Abstract
Objectives
Breast cancer (BC) is one of the most common cancers with a high mortality rate in women worldwide. The advantages of early cancer diagnosis are apparent, and it is a critical factor in increasing the patient’s life and survival. According to mounting evidence, microRNAs (miRNAs) may be crucial regulators of critical biological processes. miRNA dysregulation has been linked to the beginning and progression of various human malignancies, including BC, and can operate as tumor suppressors or oncomiRs. This study aimed to identify novel miRNA biomarkers in BC tissues and non-tumor adjacent tissues of patients with BC. Microarray datasets GSE15852 and GSE42568 for differentially expressed genes (DEGs) and GSE45666, GSE57897, and GSE40525 for differentially expressed miRNAs (DEMs) retrieved from the Gene Expression Omnibus (GEO) database were analyzed using “R” software. A protein-protein interaction (PPI) network was created to identify the hub genes. MirNet, miRTarBase, and MirPathDB databases were used to predict DEMs targeted genes. Functional enrichment analysis was used to demonstrate the topmost classifications of molecular pathways. The prognostic capability of selected DEMs was evaluated through a Kaplan-Meier plot. Moreover, the specificity and sensitivity of detected miRNAs to discriminate BC from adjacent controls were assessed by area under the curve (AUC) using the ROC curve analysis. In the last phase of this study, gene expression on 100 BC tissues and 100 healthy adjacent tissues were analyzed and calculated by using the Real-Time PCR method.
Results
This study declared that miR-583 and miR-877-5p were downregulated in tumor samples in comparison to adjacent non-tumor samples (|logFC|< 0 and P ≤ 0.05). Accordingly, ROC curve analysis demonstrated the biomarker potential of miR-877-5p (AUC = 0.63) and miR-583 (AUC = 0.69). Our results showed that has-miR-583 and has-miR-877-5p could be potential biomarkers in BC.
Similar content being viewed by others
Introduction
With an estimated 1,200,000 new cases each year, Breast cancer (BC) is the most frequently diagnosed malignancy in females [1, 2]. BC is a complex and heterogeneous disease that can be classified into several subtypes based on histopathological characteristics, tumor grade, and lymphovascular invasion [3]. However, it sometimes uncovers after signs emerge, despite many women with BC having no symptoms [4], and neither of the commonly used methods is accurate enough to meet the criteria for diagnosing BC, and the late diagnosis is one of the reasons for the high mortality rate from this cancer. The authors list surgery, chemotherapy, and radiation as main treatment plans for patient with breast cancer. However, since majority of newly-diagnosed breast cancers are hormone-receptor positive and the patients receive endocrine therapies, targeted therapies should be listed as a treatment plan [5]. Despite breakthroughs in treatment, approximately half of people with BC will have a cancer recurrence or die within 5 years [6]. Therefore, new biomarkers associated with gene expression must be found to assist valuable diagnostic procedures and treatment strategies for BC patients by increasing the analysis of molecular pathways linked to the onset and progression of the tumor [7,8,9,10].MicroRNAs (miRNAs) are small single-stranded non-coding RNAs that hinder the expression of their target genes [11, 12], are crucial regulators for essential cellular functions, and have a role in different disorders [13] and various cancers [14, 15]. MiRNAs are involved in tumorigenesis via altering the expression of tumor suppressor genes and oncogenes [16], and they are also important for the cell cycle [17], migration and invasion [18], apoptosis [19], metastasis [20], and the development of cancer [21]. Although miRNA expression patterns are consistent across all cancer types, most of them are cell-type-specific and might thus be used as cancer biomarkers. Evidence from malignant tissues and cell lines shows that miRNAs have a role in the development of all types of BC [22,23,24]. Evidence suggests that miRNAs, a form of small endogenous singlestranded RNA that binds to the 3’ untranslated region of purpose mRNAs, may play essential roles in numerous biological processes and are proven to negatively suppress gene expression. However, numerous cellular processes are impacted by miRNAs, containing proliferation, differentiation, apoptosis, migration, metabolism, and stress response [25]. High-throughput genomic technologies have greatly helped researchers in understanding the mechanisms involved in cancer pathogenesis by allowing them to explore the involvement of genetic variables in cancer. Furthermore, the development of novel methods, such as enrichment analysis and prediction tools, enables more extensive investigations of molecular networks and disease pathophysiology processes and provides an exceptional tool for identifying novel therapeutic targets. Seizing the benefit of microarray datasets and other in situ tools, we aimed to find novel markers for earlier and more accurate identification to evaluate the expression levels and diagnostic values of miRNAs and validate our data through experimental analyses between BC tissues and non-tumor adjacent samples to find novel markers for earlier and more accurate identification.
