diagnosed with hypothyroidism with levothyroxine treatment indication referred to the endocrinology clinic of Imam Reza Hospital and Sheikh Al-Reis Clinic at Tabriz University of Medical Sciences. This trial was approved by the Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran (Ethics code: TBZMED. REC.1398.457). All patients signed an informed consent form at baseline. All methods were carried out in accordance with Consort guidelines and regulations.
Patients with primary hypothyroidism or previously untreated or uncontrolled hypothyroidism aged between 18 and 65 years were included in the present study. Subjects with metabolic syndrome could also participate in this study. Diabetes mellitus (fasting blood glucose (FBG) ≥ 126 mg/dL) or familial history of diabetes, heart failure, liver and kidney diseases and polycystic ovary syndrome, pregnancy, lactation and consumption of levothyroxine, statins and contraceptive pills during the last three months before the study were also considered as exclusion criteria.
Endocrinologist decided the doses of levothyroxine substitution and the target TSH was 0.5–4.5 miu/l. In participants, all parameters were assessed at the beginning of diagnosis and three months after levothyroxine treatment (1.7 mg/kg of body weight daily). This study adheres to CONSORT guidelines.
Sample size
A total of 105 patients were calculated using the mean (± standard deviation [SD]) of TSH obtained from the study of Nada AM [11], based on a confidence interval (CI) of 95% and power of 90% in 2-sided tests. We used the following statistical formula for calculation of sample size.
Blood sampling, biochemical measurements, and anthropometric measurements
After 12–14 h overnight fasting, the participants’ blood samples were collected and centrifuged at 3,000 rpm for 5 min to extract the serum. Serum FBG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglyceride were measured using colorimetric-enzymatic methods using commercial kits (ParsAzmoon Co., Tehran, Iran). Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald equation. The enzyme-linked immunosorbent assay (ELISA) method was used to measure serum insulin using commercial kits (Monobind, Lake Forest, CA, USA). IR was also calculated by hemostasis model assessment of IR (HOMA-IR) and the quantitative insulin sensitivity check index (QUICKI). The serum TSH and free T4 (FT4) concentrations were measured by electrochemiluminescence assay.
After an overnight fasting, height and weight were measured to the nearest 0.1 cm and 0.1 kg, respectively, with light clothes and no shoes using a calibrated scale and stadiometer (Seca, Germany). Body mass index (BMI) was estimated as weight (Kg) divided by height squared (m2).
Statistical analysis
SPSS software (statistical package for social analysis, ver. 20, SPSS Inc., Chicago, IL, USA) was used for data analysis. Mean values were compared using paired T-test between groups. P < 0.05 was also considered as statistically significant.
Results
Table 1 shows the characteristics of the study participants. In this interventional clinical trial study, 105 subjects (males = 34.28% and females = 65.72%) were enrolled. Metabolic syndrome was diagnosed in 61.11% of males and 63.76% of females (p = 0.791).
Table 2 shows BMI, biochemical, IR indices and thyroid function tests (TFT) before and after levothyroxine administration. Significant differences were observed in BMI (27.18 ± 4.27 versus 26.81 ± 4.18 kg/m2, p = 0.028), cholesterol (199.79 ± 37.61 versus 178.10 ± 32.25 mg/dl, p < 0.001), triglyceride (160.41 ± 71.86 versus 146 ± 61.11 mg/dl, p = 0.012), LDL-C (123.54 ± 30.7 versus 107.08 ± 26.98 mg/dl, p < 0.001), fasting insulin (8.91 ± 3.92 versus 8.05 ± 2.65 mIU/l, p < 0.001), TSH (47.47 ± 3.4 versus 2.22 ± 1.84 µIU/ml, p < 0.001) and FT4 (0.8 ± 0.19 versus 1.20 ± 0.44 ng/dl, p < 0.001) before and after drug intervention. No significant differences were observed regarding the HDL-C, FBG, HOMA- IR and QUICKI.
Discussion
In the literature, there are two main non-invasive (HOMA-IR and QUICKI) [26] and invasive (hyperinsulinemic-euglycemic clamp technique) methods for IR assay [27]. In the present study, HOMA-IR and QUICKI were used to evaluate IR. Our data revealed an increased basal HOMA-IR level which was in line with the study of Estaghamati et al. [28] with the estimated 1.78 HOMA-IR level.
After receiving levothyroxine and euthyroidism induction, the rate of IR in our patients did not significantly change; although, a significant decreased BMI level was reached among patients. Similarly, study of Brenta et al. [10] showed that treatment with levothyroxine did not reduce the IR. Teixeira et al. [18, 29] also showed no improvement in IR and BMI levels after levothyroxine treatment. However, Stanicka et al. [8] and Handisurya et al. [9] reported a significant decrease in IR after levothyroxine treatment. Similar results were also reported by Kowalska et al. [15], Deyneli et al. [17] and Rochon et al. [20] regarding the improved IR after levothyroxine administration. In another study conducted by Brenta et al. [16], decreased IR was also observed after this drug administration which all mentioned studies used hyperinsulinemic-euglycemic clamp technique to evaluate IR. Generally, in patients with hypothyroidism before and after levothyroxine replacement therapy, no significant changes in IR have been reported using HOMA-IR as IR indicator which are in line with our finding [10, 11, 18]. However, in studies using hyperinsulinemic-euglycemic clamp technique to evaluate IR, levothyroxine replacement has led to significant reduction in IR [8, 9, 15,16,17, 20]. Hypothyroidism causes peripheral IR, and hyperinsulinemic-euglycemic clamp technique is the more exact method for its evaluation. However, the number of studies using this technique is limited because of its difficulties and side effects.
