Abstract
Objective
To assess the effectiveness and safety of hydroxychloroquine (HCQ) prophylaxis for the prevention of SARS-CoV-2 infection in healthcare workers (HCW) on duty during the COVID-19 pandemic.
Results
A total of 68 HCWs met the eligibility criteria were randomly allocated to receive HCQ (n = 36) or not (n = 32). There were no significant differences between groups in respects to age, gender, or medical history. Eight participants met the primary efficacy endpoint of SAR-CoV-2 infection during the study period; there was no difference in incidence of SARS-CoV-2 infections between both study arms (HCQ: 5 vs Control: 3, p = 0.538). The relative risk of SARS-CoV-2 infection in the HCQ arm was 1.69 compared to the control group (95%CI 0.41–7.11, p = 0.463); due to poor participant accrual, the resulting statistical power of the primary efficacy outcome was 11.54%. No serious adverse events occurred; however, two (2/36, 5.6%) participants no longer wished to participate in the study and withdrew consent due to recurring grade 1 and 2 adverse events.
Trial registration: ClinicalTrials.gov ID: NCT04414241. (Registered on June 4, 2020).
Similar content being viewed by others
Introduction
As the novel coronavirus SARS-CoV-2 spread globally, many opted to repurpose readily available medications to combat the damaging effects of COVID-19. Among the first, the misguided use of hydroxychloroquine (HCQ) gained fast recognition early in the pandemic from preliminary data from a small study with findings suggestive of possible benefit in the treatment of COVID-19 [1, 2]. Several clinical trials (at present, over 250 trials registered on ClinicalTrials.gov) were launched to evaluate its efficacy in the treatment of COVID-19, including the well-known RECOVERY and SOLIDARITY trials [3]. Soon after, trials aimed at prevention using HCQ were abundant [4].
By the first year of the pandemic, Peru was reporting an overwhleming 1239 COVID-19 deaths per million inhabitants [5]. The healthcare workers (HCW) were among the most vulnerable groups at risk of contracting the virus. A report by the Peruvian medical governing authority showed that 3676 (from approximately 58,000) physicians had contracted COVID-19 by September 2020, of which 170 had died [6]. Similarly, by January 2021, over 6000 infections and 94 deaths were reported among nurses [7]. Additionally, 2035 midwifes had contracted COVID-19 and 21 had died [6]. As a result of the inmense death toll, and given that vaccine candidates were still under research, we sought to assess the effectiveness and safety of HCQ prophylaxis for the prevention of SARS-CoV-2 infection in HCWs on duty during the COVID-19 pandemic.
Main text
Materials and methods
Trial design
The study was a pragmatic, randomised, open-label, controled, phase 3 clinical trial evaluating two parallel arms, allocated in a 1:1 ratio, for pre-exposure prevention of SARS-CoV-2 infection in healthcare workers. The study was conducted from July to November 2020. The study consisted of eight (plus baseline) weekly visits to assess seroconversion of antibodies to SARS-CoV-2 or the presence of the virus after presenting with symptoms compatible with COVID-19. The study was approved by the Transitory National Committee of Ethics in Research (CNTEI, acronym in Spanish) as process number CNTEI-003-200. The trial is registered in the Clinical Trials Registry (NCT04414241). The study adheres to the CONSORT Guidelines.
Participants
We recruited participants from four public hospitals in Lima Metropolitan Area in Peru; each study site had a designated area for all study procedures. Adult healthcare workers, without evidence of SARS-CoV-2 infection (negative PCR at enrolment), working in hospital services (triage, emergency department, hospitalisation, ICU, etc.) with direct contact with patients with COVID-19 were eligible to participate. Exclusion criteria included previous (last 30 days) or current use of hydroxychloroquine, chloroquine sulfate, or azithromycin; known allergy or intolerance to hydroxychloroquine and/or chloroquine, a history of heart disease or a known history of prolonged QT syndrome; other conditions, such as Glucose-6-phosphate dehydrogenase (G6PD) deficiency, liver or kidney failure or, presence of alterations in visual acuity or field, which made participation in the study not the most benefitial for the individual.
