Abstract
Background
Recent guidelines have recommended vancomycin trough levels of 15–20 mg/L for treatment of serious infections caused by methicillin-resistant Staphylococcus aureus (MRSA). However, high trough levels may increase risk of nephrotoxicity and mortality, and high vancomycin trough levels have not been well studied. This study was designed to combine safety and efficacy results from independent studies and to compare between high and low vancomycin trough levels in the treatment of MRSA-infected patients using meta-analysis.
Methods
From 19 eligible studies, 9 studies were included in meta-analysis to compare clinical success between high and low vancomycin trough levels, while 10 and 11 studies met criteria for comparing trough levels and nephrotoxicity and trough levels and mortality, respectively. The PubMed/Medline, Web of Science, and Scopus databases, and hand searching were used to identify eligible studies dated up to March 2016. Of 2344 subjects with MRSA infection, 1036 were assigned to trough levels ≥15 mg/L and 1308 to trough levels <15 mg/L.
Results
High vancomycin trough levels were found to be associated with risk of nephrotoxicity (odds ratio [OR] 2.14, 95 % confidence interval [CI] 1.42–3.23 and adjusted OR 3.33, 95 % CI 1.91–5.79). There was no evidence of difference between high and low vancomycin trough levels for mortality (OR; 1.09; 95 % CI 0.75–1.60) or clinical success (OR 1.07; 95 % CI 0.68–1.68).
Conclusion
In this study, high vancomycin trough levels were identified as an independent factor associated with risk of nephrotoxicity in MRSA-infected patients. Association between vancomycin trough levels and both adverse effects and clinical outcomes requires further study.
Similar content being viewed by others
Background
Vancomycin was first approved for use in 1958 by the US Food and Drug Administration (FDA) for treating penicillin-resistant Staphylococcus aureus infection. Vancomycin continues to be widely used, particularly due to recent increases in incidence of serious methicillin-resistant S. aureus (MRSA) infections. Although vancomycin has been used for over 40 years, it still remains a standard treatment for infections caused by MRSA. However, reports began to appear in 2003 describing clinical failures of vancomycin treatment due to the emergence of MRSA with reduced vancomycin susceptibility [1, 2]. Since 2003, several similar studies have been published in which vancomycin-susceptible MRSA strains were identified and clinical failure resulted, despite monitoring and maintenance of trough levels in the recommended range to ensure vancomycin efficacy [3, 4]. Since more than two decades ago and according to Clinical and Laboratory Standards Institute (CLSI) guidelines [5, 6], vancomycin MICs have increased over time—a phenomenon that is referred to as vancomycin MIC creep [7, 8]. As a result of published studies demonstrating vancomycin treatment failure in patients with S. aureus infections who had a vancomycin MIC ≥4 mg/L, the CLSI lowered pre-2006 vancomycin MIC breakpoints by broth microdilution (BMD) from ≤4 to ≤2 µg/mL for susceptible strains of S. aureus.
Early target trough levels for vancomycin were 5–10 mg/L, and then they were increased to 8–15 mg/L. Vancomycin trough levels of 15–20 mg/L (area under the curve [AUC]: minimum inhibitory concentration [MIC] ratio ≥400 in most patients if MIC is ≤1 mg/L) are recommended by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) in patients with normal renal function and serious infections [9, 10]. New guidelines and expert panel recommendations for vancomycin therapeutic drug monitoring (TDM) recommend trough levels of 15–20 mg/L to prevent development of resistance and improve clinical outcomes. Whether trough levels explain the apparent failure of vancomycin treatment remains controversial. Some studies have shown higher troughs not to be associated with increased vancomycin efficacy in patients with MRSA infections [11–16], while others studies did find association with increased efficacy [17–20]. The guideline suggests that vancomycin efficacy in invasive infections caused by MRSA is determined by adult pharmacokinetic and pharmacodynamic data that achieved AUC/MIC of ≥400 which correlates with vancomycin trough levels of 15–20 μg/mL. Because an AUC/MIC goal value is difficult to calculate and given the good comparability between AUC/MIC and vancomycin trough levels, trough levels are considered to be both the most accurate and the most practical method for therapeutic drug monitoring of vancomycin.
The new guidelines also warn that vancomycin nephrotoxicity should be considered if serum creatinine concentration increases greater than or equal to 0.5 mg/dL, or more than 50 % over the baseline value. Previously, most reports of acute kidney injury (AKI) were likely linked to impurities in vancomycin preparation, which was sometimes disparagingly referred to as ‘Mississippi mud’. However, in the 1960s, the purity of vancomycin preparation increased to 75 % with a further increase in purity to 92–95 % in 1985 [10, 21]. As a result, impurities in vancomycin preparation were no longer a concerned.