In study of Stanicka et al. [8], despite non-significant change in basal insulin levels and C-peptide, by glucose disposal rate determination, a decreased IR has been reported following levothyroxine replacement, and due to the increase in other examined hormones, they concluded that hypothyroidism may increase IR with the other mechanisms such as increased counter regulatory hormones levels. In other words, IR is usually observed as impaired glucose tolerance, and increased blood glucose and insulin levels after meals or after glucose tolerance testing are likely to have more accurate results than that of blood glucose and fasting insulin levels which are used in HOMA-IR and QUICKI tests.
Different studies have shown that levothyroxine replacement significantly improves lipid metabolism disorders, and a treatment period of 4 to 6 weeks is needed to correct dyslipidemia in hypothyroidism. The rate of dyslipidemia improvement is related to FT4 levels. Recent studies in hypothyroidism patients have also shown decreased serum total cholesterol and LDL-C levels after levothyroxine treatment.
In our study, thyroid hormones replacement improved dyslipidemia in patients. Levothyroxine replacement therapy in hypothyroid patients has previously been shown to improve cholesterol and LDL-C levels and increase HDL-C levels. In our study, levothyroxine treatment also caused a significant decrease in cholesterol, triglyceride and LDL-C levels. In the study of Brenta et al. [10] levothyroxine treatment did not correct the basal lipid derangement. In contrast, in a study conducted by Aml Mohamed Nada [11], a significant decrease in cholesterol level was observed after levothyroxine treatment; however, triglyceride level did not alter significantly. Deyneli et al. [17] also reported decreased cholesterol and LDL-C levels after levothyroxine administration.
Limitations
The limitations of our study were a small number of hypothyroid patients and the loss of nutritional and lifestyle data. Given that our study included a small number of subjects, we were unable to completely show the effects of replacement therapy. Moreover, our study duration was short; longer periods might reveal different results. Future studies with larger sample sizes and longer duration are recommended.
Conclusion
In the present study, levothyroxine replacement therapy in hypothyroid patients led to significant improvements in lipid profile and BMI. However, levothyroxine replacement therapy based on HOMA-IR and QUICKI methods did not improve IR. Since, mentioned methods may not have the enough sensitivity for evaluation of T4 therapy in mild IR cases, other tests with higher sensitivity are proposed.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zierath J, Krook A, Wallberg-Henriksson H. Insulin action and insulin resistance in human skeletal muscle. Diabetologia. 2000;43(7):821–35.
Kapadia KB, Bhatt PA, Shah JS. Association between altered thyroid state and insulin resistance. J Pharmacol Pharmacother. 2012;3(2):156–60.
Basarikatt R. Study of insulin in subclinical hypothyroidism patients. Indian J Sci Res. 2016;7(1):155–8.
Enzevaei A, Salehpour S, Tohidi M, Saharkhiz N. Subclinical hypothyroidism and insulin resistance in polycystic ovary syndrome: is there a relationship? Iran J Reprod Med. 2014;12(7):481–6.
Singh BM, Goswami B, Mallika V. Association between insulin resistance and hypothyroidism in females attending a tertiary care hospital. Indian J Clin Biochem. 2010;25(2):141–5.
Mazaheri T, Sharifi F, Kamali K. Insulin resistance in hypothyroid patients under levothyroxine therapy: a comparison between those with and without thyroid autoimmunity. J Diabetes Metab Disord. 2014;13(1):103–9.
Vyakaranam S, Vanaparthy S, Nori S, Palarapu S, Bhongir AV. Study of insulin resistance in subclinical hypothyroidism. Int J Health Sci Res. 2014;4(9):147–53.
Stanická S, Vondra K, Pelikánová T, Vlček P, Hill M, Zamrazil V. Insulin sensitivity and counter-regulatory hormones in hypothyroidism and during thyroid hormone replacement therapy. Clin Chem Lab Med (CCLM). 2005;43(7):715–20.
Handisurya A, Pacini G, Tura A, Gessl A, Kautzky-Willer A. Effects of T4 replacement therapy on glucose metabolism in subjects with subclinical (SH) and overt hypothyroidism (OH). Clin Endocrinol. 2008;69(6):963–9.
Brenta G, Berg G, Arias P, Zago V, Schnitman M, Muzzio ML, Sinay I, Schreier L. Lipoprotein alterations, hepatic lipase activity, and insulin sensitivity in subclinical hypothyroidism: response to L-T4 treatment. Thyroid. 2007;17(5):453–60.
Nada AM. Effect of treatment of overt hypothyroidism on insulin resistance. World J Diabetes. 2013;4(4):157–61.
