Abstract
Objective
A mammalian Delta-Notch signaling component, Notch1, has been suggested for its expression during the normal sperm development although its conditional deletion caused no apparent abnormalities. Since we established our original transgenic mouse system that enabled labeling of past and ongoing Notch1 signaling at a cellular level, we tried to validate that observation in vivo. Our transgenic mouse system used Cre/loxP system to induce tandem dsRed expression upon Notch1 signaling.
Results
To our surprise, we were unable to observe tandem dsRed expression in the seminiferous tubules where the sperms developed. In addition, tandem dsRed expression was lacking in the somatic cells of the next generation in our transgenic mouse system, suggesting that sperms received no Notch1 signaling during their development. To validate this result, we conducted re-analysis of four single-cell RNA-seq datasets from mouse and human testes and showed that Notch1 expression was little in the sperm cell lineage. Collectively, our results posed a question into the involvement of Notch1 in the normal sperm development although this observation may help the interpretation of the previous result that Notch1 conditional deletion caused no apparent abnormalities in murine spermatogenesis.
Similar content being viewed by others
Introduction
Notch1 is a component of the mammalian Delta-Notch signaling pathway that consists of five Delta ligands (Delta-like ligands 1, 3, and 4 and Jagged 1 and 2) and Notch receptors (Notch 1, 2, 3 and 4) [1]. Upon binding with Delta ligands, the Notch intracellular domain is cleaved, translocates to the nucleus, and acts as a transcription factor to induce specific genes. These genes include Hes/Hey family proteins that are expressed in many organs including inner ear and cerebellum [2, 3]. Although it has been suggested that the intracellular domains of Notch1 and Notch2 were similar and interchangeable with each other, the loss-of-function effect to organ development is different by the Notch receptor subtypes owing to the difference of their extracellular domains [4, 5]. Therefore, it should be important to distinguish the contribution of each Notch receptor by their subtypes.
Concerning gamete formation, this signaling pathway is involved in nematodes. For example, in Caenorhabditis elegans, genetic deletion of germline proliferation-1 (GLP-1) Notch receptor allowed all germline stem cells to enter the meiotic cell cycle, suggesting that this signaling pathway is necessary for maintenance of germline stem cells [6]. In Drosophila melanogaster, the numbers of germline cells or supporting cells were decreased by reduction of Delta ligand or Notch receptor expression, respectively [7]. In Xenopus laevis, migration of the primordial germ cells to the future gonad was disrupted by X-Delta-2 knockdown [8].
The mammalian spermatogenesis involves multiple differentiation steps including spermatogonial cells (before meiosis), spermatocytes (during meiosis), spermatids (after meiosis) and mature sperm (spermatozoa) that are supported by the gonadal cells other than testicular germ cells such as Sertoli cells, Leydig cells and interstitial cells [9]. The involvement of Notch1 in the murine spermatogenesis was studied by Huang et al. using the transgenic mice that enabled spermatocytes-specific gain-of-function and loss-of-function [10]. Concretely, since immunohistochemistry for Notch1 suggested its expression in the wild-type mouse spermatocytes, they investigated the effect of spermatocyte-specific gain-of-function of Notch1 by using stra8-iCre mouse and showed that that led to impaired spermatogenesis. In the same report, however, loss-of-function of Notch1 in the spermatocytes caused no apparent dysfunction in spermatogenesis. Given that Huang et al. used no primary antibody negative control instead of the testis samples from stra8-iCre-mediated spermatocyte-specific Notch1 conditional knockout mice, the contribution of Notch1 to spermatogenesis in the wild type mice is still unclear. In addition, Hayashi et al. reported the presence or absence of Notch1 in the spermatocytes of the rat testis or human patients with arrested spermatocyte maturation, respectively [11]. Owing to lack of knockout study in that report, however, it is still controversial whether the absence of Notch1 in those human patients was the cause of the arrested spermatocyte maturation.