Materials and methods
Microarray-based mRNA and miRNA dataset selection
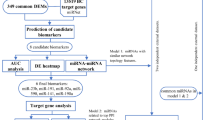
By searching for keywords “Breast Cancer and adjacent normal controls”, GSE15852 and GSE42568 mRNA datasets, and GSE45666, GSE57897, and GSE40525 miRNA datasets were screened and downloaded from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/). The information of the datasets is available in (Fig. 1).
Identification of DEMs and DEGs
The “R” software and “LIMMA” package were employed to efficiently analyze the chosen datasets utilizing correction, quantile normalization, and log2 conversion. The screening criteria were |log2 fold-change (FC)| ≤ − 0.5 and FDR adjusted p ≤ 0.05 for DEMs, and |log2 fold-change (FC)| ≥ 1 and FDR adjusted p-value ≤ 0.05 for DEGs [26].
Pathway enrichment and functional analysis
Then, DEGs were investigated utilizing the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment via the EnrichR database. The p < 0.05 was described as a significant enrichment analysis outcome. Potential roles were anticipated using KEGG pathway analysis.
Validation of the hub miRNAs
We aimed to explain a certain discrimination capacity of these miRNAs for cancer and non-cancer tissues and the diagnostic power of the hub miRNAs by plotting the receiver operating characteristic (ROC) curve and estimation of the area under the curve (AUC).
Ethics statement
All methods were performed in accordance with the guidelines of the Helsinki and was approved by the Research Ethics Committee of Azad University of Medical Sciences, Tabriz, Iran with Ethics code IR.IAU.TABRIZ.REC.1401.103. The patients’ written informed permission was gathered before participation.
Study Population
Fresh-frozen specimens from 200 breast tumors were obtained from patients with BC undergoing surgery at Tabriz’s Al-Zahra Hospital from 2020 to 2022. After that, a histological examination was used to investigate the invasion and spread of cancer cells. As controls, tumor margin samples that a pathologist deemed to be healthy were also collected from a region of the resected specimen at the farthest distance from the tumor. The specimens were stored at − 80 °C instantly. None of the patients had been treated with preoperative radiotherapy, chemotherapy, or other relevant conditions. All patients signed informed permissions and agreed to use their surgical samples for investigation. The pathological characteristics of patients are shown in (Table 1).
RNA extraction and cDNA synthesis
After homogenizing the tissues with liquid nitrogen, TRIzol reagent (Geneall) was added to extract the total RNA content from tissues. After that, a NanoDrop spectrometer (Thermo Scientific, USA) was utilized to evaluate the amount and quality of the isolated RNAs. Following extraction, the RNAs were purified in 50 µL of RNase-free water and kept at − 80 °C for storage. Reverse transcriptase (RT) enzyme (Thermo Fisher, USA), dNTP (Cinnaclone, Iran), and the specific stem-loop primers were used to create cDNA for miR-583, miR-877-5p, and RNU6 (for normalization). Three distinct stem-loop primers were created for miR-583, miR-877-5p, and RNU6, specially designed for this purpose. The reaction requirements were to hold step at 95 °C for 10 min, followed by 40 cycles of denaturation at 94 °C for 15 s, annealing at 62 °C for 30 s, and extension at 72 °C for 20 s. The final product was kept at 4 °C for preservation. The sequences of the primers are demonstrated in (Table 2).
Data analysis
The target DEMs ratio between the BC tissues and non-tumor adjacent tissues was indicated by the 2− (ΔΔCT), and the formula was as follows: R = 2− (ΔΔCT).