Gierach M, Gierach J, Junik R. Insulin resistance and thyroid disorders. Endokrynol Pol. 2014;65(1):70–6.
Eckel RH, Grundy SM, PZ Z. The metabolic syndrome. Lancet. 2005;365:1415–28.
Abdel-Gayoum A. The effect of hypothyroidism on insulin sensitivity and their influence on the serum lipid profile and renal function. Endocrinol Metab Syndr. 2016;5(248):2161–10171000248.
Kowalska I, Borawski J, Nikołajuk A, Budlewski T, Otziomek E, Górska M, Strączkowski M. Insulin sensitivity, plasma adiponectin and sICAM-1 concentrations in patients with subclinical hypothyroidism: response to levothyroxine therapy. Endocrine. 2011;40(1):95–101.
Brenta G, Celi FS, Pisarev M, Schnitman M, Sinay I, Arias P. Acute thyroid hormone withdrawal in athyreotic patients results in a state of insulin resistance. Thyroid. 2009;19(6):665–9.
Deyneli O, Akpınar İN. Meriçliler ÖS, Gözü H, Yıldız ME, Akalın NS, editors. Effects of levothyroxine treatment on insulin sensitivity, endothelial function and risk factors of atherosclerosis in hypothyroid women. Ann Endocrino 2014; 75(4):220–226.
Teixeira PF, Cabral MD, Silva NA, Soares DV, Braulio VB, Couto APC, Henriques JLM, Costa AJL, Buescu A, Vaisman A. Serum leptin in overt and subclinical hypothyroidism: effect of levothyroxine treatment and relationship to menopausal status and body composition. Thyroid. 2009;19(5):443–50.
Klieverik LP, Janssen SF, van Riel A, Foppen E, Bisschop PH, Serlie MJ, Boelen A, Ackermans T, Sauerwein MP, Fliers H, Kalsbeek E. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc Natl Acad Sci U S A. 2009;106(14):5966–71.
Rochon C, Tauveron I, Dejax C, Benoit P, Capitan P, Fabricio A, Berry C, Champredonc C, Thieblot P, Grizar J. Response of glucose disposal to hyperinsulinaemia in human hypothyroidism and hyperthyroidism. Clin Sci. 2003;104(1):7–15.
Monzani F, Caraccio N, Kozakowa M, Dardano A, Vittone F, Virdis A, Taddei S, Palombo C, Ferrannini E. Effect of levothyroxine replacement on lipid profile and intima-media thickness in subclinical hypothyroidism: a double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2004;89(5):2099–106.
Tièche M, Lupi GA, Gutzwiller F, Grob PJ, Studer H, Bürgi H. Borderline low thyroid function and thyroid autoimmunity. Risk Factors for Coronary Heart Disease? Heart. 1981;46(2):202–6.
Perk M, O’Neill BJ. The effect of thyroid hormone therapy on angiographic coronary artery disease progression. Can J Cardiol. 1997;13(3):273–6.
Bakker O, Hudig F, Meijssen S, Wiersinga WM. Effects of triiodothyronine and amiodarone on the promoter of the human LDL receptor gene. Biochem Biophys Res Commun. 1998;249(2):517–21.
Faure P, Oziol L, Artur Y, Chomard P. Thyroid hormone (T3) and its acetic derivative (TA3) protect low-density lipoproteins from oxidation by different mechanisms. Biochimie. 2004;86(6):411–8.
Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab. 2015;19(1):160–4.
Kim JK. Hyperinsulinemic–euglycemic clamp to assess insulin sensitivity in vivo. Type 2 diabetes. Springer; 2009. pp. 221–38.
Esteghamati A, Ashraf H, Khalilzadeh O, Zandieh A, Nakhjavani M, Rashidi A, Haghazli M, Asgari F. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr Metab. 2010;7(1):26–34.
Lee S, Braverman L, Pearce E. Changes in body weight after treatment of primary hypothyroidism with levothyroxine. Endocr Pract. 2014;20(11):1122–8.
Acknowledgements
We would like to appreciate the cooperation of the Clinical Research Development Unit of Imam Reza General Hospital, Tabriz, Iran in conducting this research.
Funding
This research was funded by the Endocrine Research Center of Tabriz University of Medical Sciences [Grant No.63302].
Author information
Authors and Affiliations
Contributions
A.O, T.M, M.M, F.N, and V.S designed research and contributed to the conception of the project, development of overall research plan, and study oversight. T.M contributed to data collection and was the author of the research. S.G. and H.T. drafted the manuscript. All approved the final version of this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of Tabriz University of Medical Science. In this study all subjects signed a consent form and the study protocol was approved by the Ethical Committee of Tabriz University of Medical Sciences (Ethics code: TBZMED. REC.1398.457).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ostadrahimi, A., Manzari, T., Gohari-Lasaki, S. et al. Effects of levothyroxine replacement therapy on insulin resistance in patients with untreated primary hypothyroidism. BMC Res Notes 16, 237 (2023). https://doi.org/10.1186/s13104-023-06516-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-023-06516-7