To examine the cell lineage receiving Notch1 signaling in the mammalian testis, we combined experimental and bioinformatics methods. Recently, we established a transgenic mouse system that enabled visualization of past and ongoing Notch1 signaling that consisted of three transgenic mouse lines. The first transgenic mouse expresses Notch1 receptor of which intracellular domain is replaced with a transcription factor, Gal4VP16 (N1-Gal4VP16 mouse) [12]. The second transgenic mouse expresses Cre recombinase upon the binding of Gal4VP16 to its upstream activating sequences (UAS) (UAS-Cre mouse) [13]. The third transgenic mouse expresses EGFP or tandem dsRed (tdsRed) before and after Cre-mediated recombination, respectively (R26GRR mouse) [14]. Therefore, in N1-Gal4VP16; UAS-Cre; R26GRR mouse, past and ongoing Notch1 signaling could be labeled with tdsRed or Cre recombinase, respectively. Importantly, since the transgenic mice used in the present study were the transgenic mouse in the narrowest sense (N1-Gal4VP16 mouse and UAS-Cre mouse) or ROSA26 locus-targeted knock-in mouse (R26GRR mouse), the expression levels of Notch1 were assumed to be comparable to that of the wild-type mouse. Using this transgenic mouse system, we examined past and ongoing Notch1 signaling in the murine sperm cell lineage.
Unfortunately, immunohistochemistry for Notch1 was technically difficult to us, we evaluated our result from the transgenic mouse experiments by re-analyzing four series of the previously published single-cell RNA-seq (scRNAseq) data (GSE104556 and GSE112393 from mouse testes) (GSE124263 and GSE142585 from human testes) [15,16,17,18].
Main text
Materials and methods
Animal Welfare
Animal experiments were carried out in accordance with the Regulation for Animal Experiments in our university and Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology. Approval was obtained from the Institutional Animal Care and Use Committee and the DNA Experiment Committee of the University of Tsukuba (Approval Numbers for Animal Experiments: 22–059) (Approval Number for DNA Experiments: 220,018). UAS-Cre mice are available from RIKEN through the National BioResource Project of Japan: Crl:CD1(ICR)-Tg(UAS-cre/T2A/miRFP670)216Staka (No. RBRC11716). In addition, R26GRR mice are also available from RIKEN through the National BioResource Project of Japan: C57BL/6 N-Gt(ROSA)26Sor < tm1(CAG-EGFP/tDsRed)Utr>/Rbrc (No. RBRC04874).
Animal experiment
The post-weaning mice (4–23 weeks old) were used for histological (n = 3) and PCR (n = 6) examination. PCR templates (n = 6) were derived from a single N1-Gal4VP16; UAS-Cre; R26GRR father. When sampling the embryos at embryonic day 14.5 (E14.5), the pregnant mother was euthanized by cervical dislocation and the embryos were immediately fixed in ice-cold Mildform 10 N (Cat #: 133-10311, Wako, Osaka, Japan). PCR primers for the detection of EGFP coding region were 5’-AGCAAGGGCGAGGAGCTGTTCACC-3’ (forward) and 5’-TGCCGTCGTCCTTGAAGAAGATG-3’ (reverse). In prior to sampling the organs, mice were sacrificed by cervical dislocation and perfused with PBS and Mildform 10 N. For fluorescent imaging, 10 μm thick frozen sections were counterstained with Hoechst 33,342 (Cat #: H3570, Invitrogen, Waltham, MA, USA). For immunohistochemistry, 4 μm thick paraffin sections were incubated with or a rabbit monoclonal anti-Cre recombinase antibody (clone: D7L7L, Cat #: 15,036 S, RRID: AB_2798694, Cell Signaling Technology, Danvers, MA, USA), Histofine SimpleStain (Cat #: 414,341, Nichirei Biosciences, Tokyo, Japan), and Histofine DAB Substrate Kit (Cat #: 425,011, Nichirei Biosciences), followed by counterstaining with Mayer’s hematoxylin solution (Cat #: 131–09665, Wako). Images were captured by using BIOREVO-BZ-X810 (Keyence, Osaka, Japan).
