Abstract
Background
Palliative care (PC) patients experience loss of physical function which usually impedes mobility, autonomy and quality of life. We aimed at examining the feasibility of a home-based exercise program for patients with advanced, incurable diseases after discharge.
Results
This was a single-arm pilot study (WHO-ICTRP: DRKS00005048). The 12-week home-based program comprised strength, balance, flexibility and endurance components. Patients with a presumed life expectancy of 6–12 months were recruited during a 6-months period on a specialized PC and a radiation therapy ward. We chose the De Morton Mobility Index as primary outcome. Secondary outcomes were quality of life, 6-min walk test and others. A total of 145 patients were screened, 103 (98 %) out of 105 patients on the specialized PC ward could not be included, mostly because of a low performance status [n = 94; 90 %; Eastern Cooperative Oncology Group (ECOG) >2]. The only two eligible patients declined to participate. Eleven out of 40 patients (28 %) were eligible on the radiation therapy ward. However, only one patient (9 %) participated but dropped out 2 days later (upcoming surgery). Distance to the hospital (n = 3; 30 %) and considering additional tasks as “too much” (n = 3; 30 %) were most common reasons for non-participation.
Conclusions
Establishing a home-based exercise program for inpatients after discharge was not feasible mainly due to non-eligibility and lack of demand. For future trials, we suggest that choosing (1) outpatients with (2) an ECOG of ≤2 and (3) an estimated survival of ≥9 months could enhance participation in home-based exercise programs.
Similar content being viewed by others
Background
The decrease in physical functioning impedes quality of life, mobility and autonomy of patients with terminal diseases [1–3]. Physical exercise programs are more and more advocated for patients given palliative care or suffering from advanced cancer in order to address the abovementioned aspects [4–7]. In the hospital, physical exercise is usually supervised by a physiotherapist. To ensure long-term effects of physical exercise, patients might be supported even after their discharge, e.g. by providing a manual with relevant exercises for a home-based exercise program. Interestingly, advanced cancer patients (n = 42, 84 %) stated in a previous survey that they would prefer participating in a physical activity program at home [8]. Some completely home-based exercise programs were recently conducted in patients with advanced cancer of different entities and various life expectancies [9–11]. These studies indicate an improved mobility and a decreased fatigue as a result from physical exercise [9, 11] but also point out challenges in patient recruitment and feasibility [10]. It is necessary to enhance the body of evidence for a comprehensive appraisal of the feasibility and efficacy of such home-based exercise programs.
Therefore, the primary study aim was to examine the feasibility of a home-based exercise program for patients with advanced, incurable diseases after discharge.
Methods
Patients
In this interventional single-arm pilot study, adult patients with incurable diseases, a clinician-estimated life expectancy of 6–12 months, an Eastern Cooperative Oncology Group (ECOG) Score ≤2, a numerical rating scale (NRS; 0–10) for pain ≤3 and an adequate cognitive status were included (Table 1). Patients with neurological or orthopedic diseases (that impeded the execution of our home-based exercise program), osseous metastases, heart failure of New York Heart Association (NYHA) stadium III–IV, hypertensive emergency (defined by the American Heart Association as blood pressure that damages organs or exceeds 180 systolic and 120 diastolic) in the last 12 months, bleeding tendency, and dyspnea during movement [verbal rating scale (VRS) ≥2] were excluded to ensure patients’ safety (Table 2). The decision for these inclusion criteria resulted from discussions of our multidisciplinary team (two physicians, two sport and exercise scientists, one psychologist and one theologian) and on the basis of literature [7, 10].
This pilot study was conducted in accordance to the Declaration of Helsinki. It was approved by the Freiburg Ethics Commission and subsequently registered on the World Health Organization International Clinical Trials Registry Platform (WHO-ICTRP: DRKS00005048).