Threshold Cycle (CT) identified the amplification cycle when the reaction’s real-time fluorescence intensity approximated the determined threshold, and the amplification was in the logarithmic phase. Per experiment was conducted two times to define the mean value. Logarithm 2 of Fold Change (was used in simple linear regression) and Two- sample T-test to compare two groups of data were applied to compare the expression level of selected miRNAs between demographic features of the subgroups like age, family history, abortion history, and tumor size. Moreover, survival analysis was evaluated by the Kaplan-Meier survival plot designed by GraphPad prism 8.4.3 to assess the prognostic value of selected DEMs in BC patients. All results are presented as P-value < 0.05 and mean ± SEM using the GraphPad Prism 8.4.3 and “R” software version 4.3.1.
Results
Identification of DEMs and DEGs
Considering |logFC| ≤ − 0.5 and p < 0.05 criteria for DEMs, 386 DEMs (181 upregulated and 205 downregulated) were identified in GSE57897, 213 DEMs (71 upregulated and 142 downregulated) in GSE45666, and 105 DEMs (54 upregulated and 51 downregulated) in GSE40525 (Fig. 2A and B, and 2 C). Furthermore, |logFC| ≥ 1 and p < 0.05 criteria for differentially expressed genes resulted in 410 DEGs (177 upregulated and 158 downregulated) in GSE15582 and 233 DEGs (25 upregulated and 208 downregulated) in GSE42568. Differentially expressed plot of each dataset is presented in (Fig. 3).
Detection of mRNA-miRNA network
The establishment of a miRNA-mRNA network was carried out using the detected DEMs and DEGs by miRNet [27] and String database [28] (Fig. 4). As expected, hsa-miR-583 and hsa-miR-877-5p have a direct influence on the greatest number of DEGs and are regarded as hub miRNAs.
Screening enrichment analysis of common DEGs
Each of the target genes analyzed by microarray datasets has their pathways enriched. KEGG pathway analysis [29]demonstrated that the potential target genes were mainly enriched in 10 pathways presented in (Table 3).
Validation of the hub miRNAs by the ROC curve
A ROC curve proved the diagnostic effectiveness of core miRNAs. The AUC indicated that miRNAs showed remarkable diagnostic efficiency for BC tissues and adjacent non-tumor tissues. Based on our results, miR-583 with AUC = 0.69 (specificity; 96.3%, sensitivity; 5.49%) and miR-877-5p with AUC = 0.63 (specificity; 96.3%, sensitivity; 5.49%) are good candidates for diagnosis of BC (Fig. 5).
Survival rate and prognostic value of the core DEMs
The METABRIC raw data, which possess an allied study of gene expression in the finding and confirmation of 1262 primary BC cases with ongoing clinical monitoring, were used to plot the Kaplan-Meier. The prognostic value of miR-583 and miR-877-5p was demonstrated in (Fig. 6). The outcomes showed significant values for both miRNAs.
Validation analysis using real- time PCR
To understand much more about the effects of miR-583 and miR-877-5p in patients with BC, the expression of these miRNAs in BC samples and their non-tumor adjacent control samples was examined. By using real-time PCR, the expression of the chosen miRNAs was assessed in 200 BC tissues and matched with that of non-tumor adjacent samples (Fig. 7). The expression of both hub miRNAs was aberrant and downregulated. The paired T-test findings revealed that the expression levels of miR-583 and miR-877-5p were considerably lower in human BC tissues than in non-tumor adjacent tissues, with FC = − 1.35 and FC = − 1.014, respectively.
Subgroups analysis
LFC and Two-sample T-tests respectively were applied to analyze the correlation between the expression level of miR-583 and miR-877-5p with age, family history, abortion history, and tumor size.
miR-583
Based on the results, there were no significant changes in the expression level of miR-583 in subgroups of age ≥ 50 and < 50 (p = 0.974) and in the subgroup of patients with and without family cancer history (Cancer family history means that a close relative has BC) (p = 0.0521). But there were significant differences in the expression level of miR-583 between patients with and without abortion history (***p < 0.0005) and patients with subgroups of tumor size < 10 cm3 and > 10 cm3 (*p = 0.049) (Fig. 8).