ScRNA-seq analysis
We downloaded the four datasets of the previously published scRNAseq (GSE104556 and GSE112393 from mouse testes) (GSE124263 and GSE142585 from human testes) [15,16,17,18] from NCBI Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/). We used Scanpy (v1.7.0) [19] to conduct quality control, dimension reduction with principal component analysis (“scanpy.tl.pca” function) and UMAP (“scanpy.tl.umap” function) before clustering (Leiden method or Louvain method). Single-cell RNA sequencing analysis was completed in Python 3.6.13 in an Ubuntu 20.04 LTS environment.
For the mouse dataset (GSE104556), we used 2524 cells and 2976 highly variable genes (the original dataset was provided after quality control). For the other mouse dataset (GSE112393), we used 33,468 cells and 5494 highly variable genes after removing the dead cells with more than nine mitochondrial gene counts per cell and removing the doublet cells judged by Scrublet (v0.2.3) [20]. For the human dataset (GSE124263), we selected and used 4561 cells out of 3065 highly variable genes after removing the doublet cells judged by Scrublet (v0.2.3) [20]. For the other human dataset (GSE142585), we used 13,628 cells and 6267 highly variable genes after removing the doublet cells judged by Scrublet (v0.2.3) [20]. The following marker genes were used: Spag6 (Sperm) [21], Prm1 (Sperm) [22], Hspa1l (spermatid, ST) [23], Ccna1 (spermatocyte, SC) [24], Stk31 (SC) [25], Dmrt1 (spermatogonial cell, Spg) [26], Csnk2a1 (Spg) [27], Wt1 (Sertoli cell) [28], Cyp17a1 (Leydig cell) [29], Cd68 (macrophage, macro) [30], Acta2 (interstitial cell, Int) [31], Cd34 (Blood and vascular cell, BV) [32].
Results
Animal experiments
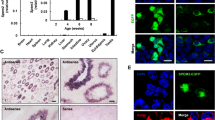
Our transgenic mouse system was summarized in Fig. 1a. We started from the fluorescent observation of the seminiferous tubules (Fig. 1b). We observed tdsRed fluorescence (past Notch1 signaling) in the stroma of N1-Gal4VP16; UAS-Cre; R26GRR mouse. Unfortunately, the EGFP or tdsRed fluorescence in the seminiferous tubules were dim (n = 3 from single-time observation), we carried out immunohistochemistry for Cre recombinase to examine ongoing Notch1 signaling (Fig. 1c). Although some of the inner ear cells at E14.5 N1-Gal4VP16; UAS-Cre mouse embryo expressed Cre recombinase (positive control), we never observed Cre-expressing cells in the seminiferous tubules. Given this result, we hypothesized that the sperms developed independently of Notch1 signaling. Since Notch1 signaling in the developing sperms would result in tdsRed expression in the somatic cells of the next generation, we examined the cerebellum of the R26GRR offspring (Fig. 1d). As expected, tdsRed fluorescence was lacking in the R26GRR offspring in contrast to that of N1-Gal4VP16; UAS-Cre; R26GRR mouse (positive control). In addition, we detected PCR bands for EGFP using the tail DNA from the R26GRR-harboring offspring as the template (Fig. 1e). Full-length blots/gels are presented in Additional File 1. Collectively, these results suggested that the sperms developed independently of Notch1 signaling.