A recent meta-analysis found association between high vancomycin trough levels and nephrotoxicity in subjects with Gram-positive infections and in patients with various other types of infections [22]. However, in that meta-analysis, the effect of vancomycin trough levels on nephrotoxicity and clinical outcomes in patients with MRSA infection was not investigated. It also remains unclear whether an increase in vancomycin trough levels could improve clinical outcomes of vancomycin treatment in MRSA infections. As such, the aim of this study was to combine safety and efficacy results from independent studies and to compare between high and low vancomycin trough levels in the treatment of MRSA-infected patients using meta-analysis.
Methods
Data sources and search strategy
Multiple electronic databases including MEDLINE/Pubmed, Web of Science, and Scopus, were searched for reports published up to March 2016. The search terms used included “vancomycin”, “trough levels”, “trough concentration”, “nephrotoxicity”, and “methicillin-resistant S. aureus”. The word vancomycin was also combined with other terms in various combinations. MeSH terms for “vancomycin” and “methicillin-resistant Staphylococcus aureus” were also included in our PubMed search. In addition, a hand search of reference lists of selected studies and grey literature (conference proceedings, dissertations, theses, and reports) was conducted to identify relevant studies not included in electronic databases. Abstract lists and conference proceedings from the 2007 to 2015 scientific meetings of the Infectious Diseases Society of America, International Society for Infectious Diseases, American Society for Microbiology, and European Society of Clinical Microbiology and Infectious Diseases were also searched to identify possibly eligible studies (see Additional file 1). No language restrictions were applied for these searches.
Study selection
A single investigator (ST) screened the titles and abstracts of potentially eligible studies, and then examined the articles to determine whether they met the established inclusion criteria. Selected articles were then double-checked in detail by the second investigator (PK). In the end, all selected articles were reviewed and approved by both investigators, with no disagreement between investigators regarding the eligibility of an article identified by one or the other investigator. All published and unpublished studies were included if they met the following criteria: (1) evaluated primary outcomes (i.e., nephrotoxicity, mortality and/or clinical success) of adult patients with MRSA infections; and, (2) the observed outcomes could be extracted and classified into two trough levels. Papers were excluded if they were characterized by one or more of the following: (1) conducted in pediatric patients; (2) focused on treatment of MRSA-infected patients with vancomycin MIC of ≥2 mg/L; (3) we could not extract information relevant to only MRSA infections; or, (4) they were reviews, guidelines, editorials, or non-human research.
Data extraction and management
Data extraction and management for included studies were performed by the first investigator (ST). Both investigators (ST, PK) met to discuss data extraction findings and characteristics of each study for purposes of ensuring clear understanding of assessment criteria. The following information was extracted from each eligible study: the first author’s last name, year of publication, study location, patient characteristic, study design, sample size (number of subjects in high and low trough groups), timing of vancomycin trough level measurement, duration of vancomycin therapy, concomitant nephrotoxic agent, outcomes and definitions of outcomes, ORs and 95 % CIs for each outcome, and covariates adjusted for in multivariable models (when they were available). The first investigator (ST) also screened and double-checked data for data entry errors.
In this meta-analysis, patients were analyzed according to their vancomycin trough levels, which were defined as <15 mg/L for low trough levels and ≥15 mg/L for high trough levels. For each eligible study, categories were recoded as follows: categories “<10” and “10–14 mg/L were collapsed into <15; “15–20 mg/L” was classified as ≥15 mg/L; and, categories “<15” and “≥15 mg/L” were retained in their original category for all analyses. If the information was available, patients with trough level >20 mg/L were eliminated from analysis so we could focus only on the 15–20 mg/L range in high trough patients. For this study, serious MRSA infection was defined as a person with any one or more of the following infections caused by MRSA: bacteremia, endocarditis, osteomyelitis, meningitis, pneumonia, and/or central nervous system infection.
Data synthesis and analysis
A funnel plot was generated to assess funnel plot asymmetry by plotting the standard error of the odds ratio on the vertical axis and the odds ratio on the horizontal axis, with degree of asymmetry tested by Egger’s test [23] and Begg’s test [24]. A p value <0.05 was considered to be statistically significant asymmetry. A forest plot was produced to show the odds ratio with 95 % CI of each study and the pooled odds ratio with the corresponding 95 % CI. Jackknife procedure-based sensitivity analysis was performed by omitting one study at a time to evaluate the effect of individual studies on the stability of the results.