The correlation of miR‑583 expression status with age, abortion history, family cancer history, and tumor size. LFC miR‑583 in patients age ≥ 50 and < 50 (p = 0.974). LFC miR‑583 in patients with abortion history and without abortion history (***p < 0.0005). LFC miR-583 in patients with family cancer history and without family cancer history (p = 0.0521). LFC miR-583 in patients with tumor size < 10 cm3 and > 10 cm3 (*p = 0.049). In four figures, the vertical axis, center line, and error bars designate LFC (i.e., base 2 logarithm of FC), median, and interquartile range, respectively
miR-877-5p
Based on the results, there were no significant changes in the expression level of miR-877-5p in subgroups of age ≥ 50 and < 50 (p = 0.767). Nevertheless, remarkably changes were seen in the expression level of the subgroup of patients with and without family cancer history (p = 0.0025), between patients with and without abortion history (p = 0.0123), and between patients with subgroups of tumor size < 10 cm3 and > 10 cm3 (p = 0.031) (Fig. 9).
The correlation of miR‑877-5p expression status with age, abortion history, family cancer history, and tumor size. LFC miR‑877-5p in patients age ≥ 50 and < 50 (p = 0.767). LFC miR‑877-5p in patients with abortion history and without abortion history (*p = 0.0123). LFC miR-877-5p in patients with family cancer history and without family cancer history (**p = 0.0025). LFC miR-877-5p in patients with tumor size < 10 cm3 and > 10 cm3 (*p < 0.031). In four figures, the vertical axis, center line, and error bars designate LFC (i.e., base 2 logarithm of FC), median, and interquartile range, respectively
Discussion
Breast cancer is a predominant cancer impacting females and resulting in high mortality [30]. MiRNAs have been determined to exert vital roles in the modulation of breast cancer. Previous studies have pinpointed several miRNAs in the regulation of breast cancer progression [31,32,33]. It has been reported that miR-513c and miR-3163 have a significant role in the development of BC [34]. Mir-335 is downregulated in breast cancer and is known as a tumor suppressor in BC [35]. Mir-30b-5p facilitates the Proliferation, migration, and invasion of breast tumors and acts as an oncomiR in breast cancer [36]. MiR-449b-5p as a tumor suppressor inhibits the growth and invasion of breast tumors by suppressing the Wnt/β-catenin signaling axis [37]. In this study, miR-583 and miR-877–5p were informed as novel breast cancer-related miRNAs that were downregulated in breast cancer tissues compared with adjacent normal tissues based on microarray and RT-qPCR results. Multiple studies’ analysis of the miRNA-mRNA network suggests that miR-583 and miR-877-5p, in association with certain proteins, contribute to the development of numerous malignancies. In 2020, Wu et al. declared that miR-877-5p inhibited gastric cancer growth and was known as a novel potential therapeutic target for gastric cancer [38]. In prostate cancer, miR-877-5p suppresses the malignant progression of cancer cells through miR-877-5p/SSFA2 axis [39]. Moreover, miR-877-5p as a tumor suppressor was detected in multiple available literatures published in 2018 [40,41,42] and 2020 [43] as a vital biomarker in patients with hepatocellular carcinoma.
Like miR-877-5p, the role of miR-583 as a biomarker has been proved in several studies. MiR-583 directly inhibits the proliferation and invasion of prostate cancer cells, providing a novel therapeutic target in prostate cancer [44]. Moreover, with the cooperation of other non-coding RNAs like circular RNAs, miR-583 hinders tumoral cells’ growth. Suppressing miR-583 through hsa_circ_0001955/miR-583/FGF21 axis promotes colorectal cancer [45]. These observations suggest miR-877-5p and miR-583 as tumor suppressors in cancer. To the best of our knowledge, miR-583 and miR-877-5p have not been reported in breast cancer.