Intervention
The program was designed to start with two instruction lessons while the patient was still in the hospital. After the patients’ discharge, the 12-week exercise program should be conducted at home with the help of an exercise manual. The home-based exercise program consisted of two parts: a strength training (ca. 35 min; three sets, 10–15 repetitions; five exercises: e.g. squats, wall push-ups) and a combined balance-endurance-flexibility training [ca. 25 min; 15–20 min walking, 5–10 min balance (e.g. tandem, semi-tandem) and flexibility exercises (e.g. pectoral and hip flexor stretch)]. No training equipment was necessary since moderate bodyweight exercises were chosen. Ratings of perceived exertion (RPE) were set between 13 (“somewhat hard”) and 14 points on the Borg RPE scale [12]. The program enabled to vary exercises in a way that an RPE of 13–14 could be theoretically achieved by each patient.
To ensure adherence and intervention fidelity, an exercise manual and a training diary were prepared. Moreover, we planned to call the patients bimonthly to ask if there were problems and barriers during the exercise program.
An extra feature of this intervention was that the patient’s caregiver was asked to participate in the exercise program as caregivers tend to neglect themselves and receive little support by the health care system [13–15]. Moreover, they could contribute to patients’ adherence regarding the home-based program.
Outcome measures
The primary study aim was to evaluate the feasibility of the home-based exercise program. However, feasibility is a broad concept with up to eight different areas [16]. We focused on the areas acceptability (patients’ and staffs’ reaction to study/intervention) and expansion. The latter was defined by Bowen et al. as “potential success of an already-successful intervention with a different population or in a different setting” [16]. Since this trial had an exploratory character, acceptability and expansion were only defined qualitatively, not quantitatively.
We planned to measure the following outcome measures at inclusion, after 6 and 12 weeksFootnote 1: primary endpoint was the De Morton Mobility Index (DEMMI) as mobility seems to be a precondition for autonomy which is mostly associated with quality of life [17, 18]. The DEMMI is a validated tool in acute medical population [19] and was considered to be appropriate for palliative care patients.
Secondary outcomes were the European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire 30 (EORTC QLQ-C30) [20], Romberg test (parallel, semi-tandem and tandem with open eyes), 6-min walk test (6MWT) [21], Barthel Index (BI) [22], five times sit-to-stand test (FTSST) [23], hand grip strength [7] and qualitative interviews (benefits, barriers) after the intervention.
Endpoints for caregivers were the “Indikatoren des Reha-Status 24” (IRES-24) [24], a questionnaire on health status and physical functioning, Romberg test, hand grip strength and 30 s chair [25] stand test.
Recruitment process
Clinicians on a specialized palliative care ward (August 2013 to January 2014) and on a radiation therapy ward (December 2013 to January 2014) screened patients for eligibility on admission. The patients were hospitalized for symptom control or radiation therapy. Eligible patients were contacted and information on the study was provided. Eligible patients who did not want to participate were asked for reasons whilst emphasizing that non-participation would not result in any disadvantage for the subsequent treatment. No personal health data of patients could be documented if no informed consent was obtained.
Analysis of results
Numbers and percent were used to present the results. Due to organizational differences during recruitment and different eligibility (mainly ECOG differences) between both wards, each ward was analyzed separately. We aimed at recruiting 25 patients for this pilot study.
Results
Specialized palliative care ward
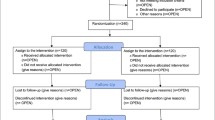
Two clinicians screened 105 patients on the specialized palliative care ward from August 2013 to January 2014 (Fig. 1). According to the criteria for inclusion and exclusion of our study only two patients (2 %) were eligible. Table 1 shows the number and percentages of patients that fulfilled (column: “yes”) the different inclusion criteria. Eleven patients (10 %) had an ECOG of ≤2 implicating that most patients on the specialized palliative care ward were too fragile for the exercise program. The most prevalent reason for non-participation concerning exclusion criteria was dyspnea with 39 patients (37 %) reaching ≥2 on the VRS (Table 2).
Two patients were eligible but they were not included in the study. The first patient reported having enough exercise by “walking regularly with the dog”. The second patient stated the “distance to the hospital” (60 km) and an “upcoming move” as a main barrier for non-participation.