Pooled odds ratio was calculated using the DerSimonian and Laird random-effects model [25]. Greenland-Robin variance formula was used to calculate confidence intervals of the pooled odds ratio. Heterogeneity among studies was evaluated using the Chi square based Q statistics (χ2), measure of inconsistency (I 2), and between-study variance (τ 2). A p value <0.10 was considered to indicate statistically significant heterogeneity while I 2 > 50 % was considered to indicate at least moderate heterogeneity. Trim and fill method [26, 27] was used to estimate overall effect size after adjusting for funnel plot asymmetry arising from publication bias [28]. All analyses were performed using R software with meta package (R Foundation, Vienna, Austria) [29].
Results
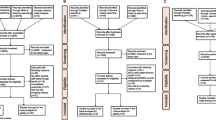
The article selection process for this meta-analysis is shown in Fig. 1. The initial search comprising three online databases, hand searching and grey literature databases for reports published up to March 2016 yielded 1170 articles. That list was narrowed to 35 potentially relevant articles after a review of their titles and abstracts. After a more detailed review, an additional 16 articles were excluded. The remaining 19 reports fully satisfied the inclusion criteria and were included in the final analysis. All included studies were reported in the English language.
Of the 19 included studies, three studies had three categories of trough levels (<15, 15–20 and >20 mg/L), one study had four categories (<10, 10–14.9, 15–20 and >20 mg/L), and the remaining 15 studies had two trough levels (<15 and ≥15 mg/L). One hundred and twelve patients with trough level >20 mg/L were excluded from this meta-analysis. However these patients were later and temporarily included for purposes of comparing outcome results against the results and conclusion with these patients excluded (data not shown). Of 19 eligible studies, meta-analysis of nine studies was per formed to investigate the link between vancomycin trough levels and clinical success, while 10 and 11 studies met the criteria for investigating association between trough levels and nephrotoxicity and trough levels and mortality, respectively. Three studies were available for analysis of the relationship between nephrotoxicity and vancomycin trough levels by combining adjusted OR estimates from multiple logistic regression analysis in order to adjust confounding variables of each included study.
The main characteristics of the studies included in this meta-analysis are presented in Table 1. Included studies were conducted and reported between 1999 and 2013. One thousand and thirty-six subjects were assigned to the high trough group (trough levels ≥15 mg/L) and 1308 subjects were assigned to the low trough group (trough levels <15 mg/L). Effect sizes of outcome measures for each included study are shown in Table 2.
The amount of heterogeneity among the included studies showed evidence of heterogeneity of ORs across the studies for nephrotoxicity (χ2 = 15.78, p = 0.072, I 2 = 43 % and τ 2 = 0.17) and clinical success (χ2 = 18.4, p = 0.018, I 2 = 56.5, and τ 2 = 0.248); whereas heterogeneity was not found for mortality (χ2 = 12.69, p = 0.242; I 2 = 21.2, and τ 2 = 0.09) (Figs. 2, 3, 4). There was no evidence of heterogeneity of adjusted OR among the three studies used to investigate the association between vancomycin trough levels and nephrotoxicity (χ2 = 0.16, p = 0.92; I 2 = 0 % and τ 2 = 0).
In our study, risk of nephrotoxicity was significantly associated with high vancomycin trough levels (OR 2.14 95 % CI 1.42–3.23; p < 0.001). There was, however, no evidence of mortality decline (OR 1.09, 95 % CI 0.75–1.60; p = 0.64) or improved clinical success (OR 1.07, 95 % CI 0.68–1.68; p = 0.761) (Figs. 2, 3, 4). Strength of association between vancomycin trough levels and nephrotoxicity was measured by combining adjusted ORs and confounding variables were adjusted for in each included study (as described in the footnotes of Table 2). After combining the adjusted ORs, the main result was still significant. Specifically, the odds of nephrotoxicity occurring in MRSA-infected patients with trough levels ≥15 mg/L were 3.33 times higher than patients with trough levels <15 mg/L (95 % CI 1.91–5.79; p < 0.0001).
For nephrotoxicity, none of the included studies influenced the results to an extent that the conclusion would have changed. The jackknife sensitivity analysis with omitted one study at a time and reevaluated association between trough levels and nephrotoxicity, consistently showed that vancomycin trough levels were associated with risk of nephrotoxicity (Fig. 5a). The sensitivity analysis also showed that the one-by-one exclusion of each study did not affect the conclusion of pooled effect size for either mortality or clinical success.