Additionally, in this study, based on Enrichr and KEGG databases, the candidate targets of miR-583 and miR-877-5p might have an important role in endocytosis and pathways in cancer. Endocytosis is a potentially vital aspect in the regulation of tumor metastasis [46] and is confirmed in BC invasion and metastasis [47, 48]. However, pathways control various physiological functions and pathological events, in BC growth [49, 50]. These results suggest that the promising targets of miR-583 and miR-877-5p could be affected in the above-mentioned pathways to impact the occurrence and development of BC. In summary, in-silico and functional analysis results of the present study revealed that the downregulation of miR-583 and miR-877-5p promotes BC. However, the results of the RT-PCR elucidated the tumor suppressor role of miR-583 (p < 0.0001 and Log FC = − 1.35) and miR-877-5p (p < 0.0001 and Log FC = − 1.014) in BC tissues compared with adjacent non-tumor tissues. Furthermore, the demographic characteristics of our patients revealed that a significant difference was observed between the expression level of miR-583 and some demographic characteristics like abortion history and tumor size. Also, the expression level between miR-877-5p and family cancer history, abortion history, and tumor size were markedly different. The Kaplan-Meier result confirmed the prognostic value of miR-583 and miR-877-5p, and the ROC analysis result confirmed miR-583 (AUC = 0.69) and miR-877-5p (AUC = 0.64) great potential to be a valuable biomarker for BC.
Conclusion
Taken together, downregulated miR-583 and miR-877-5p are potential molecular markers in BC. Our findings may suggest these miRNAs as promising diagnostic, prognostic, and therapeutic targets for BC, but further studies are needed to elucidate molecular mechanisms and validate the predicted findings using bioinformatics studies.
Limitations
Some patients completed all the questions about the age, age of their first menstrual, age of menopause, family history, weight, number of children, underlying disease, medications used, age of breast cancer diagnosis, previous treatments, etc., themselves. Therefore, it’s probable that some patients didn’t answer the questions honestly and may have toned down the seriousness of their responses. The small number of surgical samples can be mentioned as another limitation.
Availability of data and materials
All datasets supporting the conclusions of this article are available in NCBI/bioproject: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA116827, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA182286, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA195576, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA248377, and https://www.ncbi.nlm.nih.gov/bioproject/PRJNA174219. Further inquiries can be directed to the corresponding author.
Abbreviations
- AUC:
-
Curve and area under the curve
- BC:
-
Breast cancer
- DEG:
-
Differentially expressed genes
- DEM:
-
Differentially expressed miRNA
- GO:
-
Gene ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- LFC:
-
Logarithm fold change
- MiRNA:
-
MicroRNA
- NcRNA:
-
Noncoding RNA
- One-way ANOVA:
-
One-way analysis of variance
- PCR:
-
Polymerase chain reaction
- ROC:
-
Receiver operating characteristic
- RT-qPCR:
-
Reverse transcriptase-quantitative polymerase chain reaction
- SD:
-
Standard deviation
- SEM:
-
Standard error of the mean
- WHO:
-
World Health Organization
References
Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers. 2021;13(17):4287.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
Makki J. Diversity of breast carcinoma: histological subtypes and clinical relevance. Clin Med Insights Pathol. 2015;8:23–31.
Unger-Saldaña K. Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J Clin Oncol. 2014;5(3):465–77.
Spring LM, Bar Y, Isakoff SJ. The evolving role of Neoadjuvant Therapy for operable breast Cancer. J Natl Compr Canc Netw. 2022;20(6):723–34.
Altunay B, Morgenroth A, Mottaghy FM. Use of radionuclide-based imaging methods in breast cancer. In: Seminars in nuclear medicine. WB Saunders; 2022.
Szopa W, Burley TA, Kramer-Marek G, Kaspera W. Diagnostic and therapeutic biomarkers in Glioblastoma: current status and future perspectives. Biomed Res Int. 2017;2017:8013575.
Zubair M, Wang S, Ali N. Advanced approaches to breast cancer classification and diagnosis. Front Pharmacol. 2020;11:632079.