Transgenic mouse experiment revealed lack of Notch1 signaling in the sperm. (a) Schematic representation of our transgenic mouse system. Note that Notch1 signaling results in the expression of Cre recombinase (ongoing Notch1 signaling) and tdsRed (past Notch1 signaling). (b) Fluorescent examination of the seminiferous tubules. “Non-Tg” means, hereafter, the mice without any of “N1-Gal4VP16”, “UAS-Cre,” or “R26GRR” transgene elements. (c) Immunohistochemistry for Cre recombinase using the testes from adult N1-Gal4VP16; UAS-Cre mice. Right panel shows positive control (inner ear from an E14.5 N1-Gal4VP16; UAS-Cre mouse embryo). (d) Fluorescent examination of the cerebellum from mice of the indicated genotypes. (e) PCR for EGFP showed bands at the expected size (296 base pairs) in the R26GRR lane, suggesting that these cells developed devoid of Cre-mediated recombination.
An scRNAseq analysis of mouse testes (GSE104556)
To examine our experimental findings, we next carried out scRNA-seq re-analyses of two previously published mouse testes datasets (GSE104556 and GSE112393) [15, 16]. To examine species difference, we also carried out scRNA-seq re-analyses of two previously published human testes datasets (GSE124263 and GSE142585 from human testes) [17, 18] in Additional File 2. The first mouse testes dataset (GSE104556) [15] was derived from two wild-type mouse. UMAP clustering yielded five clusters (Fig. 2a). We tried to characterize each cluster using established marker genes: cluster #0 as sperms, cluster #1 as spermatids (ST), cluster #2 as spermatocytes (SC), cluster #3 as spermatogonial cells (Spg), cluster #4 as Leydig cells (Fig. 2b). Then, we plotted the expression profile of Notch receptors (Fig. 2c). Notch1 and Notch2 were rarely expressed in each cluster whereas Notch4 was observed in cluster #0 (sperm), #2 (SC), #1 (ST) at low expression levels (less than 0.1 per 10,000 mRNA counts) (Fig. 2c and d). Notch3 expression was so little that it was not considered as a highly variable gene. Among Hes/Hey family downstream target genes, Hey1 (cluster #4, Leydig cell) and Hes6 (cluster #3, Spg) were expressed at most 10% of each cluster at very low expression levels (Fig. 2e). These results implied that, at least, Notch1 was not the major receptor in the murine developing testicular germ cells.
An scRNAseq analysis of mouse testes revealed little Notch1 expression. (a) Five clusters from the mouse testes dataset (GSE104556) on UMAP. (b) Characterization of each cluster with indicated marker genes. (c) The expression levels of Notch receptors were plotted on the UMAP. (d) Dot plots for Notch receptor expressions. (e) The expression levels of the downstream targets of Notch signaling were plotted.
Another scRNAseq analysis of mouse testes (GSE112393)
To validate our experimental and scRNAseq findings, we next carried out an additional round of scRNA-seq analysis using another previously reported mouse testes dataset (GSE112393) [16]. UMAP clustering yielded six clusters characterized with following marker genes: Prm1 (sperm, cluster #1), Hspa1l (ST, cluster #1), Ccna1 (SC, cluster #0), Dmrt1 (Spg, cluster #2), Wt1 (Sertoli, cluster #4), Cyp17a1 (Leydig, cluster #3), Acta2 (Int, cluster #5) (Fig. 3a and b). We plotted the expression profiles of Notch receptors on the UMAP and dot plot (Fig. 3c and d). Notch1 and Notch4 were expressed in cluster #4 (Sertoli), but not in the testicular germ cells (cluster #0 and #1). Notch3 was expressed in cluster #5 (Int) while Notch2 was rarely expressed. Among Hes/Hey family downstream genes, expressed was Hes1 in cluster #3 (Leydig), #4 (Sertoli) and #5 (Int) (Fig. 3e). This result (GSE112393) was concordant with our previous scRNAseq re-analysis (GSE104556) in that Notch1 might not be the major receptor in the testicular germ cells.
Another mouse testes scRNAseq re-analysis supported lack of Notch1 expression in the testicular germ cells. (a) Six clusters were generated from the mouse testes dataset. (b) Characterization of each cluster with indicated marker genes. (c, d) The expression levels of the Notch receptors were plotted on the UMAP and dot plot. (e) The expression levels of the downstream targets of Notch receptors were plotted.