The funnel plot revealed some asymmetry for nephrotoxicity and clinical success (Fig. 6a, c). Formal testing for publication bias relative to two outcomes (nephrotoxicity—Begg’s test: p = 0.65 and Egger’s test: p = 0.62; and, mortality: Begg’s test: p = 0.82 and Egger’s test: p = 0.87) did not show statistical significance of asymmetry. These tests, however, yielded statistical significance of asymmetry for clinical success (Begg’s test: p = 0.095 and Egger’s test: p = 0.003).
a Funnel plot for nephrotoxicity after adjusting for missing studies using the trim and fill method; b Symmetrical funnel plot for mortality with an absence of evidence for asymmetry; c Funnel plot for clinical success after adjusting for missing studies using the trim and fill method (filled circles are original data and open circles represent estimated missing studies)
Adjustment for funnel plot asymmetry using the trim and fill method did not change the pooled OR for mortality (Fig. 6b). There was some funnel plot asymmetry for both nephrotoxicity and clinical success, and the trim and fill method indicated that the true estimates of pooled OR for nephrotoxicity and clinical success may be 1.99 (95 % CI 1.33–2.96; p < 0.001) and 1.71 (95 % CI 1.04–2.81; p = 0.034), respectively (Fig. 6a, c). However, after adjusting for funnel plot asymmetry, the conclusion of this meta-analysis regarding nephrotoxicity was not changed, while there was a significant change in the conclusion for clinical success. The trim and fill method demonstrated significant association between high trough levels and clinical success, with the odds of clinical success in MRSA-infected patients with high trough levels being 1.71 times higher than in patients with low trough levels.
Discussion
Association between vancomycin trough levels and nephrotoxicity was largely uniform across the studies included in this meta-analysis; however incidence of vancomycin-induced nephrotoxicity varied from study to study. Nephrotoxicity rates in the high and low trough groups varied from 18 to 55 % and 0 to 29 %, respectively. This variability was due to varying baseline levels among the study population, length of vancomycin therapy, receipt of other nephrotoxic agents, and renal function (creatinine clearance). From the 19 studies in this analysis, the study in patients with any type of nosocomial MRSA infection had the lowest nephrotoxicity rate [30] while the study in patients with MRSA HCAP had the highest nephrotoxicity rate in both trough level groups [31]. Patients in the study with the highest rate of nephrotoxicity had higher APACHE II scores and received more concomitant nephrotoxic agents than patients in all other studies. These results support the finding of some other studies that concomitant nephrotoxic agents were a risk factor for renal function impairment during vancomycin therapy [30–32]. The meta-analysis by van Hal and colleagues [22] found nephrotoxicity rates that varied from 7 to 67 % in the high trough group (≥15 mg/L) and from 0 to 33 % in the low trough group (<15 mg/L), which both ranges exceeding those from our study. Nephrotoxicity ranges in the van Hal, et al. meta-analysis were wider than the ranges in this study due to some differences of the included studies and no limitation regarding the type of MRSA infection included in their study.
Clinical success was extremely variable across the studies in this analysis with most differences between high and low trough levels being statistically non-significant. There was two exceptions [17, 33], however, with success rates ranging from 24 to 82 % and 39 to 87 % for high and low trough groups, respectively. This variability was due to baseline clinical status of study population and sites of MRSA infections. In the present meta-analysis, the studies with the most patients infected with MRSA pneumonia had low clinical success rates ranging from 24 to 54 % [34, 35]. MRSA-infected patients in the intensive care unit (ICU) were also more likely to have poor clinical success with vancomycin therapy, as compared to non-ICU MRSA-infected patients [36, 37].
Mortality rate also varied widely across the studies in this analysis, ranging from 5 to 20 % in most of the studies and from 36 to 47 % in two studies [36, 38]. No statistically significant differences were observed between high and low trough groups. Patients who had either MRSA bacteremia or who required intensive care had higher mortality rates. The results of this meta-analysis support the results of previous studies [15, 39], all finding that there is no evidence supporting association between higher vancomycin trough levels and improved outcome in patients with MRSA. Although we had originally planned to conduct analysis using a meta-regression model to investigate possible sources of heterogeneity of clinical success among the studies, sufficient data were not available to conduct this analysis.