Foruzandeh Z, Dorabadi DG, Sadeghi F, Zeinali-Sehrig F, Zaefizadeh M, Rahmati Y, Alivand MR. Circular RNAs as novel biomarkers in triple-negative breast cancer: a systematic review. Mol Biol Rep. 2022;49(10):9825–40.
Califf RM. Biomarker definitions and their applications. Exp Biol Med. 2018;243(3):213–21.
Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–6.
Grosshans H, Slack FJ. Micro-RNAs: small is plentiful. J Cell Biol. 2002;156(1):17–21.
Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451–65.
Javdani H, Mollaei H, Karimi F, Mahmoudi S, Farahi A, Mirzaei-Parsa MJ, Shahabi A. Review article epithelial to mesenchymal transition‑associated microRNAs in breast cancer. Mol Biol Rep. 2022;49(10):9963–73.
Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20(8):460–9.
Zhang W, Dahlberg JE, Tam W. MicroRNAs in tumorigenesis: a primer. Am J Pathol. 2007;171(3):728–38.
Mens MMJ, Ghanbari M. Cell cycle regulation of stem cells by MicroRNAs. Stem Cell Rev Rep. 2018;14(3):309–22.
He L, Zhang H. MicroRNAs in the Migration of mesenchymal stem cells. Stem Cell Rev Rep. 2019;15(1):3–12.
Huang Q, Chen L, Bai Q, Tong T, Zhou Y, Li Z, et al. The roles of microRNAs played in lung diseases via regulating cell apoptosis. Mol Cell Biochem. 2021;476(12):4265–75.
Solé C, Lawrie CH. MicroRNAs in metastasis and the tumour microenvironment. Int J Mol Sci. 2021;22(9):4859.
Pan G, Liu Y, Shang L, Zhou F, Yang S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun. 2021;41(3):199–217.
Kahraman M, Röske A, Laufer T, Fehlmann T, Backes C, Kern F, et al. MicroRNA in diagnosis and therapy monitoring of early-stage triple-negative breast cancer. Sci Rep. 2018;8(1):11584.
McGuire A, Brown JA, Kerin MJ. Metastatic breast cancer: the potential of miRNA for diagnosis and treatment monitoring. Cancer Metastasis Rev. 2015;34(1):145–55.
Arabkari V, Clancy E, Dwyer RM, Kerin MJ, Kalinina O, Holian E, et al. Relative and absolute expression analysis of MicroRNAs associated with luminal a breast cancer—a comparison. Pathol Oncol Res. 2020;26(2):833–44.
Tafrihi M, Hasheminasab E. MiRNAs: biology, biogenesis, their web-based tools, and databases. Microrna. 2019;8(1):4–27.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47.
Chang L, Zhou G, Soufan O, Xia J. miRNet 2.0: network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020;48(W1):W244–51.
Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49(D1):D605–12.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Woolston C. Breast cancer. Nature. 2015;527(7578):101.
Chen Y, Zhang J, Wang H, Zhao J, Xu C, Du Y, et al. miRNA-135a promotes breast cancer cell migration and invasion by targeting HOXA10. BMC Cancer. 2012;12:111.
Wang Y, Liu Z, Shen J. MicroRNA-421-targeted PDCD4 regulates breast cancer cell proliferation. Int J Mol Med. 2019;43(1):267–75.
Li P, Xu T, Zhou X, Liao L, Pang G, Luo W, et al. Downregulation of miRNA-141 in breast cancer cells is associated with cell migration and invasion: involvement of ANP32E targeting. Cancer Med. 2017;6(3):662–72.
Delgir S, Ilkhani K, Safi A, Rahmati Y, Montazari V, Zaynali-Khasraghi Z, et al. The expression of miR-513c and miR-3163 was downregulated in tumor tissues compared with normal adjacent tissue of patients with breast cancer. BMC Med Genomics. 2021;14(1):180.
Dong Y, Liu Y, Jiang A, Li R, Yin M, Wang Y. MicroRNA-335 suppresses the proliferation, migration, and invasion of breast cancer cells by targeting EphA4. Mol Cell Biochem. 2018;439(1–2):95–104.