Discussion
By using transgenic mouse system and scRNAseq analysis, this study raised a question to a previous report that suggested Notch1 expression in the male germ cells [10]. In this report, Huang et al. used Stra8-iCre mouse to induce gene recombination at early spermatogonial cells (postnatal day3) and suggested that overexpression of Notch1 intracellular domain (aberrant activation of Notch1 signaling) severely affected sperm development whereas Notch1 deletion had no apparent effect. Although our result was contradictory to this report that reported Notch1 expression, the main body of the report that gain-of-function, instead of loss-of-function, of Notch1 abrogated sperm development was natural given the results of our current study because the sperms might develop independently of Notch1 signaling.
Delta-Notch signaling pathway components are suggested to be involved in gamete formation in many non-mammalian species [6,7,8]. In Xenopus laevis, X-Delta-2 mediates migration of the primordial germ cells [8]. Our scRNAseq re-analyses of the mouse datasets suggested that Notch1 expressed in Sertoli cells that agreed with a previous study [33] although the testicular germ cells are clearly different from Sertoli cells. In addition, the contribution of Notch3 in mouse (GSE112393) and human (GSE142585) (See Additional File 2) testicular interstitial cells might reflect the contribution of Notch3 in the blood vessels since this gene is involved in a hereditary arteriopathy named cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) [34]. The expression levels of Notch2 and Notch4 were relatively low in our scRNAseq re-analyses of four datasets, leaving an uncertainty of their contributions in the testis physiology. Importantly, differentiating testicular germ cells lacked the Notch1 expression in all four datasets from mouse and human. Therefore, our transgenic mouse experiment suggesting that Notch1 signaling is lacking in the seminiferous tubules is reasonable.
Conclusion
Notch1 signaling is rare in the murine developing sperm and that might partially constitute the reason gain-of-function, instead of loss-of-function, of Notch1 led to abnormalities in the sperm development in a previous paper by Huang et al. [10].
Limitations
Since our scRNAsq analysis was carried out only for gonadal cells, our conclusion could not be generalized for gamete formation as a whole, except for Notch1 signaling in mouse.
Data Availability
The datasets generated and/or analyzed during the current study are available at our GitHub repositories (https://github.com/Naoto-Sambe/sperm) (https://github.com/MasaharuYoshihara/Sambe_Sperm_Original_Data).
Abbreviations
- BV:
-
Blood and vascular cell
- CADASIL:
-
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
- EGFP:
-
Enhanced green fluorescent protein
- Int:
-
Interstitial cell
- macro:
-
Macrophage
- ST:
-
Spermatid
- SC:
-
Spermatocyte
- Spg:
-
Spermatogonial cell
- scRNAseq:
-
Single-cell RNA-seq
- tdsRed:
-
Tandem dsRed
- UAS:
-
Upstream activating sequence
- UMAP:
-
Uniform manifold approximation and projection
References
Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18(5):698–712.
Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, et al. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21(13):4712–20.
Sasai Y, Kageyama R, Tagawa Y, Shigemoto R, Nakanishi S. Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and enhancer of split. Genes Dev. 1992;6(12B):2620–34.
Liu Z, Chen S, Boyle S, Zhu Y, Zhang A, Piwnica-Worms DR, et al. The extracellular domain of Notch2 increases its cell-surface abundance and ligand responsiveness during kidney development. Dev Cell. 2013;25(6):585–98.
Kopan R, Chen S, Liu Z, Alagille. Notch, and robustness: why duplicating systems does not ensure redundancy. Pediatr Nephrol. 2014;29(4):651–7.
Robinson-Thiewes S, Kershner AM, Shin H, Haupt KA, Kroll-Connor P, Kimble J. A sensitized genetic screen to identify regulators of Caenorhabditis elegans germline stem cells. G3 (Bethesda). 2022 4;12(3):jkab439.