Interventions
Participants in the control group were asked to adhere to standard personal protection measures against COVID-19, as recommended in the hospital. The type and quantity of personal protection equipment (PPE) were dependent on hospital and services; the trial did not provide additional PPE. No placebo was used. Patients in the intervention group were assigned HCQ and also to adhere to standard personal protection measures. The intervention consisted of a loading dose of 600 mg of HCQ orally on the first day, followed by 400 mg orally every-other-day. Participants assigned to HCQ underwent a electrocardiogram (ECG) prior to first dose to evaluate QT interval duration; if above or equal to 500 ms (Frediricia-corrected) no doses would be given. Repeat ECG were performed at week 4 and 8 of the study.
Randomisation
Participants were randomly allocated in a 1:1 ratio to the intervention or control group using a random number sequence generated in Excel, stratified by study site. The generated sequence was uploaded to the study project in REDCap in order to conceal sequence and correctly allocate participants after informed consent and confirming eligibility.
Outcomes
The primary outcome measures pertained to efficacy and safety of HCQ to prevent SARS-CoV-2 infection up to 4 weeks after randomisation. The primary efficacy endpoint was a positive polymerase chain reaction (PCR) or serological test for SARS-CoV-2 and the primary safety endpoint was grade 3 or greater adverse event during follow-up. The secondary outcome concerned the tolerability of the prophylactic treatment and was measured as the proportion of participants opting out of treatment due to the presence of grade 1 or 2 adverse events.
Sample size
Based on the scarce information available at the time of the design of the study, we assumed that the risk in HCWs of contracting COVID-19 was 30%. Furthermore, a 50% relative reduction in the incidence of SARS-CoV-2 infection was considered to be the minimum desirable for this intervention. For a 50% reduction, 95% confidence (type 1 error, α = 0.05), 80% power, and with a potential loss to follow-up of 20%, it was estimated that 160 participants per arm were needed.
Statistical analysis
The analysis was by an intention-to-treat approach, including all participants who were randomised to a study arm. Categorical variables describing baseline data were presented in frequencies and percentages, and continuous variables with means and standard deviation (SD). Chi-square or Fisher’s exact test were used to evaluate differences in categorical variables between arm groups; for continuous variables, a Student’s t-test was used. For the primary efficacy endpoint an incidense density and ratio was calculated. Frequencies were reported for the primary safety endpoint and secondary outcome. Data analysis was performed in Stata SE 16.1 (StataCorp, USA).
Results
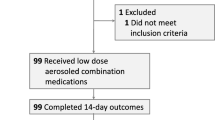
Out of four planned study sites only three opted to enrol and follow participants. A total of 75 HCWs from were invited to participate, of which only 68 met the eligibility criteria and were randomly allocated (Fig. 1). Most participants were female (59%), with a mean age of 39.2 years (SD:9.36). There was greater participation of medical staff (57%). Comparative demographic information, medical history, and baseline exposure data between both arms are shown in Table 1. There were no significant differences between groups in respects to age, gender, or medical history. A difference was found with the use of the face shield, with the HCQ group reporting greater use compared to the control group (p = 0.025).
Eight participants met the primary efficacy endpoint of SAR-CoV-2 infection during the study period: four by rapid serological test and four by PCR from nasopharyngeal swab prompted by the presence of symptoms. Six infections were reported in participants from CMN-CMST, and two from HCH. There was no difference in incidence of SARS-CoV-2 infections between both study arms (HCQ: 5 vs Control: 3, p = 0.538). The relative risk of SARS-CoV-2 infection in the HCQ arm was 1.69 compared to the control group (95% CI 0.41–7.11, p = 0.463). Due to poor participant accrual, the resulting statistical power of the primary efficacy outcome was 11.54%.
Regarding drug safety, no serious adverse events occurred. However, a grade 1 adverse cardiac event was reported in one participant who presented with a 48 ms increase in the QTc interval (Baseline: 389 ms) associated with palpitations. Palpitations occurred after receiving 15 doses of HCQ. Based on the recommendation of the study cardiologist, the regimen was modified to receive HCQ every 3 days. A week after, in the control ECG, a QTc interval of 399 ms was observed and the study regimen was continued. Regarding the tolerability of HCQ prophylaxis, two (2/36, 5.6%) participants no longer wished to participate in the study and withdrew consent due to recurring grade 1 and 2 adverse events (mainly headache and dyspepsia). The list of reported adverse event reports is shown in Table 2.
Discussion
We observed that there is no difference in SARS-CoV-2 infection incidence between the group that received HCQ prophylaxis and the control group. Despite the low statistical power of our study, this finding aligns with other trials evaluating the effectiveness of HCQ to prevent SARS-CoV-2 infection or treat COVID-19 [8, 9].