Wu T, Song H, Xie D, Hua K, Hu J, Deng Y, et al. Mir-30b-5p promotes Proliferation, migration, and invasion of breast cancer cells via targeting ASPP2. Biomed Res Int. 2020;2020:7907269.
Jiang J, Yang X, He X, Ma W, Wang J, Zhou Q, et al. MicroRNA-449b-5p suppresses the growth and invasion of breast cancer cells via inhibiting CREPT-mediated Wnt/β-catenin signaling. Chem Biol Interact. 2019;302:74–82.
Wu K, Yu Z, Tang Z, Wei W, Xie D, Xie Y, et al. Mir-877-5p suppresses gastric Cancer Cell Proliferation through Targeting FOXM1. Onco Targets Ther. 2020;13:4731–42.
Wang W, Yi J, Dong D, Mao W, Wang X, Yan Z. miRNA-877-5p inhibits malignant progression of prostate cancer by directly targeting SSFA2. Eur J Histochem. 2021;65(3):3243.
Yan TH, Qiu C, Sun J, Li WH. MiR-877-5p suppresses cell growth, migration and invasion by targeting cyclin dependent kinase 14 and predicts prognosis in hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2018;22(10):3038–46.
Pafundi PC, Caturano A, Franci G. Comment on: MiR-877-5p suppresses cell growth, migration and invasion by targeting cyclin dependent kinase 14 and predicts prognosis in hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2018;22(14):4401–2.
Xiong DD, Dang YW, Lin P, Wen DY, He RQ, Luo DZ, et al. A circRNA-miRNA-mRNA network identification for exploring underlying pathogenesis and therapy strategy of hepatocellular carcinoma. J Transl Med. 2018;16(1):220.
Liu Y, Guo J, Shen K, Wang R, Chen C, Liao Z, et al. Paclitaxel suppresses Hepatocellular Carcinoma Tumorigenesis through regulating Circ-BIRC6/miR-877-5p/YWHAZ Axis. Onco Targets Ther. 2020;13:9377–88.
Li Z, Chen J. miR–583 inhibits the proliferation and invasion of prostate cancer cells by targeting JAK1. Mol Med Rep. 2021;23(3):199.
Gao X, Yin J, Yao Y. hsa_circ_0001955 promotes colorectal cancer progression by regulating miR-583/FGF21 Axis. J Oncol. 2022;2022:4288474.
Khan I, Steeg PS. Endocytosis: a pivotal pathway for regulating metastasis. Br J Cancer. 2021;124(1):66–75.
Moreno-Layseca P, Jäntti NZ, Godbole R, Sommer C, Jacquemet G, Al-Akhrass H, et al. Cargo-specific recruitment in clathrin- and dynamin-independent endocytosis. Nat Cell Biol. 2021;23(10):1073–84.
Park S, Shi Y, Kim BC, Jo MH, Cruz LO, Gou Z, et al. Force-dependent trans-endocytosis by breast cancer cells depletes costimulatory receptor CD80 and attenuates T cell activation. Biosens Bioelectron. 2020;165:112389.
Xu X, Zhang M, Xu F, Jiang S. Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol Cancer. 2020;19(1):165.
Ellis H, Ma CX. PI3K inhibitors in breast Cancer therapy. Curr Oncol Rep. 2019;21(12):110.
Acknowledgements
We would like to express our gratitude to personnel of medical genetic lab at department of Medical Genetics and Clinical Research Development Unit and all the patients and the pathological board of Al-Zahra Hospital who had participant in our work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: ZF and MRA, computational analyses, and experiments: ZF, MGR, MZ, and SG, writing manuscript: ZF, MZ, supervision and finalization: MRA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were performed in accordance with the guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of Azad University of Medical Sciences, Tabriz, Iran with Ethics code IR.IAU.TABRIZ.REC.1401.103. All patients enrolled signed an informed written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Foruzandeh, Z., Alivand, M.R., Ghiami-Rad, M. et al. Identification and validation of miR-583 and mir-877-5p as biomarkers in patients with breast cancer: an integrated experimental and bioinformatics research. BMC Res Notes 16, 72 (2023). https://doi.org/10.1186/s13104-023-06343-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-023-06343-w