Ng CL, Qian Y, Schulz C. Notch and Delta are required for survival of the germline stem cell lineage in testes of Drosophila melanogaster. PLoS ONE. 201912;14(9). e0222471.
Morichika K, Kataoka K, Terayama K, Tazaki A, Kinoshita T, Watanabe K, et al. Perturbation of Notch/Suppressor of hairless pathway disturbs migration of primordial germ cells in Xenopus embryo. Dev Growth Differ. 2010;52(2):235–44.
Griswold MD. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96(1):1–17.
Huang Z, Rivas B, Agoulnik AI. NOTCH1 gain of function in germ cells causes failure of spermatogenesis in male mice. PLoS ONE. 2013;8(7):e71213.
Hayashi T, Kageyama Y, Ishizaka K, Xia G, Kihara K, Oshima H. Requirement of notch 1 and its ligand jagged 2 expressions for spermatogenesis in rat and human testes. J Androl. 2001;22(6):999–1011.
Smith E, Claudinot S, Lehal R, Pellegrinet L, Barrandon Y, Radtke F. Generation and characterization of a Notch1 signaling-specific reporter mouse line. Genesis. 2012;50(9):700–10.
Yoshihara M, Nishino T, Sambe N, Nayakama T, Radtke F, Mizuno S, et al. Generation of a Gal4-dependent gene recombination and illuminating mouse. Exp Anim. 2022;71(3):385–90.
Hasegawa Y, Daitoku Y, Sekiguchi K, Tanimoto Y, Mizuno-Iijima S, Mizuno S, et al. Novel ROSA26 cre-reporter knock-in C57BL/6 N mice exhibiting Green Emission before and Red Emission after cre-mediated recombination. Exp Anim. 2013;62(4):295–304.
Lukassen S, Bosch E, Ekici AB, Winterpacht A. Single-cell RNA sequencing of adult mouse testes. Sci Data. 2018;5:180192.
Green CD, Ma Q, Manske GL, Shami AN, Zheng X, Marini S, et al. A Comprehensive Roadmap of Murine Spermatogenesis defined by single-cell RNA-Seq. Dev Cell. 2018;46(5):651–667e10.
Sohni A, Tan K, Song HW, Burow D, de Rooij DG, Laurent L, et al. The neonatal and adult human testis defined at the single-cell level. Cell Rep. 2019;26(6):1501–1517e4.
Shami AN, Zheng X, Munyoki SK, Ma Q, Manske GL, Green CD, et al. Single-cell RNA sequencing of Human, Macaque, and mouse testes uncovers conserved and divergent features of mammalian spermatogenesis. Dev Cell. 2020;54(4):529–547e12.
Wolf FA, Angerer P, Theis FJ. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19(1):15.
Wolock SL, Lopez R, Klein AM, Scrublet. Computational identification of cell doublets in single-cell Transcriptomic Data. Cell Syst. 2019;8(4):281–291e9.
Sapiro R, Kostetskii I, Olds-Clarke P, Gerton GL, Radice GL, Strauss JF III. Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol Cell Biol. 2002;22(17):6298–305.
Ariel M, McCarrey J, Cedar H. Methylation patterns of testis-specific genes. Proc Natl Acad Sci U S A. 1991;88(6):2317–21.
Wang X, Xie W, Yao Y, Zhu Y, Zhou J, Cui Y, et al. The heat shock protein family gene Hspa1l in male mice is dispensable for fertility. PeerJ. 2020;8:e8702.
Panigrahi SK, Manterola M, Wolgemuth DJ. Meiotic failure in cyclin A1-deficient mouse spermatocytes triggers apoptosis through intrinsic and extrinsic signaling pathways and 14-3-3 proteins. PLoS ONE. 2017;12(3):e0173926.