At the beginning of the pandemic, a call was made for well-designed, robust trials to combat the growing number of observational studies and anecdotal evidence that hinted at the benefits of HCQ. Among the first published, a trial on post-exposure (within 4 days) HCQ prophylaxis found no difference in the incidence of illness compatible with COVID-19 [10].The strength of the study was the clear exposition to SARS-CoV-2, subcategorised as moderate or high [10]; thus, allowing the researchers to observe if there was resolution of clinical symptoms or prevention of severe disease. A post-exposure non-randomised trial among HCWs also failed to show a substantial benefit associated with the use of HCQ [11]. For many researchers the intended purpose of HCQ was as pre-exposure prophylaxis; however, other trials focusing on pre-exposure and HCWs, also found no clinical benefit from the use of HCQ [12, 13]. Our findings add, albeit slightly, to the growing evidence of no benefit in the use of HCQ against SARS-CoV-2 infection. Furthermore, result reporting has been notably deficient with COVID-19 trials, especially in the case of discontinued trials [14]; thus, presenting these findings add transparency to the topic.
Fortunately, the trials did not report an increase in serious adverse events, in particular cardiac, associated with the use of HCQ [10,11,12,13]. Concerns due to prolongation of QT interval and potentially lethal arrhythmias have been mostly associated with the concomitant use of HCQ and azithromycin or other contraindications to HCQ [15], which is why HCWs who had received these drugs were excluded from participating. Lower grade adverse events due to HCQ were common, and as shown in our findings, appear to be an obstacle in participant adherence and retention in the study [10,11,12,13].
Within the first 100 days of the pandemic, a vast number of trials had already been registered on ClinicalTrials.gov and the World Health Organization Inter- national Clinical Registry Platform [14]. Our study is one of many evaluating HCQ for COVID-19 prevention, which ultimately resulted in excessive duplication of research in a pharmacological intervention that was not beneficial [16]. Although this was done to rapidly obtain a therapeutic agent against SARS-CoV-2 infection, many of the trials—including ours—were small (< 500 sample size) and ultimately contributed to research waste or misleading conclusions [16]. Adaptive and pragmatic platform trials that were embedded in clinical care, such as SOLIDARITY or RECOVERY, were key in generating evidence with ample collaboration across multiple sites, allowing for a large sample size and representativeness of patients [16]. Looking forward, having these research platforms in place, with sites ready to enrol and speedy ethical approval processes, would increase pandemic preparedness. Additionally, robust evidence from such trials, arriving at a prompt time would limit and discourage self-medication of untested drugs.
Limitations
The main limitation of our study was the poor participant accrual. Despite the pragmatic design of the trial, we only managed to enrol 21% (68/320) of the intended sample size, from three out of four planned study sites. This design was intended to increase generalisability of the findings and, if beneficial, a rapid uptake into clinical practice [17]. One major reason why recruitment was difficult was that the study period coincided with the publishing of studies that showed no benefit associated with the use of HCQ. Of note, HCWs in our study were seeing first-hand the (lack of) effect of HCQ in hospitalised patients with COVID-19. In addition, safety concerns about QT prolongation (and even Torsades de Pointes) were prominent among HCWs [15, 18]. As a result, after the initial influx of participants, accrual became difficult as HCWs were no longer interested in participating in a trial testing a drug that no longer had any perceivable clinical equipoise.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- BCG:
-
Bacillus Calmette-Guerin
- CMN-CMST:
-
Centro Médico Naval—Cirujano Mayor Santiago Tavara
- CNTEI (acronym in Spanish):
-
Transitory National Committee of Ethics in Research
- COVID-19:
-
Coronavirus disease 2019
- ECG:
-
Electrocardiogram
- G6PD:
-
Glucose-6-phosphate dehydrogenase
- HCH:
-
Hospital Cayetano Heredia
- HCQ:
-
Hydroxychloroquine
- HCW:
-
Healthcare worker
- HNAL:
-
Hospital Nacional Arzobispo Loayza
- ICU:
-
Intensive care unit
- PCR:
-
Polymerase chain reaction
- PPE:
-
Personal protective equipment
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SD:
-
Standard deviation
References
Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA J Am Med Assoc. 2020;324:2161–2.
Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949.
Xu J, Cao B. Lessons learnt from hydroxychloroquine/azithromycin in treatment of COVID-19. Eur Respir J. 2022. https://doi.org/10.1183/13993003.02002-2021.
Jorge A. Hydroxychloroquine in the prevention of COVID-19 mortality. Lancet Rheumatol. 2021;3:e2-3.
Schwalb A, Seas C. The COVID-19 pandemic in Peru: what went wrong? Am J Trop Med Hyg. 2021;104:1176–8.
Neyra-León J, Huancahuari-Nuñez J, Díaz-Monge JC, Pinto JA. The impact of COVID-19 in the healthcare workforce in Peru. J Public Health Policy. 2021;42:182–4.
Iturri de la Mata JA, Gallegos Pacheco RA, Brou Gonzáles PS, Roberto Rovere M. Enfermería y COVID-19 en el Perú. Colegio de Enfermeros del Perú; 2021.
Schwartz IS, Boulware DR, Lee TC. Hydroxychloroquine for COVID19: the curtains close on a comedy of errors. Lancet Reg Health Am. 2022;11:100268.
Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. 2020;173:287–96.
Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383:517–25.
Agusti A, Guillen E, Ayora A, Anton A, Aguilera C, Vidal X, et al. Efficacy and safety of hydroxychloroquine in healthcare professionals with mild SARS-CoV-2 infection: prospective, non-randomized trial. Enferm Infecc Microbiol Clin. 2022;40:289–95.
Rajasingham R, Bangdiwala AS, Nicol MR, Skipper CP, Pastick KA, Axelrod ML, et al. Hydroxychloroquine as Pre-exposure prophylaxis for coronavirus disease 2019 (COVID-19) in healthcare workers: a randomized trial. Clin Infect Dis. 2021;72:e835–43.
Naggie S, Milstone A, Castro M, Collins SP, Seetha L, Anderson DJ, et al. Hydroxychloroquine for pre-exposure prophylaxis of COVID-19 in health care workers: a randomized, multicenter, placebo-controlled trial (HERO-HCQ). bioRxiv. 2021;129:60.
Janiaud P, Axfors C, Ioannidis JPA, Hemkens LG. Recruitment and results reporting of COVID-19 randomized clinical trials registered in the first 100 days of the pandemic. JAMA Netw Open. 2021;4:e210330.
Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1036–41.
Janiaud P, Hemkens LG, Ioannidis JPA. Challenges and lessons learned from COVID-19 trials: should we be doing clinical trials differently? Can J Cardiol. 2021;37:1353–64.
Casey JD, Beskow LM, Brown J, Brown SM, Gayat É, Ng Gong M, et al. Use of pragmatic and explanatory trial designs in acute care research: lessons from COVID-19. Lancet Respir Med. 2022. https://doi.org/10.1016/S2213-2600(22)00044-3.
Hooks M, Bart B, Vardeny O, Westanmo A, Adabag S. Effects of hydroxychloroquine treatment on QT interval. Heart Rhythm. 2020;17:1930–5.
Acknowledgements
The authors would like to acknowledge the work of Ana Quispe, who was a constant aid in the follow-up of participants in HCH.
Funding
None.
Author information
Authors and Affiliations
Contributions
AL-C: conceptualisation, methodology, supervision. AS: conceptualisation, formal analysis, methodology, writing-original draft. JLQ: investigation, data curation. BD: investigation. FA: investigation. CU-G: conceptualisation, formal analysis, methodology. RIGG: investigation. AL: investigation. MG: methodology, supervision. EG: conceptualisation, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the independent ethics committee (IEC) Transitory National Committee of Ethics in Research (CNTEI, acronym in Spanish) from Peru, as process number CNTEI-003-200. This trial was conducted in compliance with the International Conference on Harmonisation (ICH-GCP) E6 on Good Clinical Practice and the clinical trial regulations for COVID-19 (Supreme Decree No. 014-2020 SA) from the National Health Institute (INS, acronym in Spanish). All participants gave written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Llanos-Cuentas, A., Schwalb, A., Quintana, J.L. et al. Hydroxychloroquine to prevent SARS-CoV-2 infection among healthcare workers: early termination of a phase 3, randomised, open-label, controlled clinical trial. BMC Res Notes 16, 22 (2023). https://doi.org/10.1186/s13104-023-06281-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-023-06281-7