Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a chronic, irreversible disease and a leading cause of worldwide morbidity and mortality. In Canada, COPD is the fourth leading cause of death. This systematic review was undertaken to update healthcare professionals and decision makers regarding the recent clinical, humanistic and economic burden evidence in Canada.
Methods
A systematic literature search was conducted in PubMed, EMBASE, and Cochrane databases to identify original research published January 2000 through December 2012 on the burden of COPD in Canada. Each search was conducted using controlled vocabulary and key words, with “COPD” as the main search concept and limited to Canadian studies, written in English and involving human subjects. Selected studies included randomized controlled trials, observational studies and systematic reviews/meta-analyses that reported healthcare resource utilization, quality of life and/or healthcare costs.
Results
Of the 972 articles identified through the literature searches, 70 studies were included in this review. These studies were determined to have an overall good quality based on the quality assessment. COPD patients were found to average 0–4 annual emergency department visits, 0.3–1.5 annual hospital visits, and 0.7–5 annual physician visits. Self-care management was found to lessen the overall risk of emergency department (ED) visits, hospitalization and unscheduled physician visits. Additionally, integrated care decreased the mean number of hospitalizations and telephone support reduced the number of annual physician visits. Overall, 60–68 % of COPD patients were found to be inactive and 60–72 % reported activity restriction. Pain was found to negatively correlate with physical activity while breathing difficulties resulted in an inability to leave home and reduced the ability to handle activities of daily living. Evidence indicated that treating COPD improved patients’ overall quality of life. The average total cost per patient ranged between CAN $2444–4391 from a patient perspective to CAN $3910–6693 from a societal perspective. Furthermore, evidence indicated that COPD exacerbations lead to higher costs.
Conclusions
The clinical, humanistic and economic burden of COPD in Canada is substantial. Use of self-care management programs, telephone support, and integrated care may reduce the overall burden to Canadian patients and society.
Similar content being viewed by others
Background
Chronic obstructive pulmonary disease (COPD) is a persistent, irreversible, progressive disease exacting a heavy toll on patients and caregivers and is a leading cause of morbidity and mortality worldwide [1–4]. Estimates indicate that more than 10 % of the adult population are affected by COPD, and one in four adults over the age of 35 will develop COPD in their lifetime [5, 6]. In Canada, COPD is project to be the fourth leading cause of death behind heart disease, cancer and stroke and is expected to be the third leading cause of death by 2020 [3]. Exposure to environmental factors is thought to be the major underlying cause of COPD, with smoking being the most important risk factor [7–9]. Comorbidities, such as cardiovascular disease, are very common and are thought to contribute to the vast majority of COPD deaths [10–12].
The unique features of the Canadian universal healthcare system provide different challenges for government and health care providers alike in the delivery and implementation of health services. With the substantial burden and societal importance of COPD, it is important for Canadian healthcare professionals and decision makers to remain up to date with evidence of managing and treating COPD. A sizeable body of research on the burden of COPD in Canada has been conducted in recent years; however, a systematic review of recent evidence is lacking. The overall purpose of this systematic review is to update the knowledge of the burden of COPD in Canada by summarizing the most current, evidence-based information. The specific objective is to summarize the recent literature describing the clinical, humanistic and economic burden of COPD among Canadians.
Methods
Literature search
We conducted a search of the PubMed, EMBASE, and Cochrane databases to identify original research (observational and interventional studies, burden of illness studies, and cost of illness studies) published January 2000 through December 2012 on the burden of COPD in Canada. Non-systematic review articles, letters, editorials, commentaries, studies reporting summaries of meeting proceedings or conferences, abstracts or posters presented at scientific meetings, and studies examining the efficacy or effectiveness of specific pharmacotherapy interventions were not included. Each search was conducted using controlled vocabulary and key words and was limited to articles published in English, studies conducted with Canadian data, and studies involving humans. Additional articles were identified and added to each review through a review of the bibliographies of included articles and if identified in the other literature search (i.e. article with economic data found in humanistic literature search).
Study selection
Titles and abstracts of articles identified were carefully screened in the initial review for relevance to the topic by a single reviewer. Articles were selected for inclusion based on predefined acceptance criteria, which included relevant patient population (i.e., adults/children diagnosed with COPD), study design [randomized controlled trial (RCT), observational study, systematic review/meta-analyses] and outcome measures (healthcare resource utilization, quality of life, healthcare costs). Complete articles were obtained for any article that categorized as ‘included’ or ‘unsure’ after the title and abstract review. All ‘unsure’ articles were then reviewed to make a final determination of inclusion or exclusion. A second, independent reviewer performed a check on a random sample of 20 % of the articles with discrepancies resolved through consensus. Articles identified as potentially relevant were obtained in full text for further evaluation.