Based on our review of the literature, this study is the first meta-analysis to compare the safety and efficacy of high vs. low vancomycin trough levels in MRSA-infected patients that were treated according to the current guidelines for treating MRSA infections. The results of this analysis also confirm the results of previous studies that were not included in this meta-analysis, which showed that higher vancomycin trough levels (or higher vancomycin doses) were associated with increase risk of nephrotoxicity [8, 30, 40–46]. Considerable controversy exists concerning the relationship between vancomycin MICs and clinical outcomes. Several studies have reported association between higher vancomycin MIC and increased risk of treatment failure or mortality [30, 47–54], with others finding no significant association with poor outcomes [37, 55–59]. However, recent meta-analysis study in high vancomycin MIC and clinical outcomes in adults with MRSA infections by Jacob and DiazGranados [60] found that high vancomycin MIC was associated with increased mortality and treatment failure.
In this study, the random effects model was used to combine the odds ratios for the outcomes of interest, even though there was no evidence of heterogeneity. The decision to use the random effects model was made a priori, depending upon the nature of the eligible studies and our goals for the following reasons: individual eligible studies were collected from several independent studies; included studies were heterogeneous for study design; and, inferences based on the random effects model can be generalized beyond the studies included in the meta-analysis.
This study has several notable strengths. First, studies that were included in this meta-analysis were independent studies that were conducted during different periods of observation. The results, both individually and collectively, strongly support the fact that there is evidence that higher trough levels are more harmful than low trough levels in terms of nephrotoxicity. Secondly, the results of influence analysis on all outcomes in which each study was removed from the analysis one by one to determine the magnitude of influence on overall effect size, showed that overall effect size was relatively independent of any one particular study. Third, adjusting for asymmetric funnel plots using the trim and fill method did not significantly change the results of this meta-analysis for nephrotoxicity and mortality, indicating that the missing studies were unlikely to have changed the conclusions relating to these outcomes. However, the results of trim and fill analysis showed substantial impact of publication bias on the conclusion for clinical success. Specifically, after trim and fill, the association between high trough level and clinical success was no longer non-significant. Finally, the conclusions of the present meta-analysis were similar to the conclusions from our exploratory meta-analysis that included the excluded 112 patients that had vancomycin trough levels of >20 mg/L.
This study also has some mentionable limitations. First, the definition of mortality and/or clinical success varied slightly among some of the studies included in this meta-analysis, according to the stated protocol of each study. Second, this meta-analysis included a combination of different study designs, including: nine retrospective cohort studies, four prospective cohort studies, two retrospective studies, one non-randomized comparative study, one retrospective quasi-experimental study, one prospective surveillance study, and one multicenter prospective study. Third, only one study met the criteria for combining adjusted OR from different studies for the outcomes of clinical success and mortality; thus, these analyses were not performed. Finally, we did not evaluate the other factors that might associate with high vancomycin trough levels due to lack of information.
The inclusion of grey literature that meets the predefined inclusion criteria of a meta-analysis may help to reduce the effect of publication bias and/or funnel plot asymmetry, whereas the exclusion of grey literature could lead to bias, most notably the overestimation of effect sizes [61, 62]. The research cited in published reviews demonstrates that studies published in journals have a tendency to report larger effect sizes than studies published in grey literature [63, 64].
Conclusion
Based on pooled adjusted OR, high vancomycin trough level is the variable that was identified as the independent factor associated with risk of nephrotoxicity in MRSA infections. MRSA-infected patients with trough levels ≥15 mg/L had greater odds of nephrotoxicity than those with trough levels <15 mg/L. There was no evidence of difference in mortality or clinical success between patients with trough levels ≥15 and <15 mg/L. However, we need to acknowledge that this conclusion does not take into account vancomycin MIC data, which were not available for analysis in this study. Since adjustment of funnel plot asymmetry using the trim and fill method yielded significant change in pooled OR for clinical success, association between high vancomycin trough levels and both risk for adverse events and improvement in clinical outcomes in patients with MRSA infection requires further study.
Abbreviations
- MRSA:
-
methicillin-resistant Staphylococcus aureus
- MIC:
-
minimum inhibitory concentration
References
Lutz L, Machado A, Kuplich N, Barth AL. Clinical failure of vancomycin treatment of Staphylococcus aureus infection in a tertiary care hospital in southern Brazil. Braz J Infect Dis. 2003;7:224–8.
Sakoulas G, Eliopoulos GM, Moellering RC Jr, Novick RP, Venkataraman L, Wennersten C, et al. Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? J Infect Dis. 2003;187:929–38.
Gould IM. Is vancomycin redundant for serious staphylococcal infection? Int J Antimicrob Agents. 2010;36(Suppl 2):55–7.