Radiation therapy ward
A total of 40 patients were screened for eligibility by three clinicians of the radiation therapy ward from December 2013 to January 2014 (Fig. 1). Twenty-nine patients (73 %) in the radiation therapy ward had an estimated life expectancy over 12 months and, therefore, were not eligible. Eligible patients (n = 11, 28 %; six female and five male; aged 62–86 years, mean: 67.1, standard deviation: 9.4) were contacted by the study investigator. Three out of eleven (27 %) patients did not participate in the exercise program because they felt it would be “too much” in addition to all other burdens. Another three patients (27 %) resigned because of the large distance to the revaluation site (University Medical Center) (see Fig. 1 for additional resaons). Only one (1/11, 9 %) male, 72 years old, patient with lung cancer and his caregiver signed the informed consent but withdrew 2 days later because of the decision for surgery.
Discussion
Feasibility
The acceptability for participation on the part of the patients can be considered as low because only one patient out of 11 eligible patients gave informed consent (Fig. 1). However, the decision for not participating is multifactorial and could probably not be summarized in a single reason. It is hardly possible to judge whether the given reason, the complex overall situation, the study design, the intervention, other reasons or a combination of these factors have led to the patients’ decision. The low number of eligible patients can be traced back to our eligibility criteria, which apparently was not appropriate, and the low acceptability may be a consequence of different recruitment barriers (see paragraphs below).
No patient completed the study. Therefore, no judgement can be made on the efficacy of the intervention for this population (expansion) [16].
Feasibility (here: acceptability and expansion) was not defined in a quantitative way because there is a lack of studies with comparable inclusion criteria (especially for life expectancy) [10]. This decision can be criticized. However, an a priori quantitative definition, though arbitrary, is important in order to make a clear and transparent decision.
Non-eligibility
This pilot study confirms some previously identified recruitment difficulties in palliative care like low eligibility and severe patient illness [26]. Non-eligibility was the main problem on the specialized palliative care ward (n = 103; 98 %) whereas the acceptability and demand [16] for the home-based exercise program was low for eligible patients (1/11; 9 %) of the radiation therapy ward. In a study by Lowe et al. [10] 524 outpatients were screened, nine (2 %; median survival: 92 days) consented to participate and just three (dropout rate 67 %) completed the 6 week exercise program. It is suggested that patients with a better performance status and longer (median) survival clearly contribute to a study’s feasibility as seen from the example of Cheville et al. [93 patients screened, 66 (71 %) randomized; dropout rate: 7/33; 21 %] [9].
Recruitment barriers
We tried to recruit inpatients for a home-based program after their discharge. It is noticeable that (especially palliative care) inpatients experience probably more burden by symptoms, psychosocial problems and are more confronted with diagnostic and therapeutic interventions than outpatients that were recruited in two comparable studies [9, 10]. In addition, being not at home and spending thoughts (i.e. cognitive capacity) on further treatments or different psychosocial questions could have contributed to the low participation of eligible patients especially from the radiation therapy ward.
Eligible patients on the radiation therapy ward were possibly confused by the term “palliative” as conversations about patients’ prognosis are often neglected [27]. Moreover, advanced cancer patients are often not aware that their situation or treatment is non-curative [28, 29].
Lowe’s [10] and our recruitment difficulties are in contrast to an interviewer-administered needs assessment [8] where 39 of 50 terminally ill patients (78 %) stated to be interested in a physical activity program. Social desirability bias may have led to the overoptimistic survey results [30, 31].
Implications and suggestions for future research
Acceptability, demand (radiation therapy ward) and especially non-eligibility (specialized palliative care ward) were the main problems for non-participation in this study [16]. Several studies show that these problems can be reduced by using wider limits (than in this study) with respect to life expectancy or performance status [4, 7, 32–34].
A reasonable combination of modulating factors (Table 3) could contribute to a higher demand or feasibility for home-based exercise programs. However, emerging costs and practicability should be taken into account to enable transfer in daily clinical practice [16].