Data abstraction
Data abstraction forms were designed a priori. For articles that met predefined inclusion/exclusion criteria, key outcomes were abstracted and tabulated in summary tables. Key outcomes extracted included: emergency department visits, hospitalization and office visits in the clinical burden literature; quality of life measures in the humanistic burden literature; patient and population costs in the economic burden literature. In the economic burden section, reported costs were inflated to 2012 Canadian dollars using the Consumer Price Index from Statistics Canada (http://www.statcan.gc.ca). A second, independent reviewer performed a check on a random sample of the data abstracted from 20 % of the articles.
Quality assessment
Quality was assessed by using internationally recognized methodological checklists from the National Institute for Health and Care Excellence (NICE) Guidelines Manual for RCT [13], the strengthening the reporting of observational studies in epidemiology (STROBE) statement [14] for observational studies, and the PRISMA checklist for systematic reviews and meta-analyses [15]. The NICE RCT checklist provides an assessment of potential bias in 4 categories: selection, performance, attrition and detection. The STROBE checklist contains 22 items that assess completeness of reporting in observational studies and the 27-item PRISMA checklist provides a similar assessment for systematic reviews and meta-analyses. The information collected in these checklists enabled a decision to be made about the eligibility of the studies for inclusion in this project. A second, independent reviewer performed a quality review check on a random sample of 20 % of the articles.
Results
Literature search
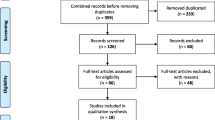
A total of 495 studies were identified by the clinical and economic burden literature searches with 58 studies being suitable for inclusion (Fig. 1). The 58 studies included: 3 systematic review/meta-analyses, 5 RCTs, and 27 cohort, 18 cross-sectional, and 5 case–control studies. A total of 477 studies were identified by the humanistic burden literature searches of which 12 studies were ultimately included (Fig. 2). The study designs of the 12 included articles were 6 RCTs, 4 cross-sectional and 2 case–control studies.
Quality assessment
The clinical and economic burden literature included 3 systematic review/meta-analyses which met most of the PRISMA checklist criteria [16–18]. The criteria that were not met included: no description of methods for combining studies (100 %), not addressing risk of bias across studies (67 %) or individual studies (33 %) and not describing study limitations (67 %). Of the 5 RCTs appraised using the NICE RCT methodology checklist, most were rated as having a low risk of bias; however, a high risk of attrition bias was noted for three studies [19–21]. Lastly, the 50 remaining studies were assessed using the STROBE checklist. Many of the cohort studies did not indicate the study design (36 %), lacked reporting sensitivity or sub-group analyses (71 %), and missing or follow-up data was infrequently addressed (68 and 39 % respectively). The methodological limitations identified for the cross-sectional and case–control studies were very similar.
The humanistic burden literature included a total of 6 RCTs which were appraised by the NICE RCT methodology, all of which had an overall low risk of bias. The remaining 6 studies met most of the STROBE criteria; however, only 2 of the 6 studies adequately described the study setting [22, 23], 2 studies discussed efforts to address sources of bias [22, 24], and there was an overall lack of reporting on how missing data was addressed as well as sub-group and sensitivity analysis [23–27].
Clinical burden evidence results
Overview
Of the 57 articles with clinical burden data (Tables 1, 2 and 3), the primary data source for 60 % of the studies (retrospective cohort and cross-sectional designs) was the provincial healthcare databases containing hospital records and pharmaceutical claims. The time frame of the included studies varied based on the study design. In general, the prospective designed studies included a much shorter time frame than systematic reviews or retrospective database analyses which often spanned decades.
Emergency department (ED) visits
Emergency department visits were reported as an outcome in 23 out of the 58 studies (Table 1). A number of studies reported the mean number of emergency department visits which ranged from 0.1 to 2.20 per year [1, 17, 28–39]. Eleven studies reported that 7.2–63.2 % of patients with COPD visited the emergency department [1, 17, 21, 28, 30, 35, 40–44]. Johnston [32] reported the mean annual number of ED visits by disease severity. The instrument used to assess disease severity was developed by the global initiative for chronic obstructive lung disease (GOLD) and categorizes patients from mild to very severe in 4 levels (GOLD 1–4 stratum). The mean number of annual ED visits ranged from 1.4 (GOLD stratum 1 and 2) to 1.8 (GOLD stratum 3 and 4) in COPD patients with an exacerbation [32].
Three studies reported how different pre/post interventions affected ED visits in COPD patients. Overall ED visits were less in COPD patients with self-management education or self-care management programs; however, integrated care appeared to provide no benefit on the annual mean number of ED visits [28, 29, 44].