Garau J, Bouza E, Chastre J, Gudiol F, Harbarth S. Management of methicillin-resistant Staphylococcus aureus infections. Clin Microbiol Infect. 2009;15:125–36.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; sixteenth international supplement. CLSI document M100-S16. Wayne: CLSI; 2006.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, twenty-second informational supplement. CLSI document M100–S22. Wayne: CLSI; 2012.
Weber SG, Salgado C. Healthcare associated infections: a case-based approach to diagnosis and management. New York: Oxford University Press; 2013.
Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother. 2007;60:788–94.
Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82–98.
American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
Ok HS, Lee HS, Park MJ, Kim KH, Kim BK, Wi YM, et al. Predictors and clinical outcomes of persistent methicillin-resistant Staphylococcus aureus bacteremia: a prospective observational study. Korean J Intern Med. 2013;28:678–86.
Ackerman BH, Guilday RE, Reigart CL, Patton ML, Haith LR. Evaluation of the relationship between elevated vancomycin trough concentrations and increased efficacy and/or toxicity. J Burn Care Res. 2013;34:e1–9.
Choi W, Noh J, Huh J, Youn Y, Song JY, Sohn J, et al. Clinical response and nephrotoxicity according to the trough serum vancomycin concentration among patients with methicillin-resistant Staphylococcus aureus bacteraemia. In: 21st European Congress of Clinical Microbiology and Infectious Diseases (ECCMID). Milan; 2011. Abstract P 1290.
Itani KM, Dryden MS, Bhattacharyya H, Kunkel MJ, Baruch AM, Weigelt JA. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be caused by methicillin-resistant Staphylococcus aureus. Am J Surg. 2010;199:804–16.
Jeffres MN, Isakow W, Doherty JA, McKinnon PS, Ritchie DJ, Micek ST. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation for vancomycin pharmacokinetic indices. Chest. 2006;130:947–55.
Kettle JK, Grace JW, Schaefer S, Desai A. Treatment of methicillinresistant Staphylococcus aureus with a vancomycin minimum inhibitory concentration of 2 mcg/ml. Hosp Pharm. 2010;45:375–80.
Kullar R, David SL, Taylor TN, Kaye KS, Rybak MJ. Effects of targeting higher vancomycin trough levels on clinical outcomes and costs in a matched patient cohort. Pharmacotherapy. 2012;32:195–201.
Cheong JY, Makmor-Bakry M, Lau CL, Abdul Rahman R. The relationship between trough concentration of vancomycin and effect on methicillin-resistant Staphylococcus aureus in critically ill patients. S Afr Med J. 2012;102:616–9.
Lomaestro BM. Vancomycin dosing and monitoring 2 years after the guidelines. Expert Rev Anti Infect Ther. 2011;9:657–67.
Forstner C, Dungl C, Tobudic S, Mitteregger D, Lagler H, Burgmann H. Predictors of clinical and microbiological treatment failure in patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia: a retrospective cohort study in a region with low MRSA prevalence. Clin Microbiol Infect. 2013;19:E291–7.
Mohammedi I, Descloux E, Argaud L, et al. Loading dose of vancomycin in critically ill patients: 15 mg/kg is a better choice than 500 mg. Int J Antimicrob Agents. 2006;27:259–62.
van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 mg per liter. Antimicrob Agents Chemother. 2013;57:734–44.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Taylor SJ, Tweedie RL. Practical estimates of the effect of publication bias in meta-analysis. Aust Epidemiol. 1998;5:14–7.
R Development Core Team R. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. ISBN 3-900051-07-0. http://www.R-project.org/.
Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166:2138–44.
Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther. 2007;29:1107–15.
Hanrahan TP, Harlow G, Hutchinson J, Dulhunty JM, Lipman J, Whitehouse T, Roberts J. Vancomycin-associated nephrotoxicity in the critically ill: a retrospective multivariate regression analysis. Crit Care Med. 2014;42:2527–36.
Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52:975–81.
Leu WJ, Liu YC, Wang HW, Chien HY, Liu HP, Lin YM. Evaluation of a vancomycin dosing nomogram in achieving high target trough concentrations in Taiwanese patients. Int J Infect Dis. 2012;16:e804–10.
Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9:9–14.
Chung J, Oh JM, Cho EM, Jang HJ, Hong SB, Lim CM, et al. Optimal dose of vancomycin for treating methicillin-resistant Staphylococcus aureus pneumonia in critically ill patients. Anaesth Intensive Care. 2011;39:1030–7.