Based on our experience from this trial and on two comparable studies [9, 10], we suggest that the following criteria could enhance participation and enable evaluation of the benefits of a home-based exercise program:
-
palliative care outpatients
-
ECOG of ≤2
-
estimated survival >9 months
Conclusion
Implementing a home-based exercise program was not feasible for patients with advanced, incurable diseases after discharge from a specialized palliative care ward and a radiation therapy ward. Patients on specialist palliative care and radiotherapy wards might be too sick and burdened by other symptoms and medical interventions to feel comfortable in engaging themselves in a home-based exercise program.
Notes
As a consequence of the non-feasibility of this study and for the upcoming future trial we slightly changed the study duration to six weeks and chose a interim measurement of 3 weeks (http://apps.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00005048; http://drks-neu.uniklinik-freiburg.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00005048).
Abbreviations
- BI:
-
Barthel Index
- DEMMI:
-
De Morton Mobility Index
- ECOG:
-
Eastern Cooperative Oncology Group
- EORTC QLQ-C30:
-
European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire 30
- FTSST:
-
five times sit-to-stand test
- IRES-24:
-
Indikatoren des Reha-Status 24
- PC:
-
palliative care
- RPE:
-
ratings of perceived exertion
- VRS:
-
verbal rating scale
- WHO-ICTRP:
-
World Health Organization International Clinical Trials Registry Platform
- 6MWT:
-
6-min walk test
References
Helbostad JL, Hølen JC, Jordhøy MS, Ringdal GI, Oldervoll L, Kaasa S. A first step in the development of an international self-report instrument for physical functioning in palliative cancer care: a systematic literature review and an expert opinion evaluation study. J Pain Symptom Manage. 2009;37:196–205.
Lowe SS, Watanabe SM, Baracos VE, Courneya KS. Associations between physical activity and quality of life in cancer patients receiving palliative care: a pilot survey. J Pain Symptom Manage. 2009;38:785–96.
Cohen SR, Leis A. What determines the quality of life of terminally ill cancer patients from their own perspective? J Palliat Care. 2002;18:48–58.
Jensen W, Baumann FT, Stein A, Bloch W, Bokemeyer C, de Wit M, et al. Exercise training in patients with advanced gastrointestinal cancer undergoing palliative chemotherapy: a pilot study. Support Care Cancer. 2014;22:1797–806.
Jensen W, Bialy L, Ketels G, Baumann FT, Bokemeyer C, Oechsle K. Physical exercise and therapy in terminally ill cancer patients: a retrospective feasibility analysis. Support Care Cancer. 2014;22:1261–8.
Albrecht TA, Taylor AG. Physical activity in patients with advanced-stage cancer: a systematic review of the literature. Clin J Oncol Nurs. 2012;16:293–300.
Oldervoll LM, Loge JH, Lydersen S, Paltiel H, Asp MB, Nygaard UV, et al. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. Oncologist. 2011;16:1649–57.
Lowe SS, Watanabe SM, Baracos VE, Courneya KS. Physical activity interests and preferences in palliative cancer patients. Support Care Cancer. 2010;18:1469–75.
Cheville AL, Kollasch J, Vandenberg J, Shen T, Grothey A, Gamble G, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with Stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;45:811–21.
Lowe SS, Watanabe SM, Baracos VE, Courneya KS. Home-based functional walking program for advanced cancer patients receiving palliative care: a case series. BMC Palliat Care. 2013;12:22.
Headley JA, Ownby KK, John LD. The effect of seated exercise on fatigue and quality of life in women with advanced breast cancer. Oncol Nurs Forum. 2004;31:977–83.
Borg G. Borg’s perceived exertion and pain scales. Champaign: Human Kinetics; 1998.
Blum K, Sherman DW. Understanding the experience of caregivers: a focus on transitions. Semin Oncol Nurs. 2010;26:243–58.
Glajchen M. The emerging role and needs of family caregivers in cancer care. J Support Oncol. 2004;2:145–55.
Le T, Leis A, Pahwa P, Wright K, Ali K, Reeder B. Quality-of-life issues in patients with ovarian cancer and their caregivers: a review. Obstet Gynecol Surv. 2003;58:749–58.
Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, et al. How we design feasibility studies. Am J Prev Med. 2009;36:452–7.