Zhou J, Leu NA, Eckardt S, McLaughlin KJ, Wang PJ. STK31/TDRD8, a germ cell-specific factor, is dispensable for reproduction in mice. PLoS ONE. 2014;9(2):e89471.
Looijenga LH, Hersmus R, Gillis AJ, Pfundt R, Stoop HJ, van Gurp RJ, et al. Genomic and expression profiling of human spermatocytic seminomas: primary spermatocyte as tumorigenic precursor and DMRT1 as candidate chromosome 9 gene. Cancer Res. 2006;66(1):290–302.
Xu X, Toselli PA, Russell LD, Seldin DC. Globozoospermia in mice lacking the casein kinase II alpha’ catalytic subunit. Nat Genet. 1999;23(1):118–21.
Wang XN, Li ZS, Ren Y, Jiang T, Wang YQ, Chen M, et al. The Wilms tumor gene, Wt1, is critical for mouse spermatogenesis via regulation of sertoli cell polarity and is associated with non-obstructive azoospermia in humans. PLoS Genet. 2013;9(8):e1003645.
Laurich VM, Trbovich AM, O’Neill FH, Houk CP, Sluss PM, Payne AH, et al. Müllerian inhibiting substance blocks the protein kinase A-induced expression of cytochrome p450 17alpha-hydroxylase/C(17–20) lyase mRNA in a mouse leydig cell line independent of cAMP responsive element binding protein phosphorylation. Endocrinology. 2002;143(9):3351–60.
Chistiakov DA, Killingsworth MC, Myasoedova VA, Orekhov AN, Bobryshev YV. CD68/macrosialin: not just a histochemical marker. Lab Invest. 2017;97(1):4–13.
Wang J, Zohar R, McCulloch CA. Multiple roles of alpha-smooth muscle actin in mechanotransduction. Exp Cell Res. 2006;312(3):205–14.
Lai JH, Zhou YJ, Bin D, Qiangchen, Wang SY. Clinical significance of detecting lymphatic and blood vessel invasion in stage II colon cancer using markers D2-40 and CD34 in combination. Asian Pac J Cancer Prev. 2014;15(3):1363–7.
Hasegawa K, Okamura Y, Saga Y. Notch signaling in sertoli cells regulates cyclical gene expression of Hes1 but is dispensable for mouse spermatogenesis. Mol Cell Biol. 2012;32(1):206–15.
Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707–10.
Acknowledgements
The authors are grateful to all members of the Department of Anatomy and Embryology, Institute of Medicine, University of Tsukuba, and American Journal Experts (https://www.aje.com/) for English language editing.
Funding
This work was supported by the PhD Program in Humanics, University of Tsukuba (Doctoral Program for World-leading Innovative and Smart Education, Ministry of Education, Culture, Sports, Science and Technology, Japan), by the Japan Society for the Promotion of Science through JSPS KAKENHI Grant-in-Aid for Research Activity Start-up (JP22K20734) and by YOKOYAMA Foundation for Clinical Pharmacology (YRY-2207). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of these funding agencies.
Author information
Authors and Affiliations
Contributions
NS analyzed the scRNAsq data and wrote the draft. MY designed the study, revised the draft, and supervised the work. TN1 wrote python code for scRNAseq. RS and TN2 analyzed the scRNAseq data. CL carried out immunohistochemistry for Cre recombinase. ST substantially revised the draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal experiments were carried out in accordance with the Regulation for Animal Experiments in our university and Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology. Approval was obtained from the Institutional Animal Care and Use Committee and the DNA Experiment Committee of the University of Tsukuba (Approval Numbers for Animal Experiments: 22–059) (Approval Number for DNA Experiments: 220018).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sambe, N., Yoshihara, M., Nishino, T. et al. Analysis of Notch1 signaling in mammalian sperm development. BMC Res Notes 16, 108 (2023). https://doi.org/10.1186/s13104-023-06378-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-023-06378-z