Clemens EC, Chan JD, Lynch JB, Dellit TH. Relationships between vancomycin minimum inhibitory concentration, dosing strategies, and outcomes in methicillin-resistant Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis. 2011;71:408–14.
Rojas L, Bunsow E, Muñoz P, Cercenado E, Rodríguez-Créixems M, Bouza E. Vancomycin MICs do not predict the outcome of methicillin-resistant Staphylococcus aureus bloodstream infections in correctly treated patients. J Antimicrob Chemother. 2012;67:1760–8.
Hall RG II, Giuliano CA, Haase KK, Hazlewood KA, Frei CR, Forcade NA, et al. Empiric guideline-recommended weight-based vancomycin dosing and mortality in methicillin-resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Infect Dis. 2012;12:104.
Haque NZ, Kiyan PO, Reyes K, Moore CL, Peyrani P, Ramirez JA, et al. Nephrotoxicity in intensive care unit patients with hospital-acquired pneumonia: the IMPACT-HAP Project. In: 47th Annual Meeting of Infectious Diseases Society of America. Arlington: IDSA; 2009. Abstract 388.
Kralovicová K, Spanik S, Halko J, Netriova J, Studena-Mrazova M, Novotny J, et al. Do vancomycin serum levels predict failures of vancomycin therapy or nephrotoxicity in cancer patients? J Chemother. 1997;9:420–6.
Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52:1330–6.
Nguyen M, Wong J, Lee C, Nguyen L, Quist R, Hirokawa C, et al. Nephrotoxicity associated with high dose vs. standard dose vancomycin therapy. In: 47th interscience conference on antimicrobial agents and chemotherapy (ICAAC). Washington: ASM Press; 2007. Abstract K-1096.
Prabaker K, Tran T, Pratummas T, Goetz M, Graber C. Association of vancomycin trough levels with nephrotoxicity. In: 47th Annual Meeting of Infectious Diseases Society of America. Arlington: IDSA; 2009. Abstract 192.
Pritchard L, Baker C, Leggett J, Sehdev P, Brown A, Bayley KB. Increasing vancomycin serum trough concentrations and incidence of nephrotoxicity. Am J Med. 2010;123:1143–9.
Zimmermann AE, Katona BG, Plaisance KI. Association of vancomycin serum concentrations with outcomes in patients with Gram-positive bacteremia. Pharmacotherapy. 1995;15:85–91.
de Sanctis JT, Sawarynski K, Gerasymchuk L, Gerasymchuk L, Powell K, Robinson-Dunn B, et al. Is there a clinical association of vancomycin MIC creep, agr group II locus, and treatment failure in MRSA bacteremia? Diagn Mol Pathol. 2011;20:184–8.
Haque NZ, Zuniga LC, Peyrani P, Reyes K, Lamerato L, Moore CL, et al. Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-acquired, or health-care associated pneumonia. Chest. 2010;138:1356–62.
Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008;52:3315–20.
Moise-Broder P, Sakoulas G, Eliopoulos G, Schentag J, Forrest A, Mollering RJ. Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin Infect Dis. 2004;38:1700–5.
Neoh H-M, Hori S, Komatsu M, Oguri T, Takeuchi F, Cui L, et al. Impact of reduced vancomycin susceptibility on the therapeutic outcome of MRSA bloodstream infections. Ann Clin Microbiol Antimicrob. 2007;6:13.
Sakoulas G, Moise-Broder P, Schentag J, Forrest A, Moellering RJ, Elipopoulos G. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42:2398–3402.
Soriano A, Marco F, Martínez J, Pisos E, Almela M, Dimova VP, et al. Influence of vancomicina minimum inhibitory concentration on the treatment of methicillinresistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200.
Yoon Y, Kim J, Park D, Sohn J, Kim M. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J Antimicrob Chemother. 2010;65:1015–8.
Beeston C, Gupta R, Chadwick P, Young R. Methicillin-resistant Staphylococcus aureus bacteraemia and mortality in a teaching hospital. Eur J Clin Microbiol Infect Dis. 2009;28:585–90.
Lalueza A, Chaves F, San Juan R, Daskalaki M, Otero J, Aguado J. Is high vancomycin minimum inhibitory concentration a good marker to predict the outcome of methicillin-resistant Staphylococcus aureus bacteremia?. J Infect Dis. 2010;201:311–312 (author reply 312–313).
Liao CH, Chen SY, Huang YT, Hsueh PR. Outcome of patients with meticillin resistant Staphylococcus aureus bacteraemia at an Emergency Department of a medical centre in Taiwan. Int J Antimicrob Agents. 2008;32:326–32.