Strömgren AS, Sjogren P, Goldschmidt D, Petersen MA, Pedersen L, Groenvold M. Symptom priority and course of symptomatology in specialized palliative care. J Pain Symptom Manage. 2006;31:199–206.
Jäger E. Körperliche Bewegung in der onkologischen Palliativmedizin. In: Baumann FTJEBW, editor. Sport und körperliche Aktivität in der Onkologie. Berlin, Heidelberg: Springer; 2012. p. 215–224.
de Morton NA, Davidson M, Keating JL. Validity, responsiveness and the minimal clinically important difference for the de Morton Mobility Index (DEMMI) in an older acute medical population. BMC Geriatr. 2010;10:72.
Nicklasson M, Bergman B. Validity, reliability and clinical relevance of EORTC QLQ-C30 and LC13 in patients with chest malignancies in a palliative setting. Qual Life Res. 2007;16:1019–28.
Schmidt K, Vogt L, Thiel C, Jäger E, Banzer W. Validity of the Six-Minute Walk Test in cancer patients. Int J Sports Med. 2013;34:631–6.
Godfrey J, Poole L. An audit of the use of the Barthel Index in palliative care. Int J Palliat Nurs. 2007;13:543–8.
Zhang F, Ferrucci L, Culham E, Metter EJ, Guralnik J, Deshpande N. Performance on five times sit-to-stand task as a predictor of subsequent falls and disability in older persons. J Aging Health. 2013;25:478–92.
Meffert C, Gerdes N. Eignung des Kurzfragebogens IRES-24 zur Evaluation gesundheitlicher Präventionsmaßnahmen: Das Beispiel Gewichtsreduktion. Diagnostica. 2013;59:130–41.
Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70:113–9.
Hanson LC, Bull J, Wessell K, Massie L, Bennett RE, Kutner JS, et al. Strategies to support recruitment of patients with life-limiting illness for research: the palliative care research cooperative group. J Pain Symptom Manage. 2014;48:1021–30.
Peppercorn JM, Smith TJ, Helft PR, Debono DJ, Berry SR, Wollins DS, et al. American society of clinical oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol. 2011;29:755–60.
Pawlik TM, Devon KM, Fields CA, Hinshaw DB. What are patients’ expectations about the effects of chemotherapy for advanced cancer? J Am Coll Surg. 2014;219:588–90.
Weeks JC, Catalano PJ, Cronin A, Finkelman MD, Mack JW, Keating NL, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367:1616–25.
Kaushal K. Social desirability bias in face to face interviews. J Postgrad Med. 2014;60:415–6.
Krumpal I. Determinants of social desirability bias in sensitive surveys: a literature review. Qual Quant. 2013;47(2025–47):32.
Kuehr L, Wiskemann J, Abel U, Ulrich CM, Hummler S, Thomas M. Exercise in patients with non-small cell lung cancer. Med Sci Sports Exerc. 2014;46:656–63.
van Dungen IAD, Verhagen CA, van der Graaf WT, van den Berg J, Vissers KC, Engels Y. Feasibility and impact of a physical exercise program in patients with advanced cancer: a pilot study. J Palliat Med. 2014;17:1091–8.
Quist M, Rørth M, Langer S, Jones LW, Laursen JH, Pappot H, et al. Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: a pilot study. Lung Cancer. 2012;75:203–8.
Authors’ contributions
WS, GB, PD and AW conceived the study. WS designed the protocol with substantial input by AW and JG. WS drafted the manuscript with significant intellectual revision by AW, JG, PD, MH and GB. All authors read and approved the final manuscript.
Acknowledgements
The article processing charge was funded by the German Research Foundation (DFG) and the Albert Ludwigs University Freiburg in the funding program Open Access Publishing.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Siemens, W., Wehrle, A., Gaertner, J. et al. Implementing a home-based exercise program for patients with advanced, incurable diseases after discharge and their caregivers: lessons we have learned. BMC Res Notes 8, 509 (2015). https://doi.org/10.1186/s13104-015-1523-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-015-1523-z