Crompton JA, North DS, Yoon M, Steenbergen JN, Lamp KC, Forrest GN. Outcomes with daptomycin in the treatment of Staphylococcus aureus infections with a range of vancomycin MICs. J Antimicrob Chemother. 2010;65:1784–91.
Musta A, Riederer K, Shemes S, Chase P, Jose J, Johnson LB, et al. Vancomycin MIC plus heteroresistance and outcome of methicillin-resistant Staphylococcus aureus bacteremia: trends over 11 years. J Clin Microbiol. 2009;47:1640–4.
Jacob JT, DiazGranados CA. High vancomycin minimum inhibitory concentration and clinical outcomes in adults with methicillin-resistant Staphylococcus aureus infections: a meta-analysis. Int J Infect Dis. 2013;17:e93–100.
Tongsai S, Thamlikitkul V. The safety of early versus late ambulation in the management of patients after percutaneous coronary interventions: a meta-analysis. Int J Nurs Stud. 2012;49:1084–90.
Wilson DB. Missing a critical piece of the pie: simple document search strategies inadequate for systematic reviews. J Exp Criminol. 2009;5:429–40.
Conn VS, Valentine JC, Cooper HM, Rantz MJ. Grey literature in meta-analyses. Nurs Res. 2003;52:256–61.
Holly C, Salmond SW, Saimbert MK. Comprehensive systematic review for advanced nursing practice. New York: Springer; 2012.
Casapao AM, Patel S, Kullar R, Davis SL, Levine DP, Rybak MJ. Evaluation of vancomycin exposure on outcomes in a cohort of patients with methicillin-resistant Staphylococcus aureus (MRSA) infective endocarditis (IE). In: 23rd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID). Berlin; 2013. Abstract O408.
Casapao AM, Leonard SN, Davis SL, Lodise TP, Patel N, Goff DA, et al. Clinical outcomes in patients with heterogeneous vancomycin-intermediate Staphylococcus aureus bloodstream infection. Antimicrob Agents Chemother. 2013;5:4252–9.
Zelenitsky S, Rubinstein E, Ariano R, Iacovides H, Dodek P, Mirzanejad Y, Cooperative Antimicrobial Therapy of Septic Shock-CATSS Database Research Group, et al. Vancomycin pharmacodynamics and survival in patients with methicillin-resistant Staphylococcus aureus-associated septic shock. Int J Antimicrob Agents. 2013;41:255–60.
Tadros M, Williams V, Coleman BL, McGeer AJ, Haider S, Lee C, et al. Epidemiology and outcome of pneumonia caused by methicillin-resistant Staphylococcus aureus (MRSA) in Canadian Hospitals. PLoS One. 2013;8:e75171.
Arshad S, Shoyinka A, Chen A, Jacobsen G, Zervos M. Evaluation of vancomycin serum trough concentrations and outcomes in meticillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2012;40:474–5.
Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621–9.
Bosso JA, Nappi J, Rudisill C, Wellein M, Bookstaver PB, Swindler J, et al. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother. 2011;55:5475–9.
Chan JD, Pham TN, Wong J, Hessel M, Cuschieri J, Neff M, et al. Clinical outcomes of linezolid vs vancomycin in methicillin-resistant Staphylococcus aureus ventilator-associated pneumonia: retrospective analysis. J Intensive Care Med. 2011;26:385–91.
Honda H, Doern CD, Michael-Dunne W Jr, Warren DK. The impact of vancomycin susceptibility on treatment outcomes among patients with methicillin resistant Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:335.
Authors’ contributions
ST and PK conceived, designed, and conducted the study. ST conducted the literature search, performed data extraction and analysis, and interpreted the results. ST and PK mutually participated in the identification of appropriate studies that satisfied the inclusion criteria. ST drafted and wrote the manuscript. ST and PK critically reviewed the manuscript for important intellectual content and revised the manuscript. ST and PK read and approved the final version of the manuscript. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
This study was presented in part at the 23rd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), on 30 April 2013 in Berlin, Germany.
Funding disclosures
This was an unfunded study.
Author information
Authors and Affiliations
Corresponding author
Additional file
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tongsai, S., Koomanachai, P. The safety and efficacy of high versus low vancomycin trough levels in the treatment of patients with infections caused by methicillin-resistant Staphylococcus aureus: a meta-analysis. BMC Res Notes 9, 455 (2016). https://doi.org/10.1186/s13104-016-2252-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-016-2252-7