Hospitalization
Hospitalization was reported as an outcome in 38 of the 58 studies (Table 2). The rates were reported as either pre- or post- index hospitalizations. The mean number of annual hospital visits per COPD patient per year ranged from: 0–1.5 pre-index to 0–5.19 post-index [1, 28, 29, 32, 34, 41, 43, 45–48]. Three studies reported the rates of hospitalization according to disease severity and/or COPD exacerbations and found higher rates of hospitalization in more severe patients (GOLD stratum 3 or 4) and those with more severe exacerbations [3, 32, 43]. Hospital readmission rates varied between three studies with Sin [49] reporting a rate of 25 % for COPD patients ≥65 years of age, Chen [50] reporting a rate of 49.1 % in patients ≥40 years of age, and Wong [47] reporting 3.3 mean annual number of hospital readmissions in patients with a diagnosis of AECOPD.
The relationship of COPD hospitalization rates to patient demographic characteristics was examined in three studies. A higher rate of hospitalization was found in male COPD patients [126.1/1000 patient years (PY)] than females (74.3/1000 PY) and in those >65 years of age (5.19 visits/patient annually) versus those 45–64 years of age (3.45 visits/patient annually) [46, 51]. One study found that COPD patients’ body mass index (BMI) status had no effect on hospitalization rates [45].
Lastly, three studies examined the effects of different interventions on hospitalization rates in COPD patients. Moullec [28] found that integrated care (a combination of self-management education and case management) resulted in a decreased mean number of hospitalizations compared to usual care. Lebrecque [29] and Sedeno [21] found that self-management interventions also reduced hospitalizations compared to usual care.
Physician visits
A total of 24 studies reported the rate of physician visits for COPD (Table 3). The annual rate of physician visits post-index for COPD patients ranged between 1.57 and 28 visits annually [41, 46, 52]. Two studies found that elderly COPD patients (>65 years) had high rates of physician visits compared to younger patients (from 4.1 to 8.1 visits/year) [38, 46], one study found those at high risk for CV-related comorbidities had higher physician visit rates compared to those with low risk (20 vs. 5 visits per year) [53], and one study reported that COPD patients diagnosed with GOLD stratum 1–4 had a higher number of exacerbations requiring a physician visit compared to those with GOLD stratum 0 (15 vs. 9 visits, respectively) [32]. Goodridge [52] found the highest rate of physician visits for COPD patients was within 12 months of death (28 visits/year) and Rowe [34] found that Canadian and US stable COPD patients had similar mean annual urgent clinic visit rates. Lastly, two studies found that self-management interventions reduced the number of unscheduled physician visits [21, 44] and a review article found a reduction in the number of annual physician visits for patients receiving telephone support [17].
Humanistic burden evidence
Overview
A total of 12 studies were identified describing the humanistic burden by measuring the effect of COPD on a patient’s health-related quality of life (HRQoL) and physical activity (Table 4). Study timeframes were not reported in three studies and variation was found in the definition of COPD across all studies. With regard to the type of HRQoL instruments used, 4 studies [22, 25, 54, 55] reported outcomes for the 36-item short form health survey (SF-36) and 5 studies reported results for The St. George Respiratory Questionnaire (SGRQ) [20, 22, 27, 54, 56]. Other scales that were used to assess HRQoL were the chronic respiratory disease (CRD) Index Questionnaire, the sickness impact profile (SIP) and the Chronic Respiratory Questionnaire (CRQ).
Sf-36
Of the 4 studies reporting SF-36 evidence, one study found that COPD patients receiving salmeterol did not experience significant improvement in their SF-36 mental or physical health summary scores compared to baseline [54]. In contrast, a case–control study reported an absolute mean difference of 16.9 in the SF-36 physical health summary score and 12.8 in the mental component score for COPD patients compared to healthy controls. The study also indicated a significantly worse (p < 0.001) level of functioning for patients with COPD [25].
St George’s Respiratory Questionnaire (SGRQ)
Four of the five studies reporting SGRQ data compared an intervention to placebo or usual care in a COPD population [20, 22, 54, 56], while one study reported data for COPD patients versus their spouses [27]. Three RCTs found pharmaceutical agents (tiotropium, salmeterol, tiotropium plus salmeterol and tiotropium plus fluticasone/salmeterol) significantly improved patients’ quality of life as measured by the SGRQ score [20, 54, 56]. Of the remaining two studies, one cross-sectional survey found a significant mean difference (5.6, p = 0.002) for the SGRQ impact of disease scores between COPD patients and their non-COPD spouse [27] and a prospective, observational study reported no significant differences in SGRQ scores at baseline between the self-management education program and usual care groups [22].
Chronic Respiratory Questionnaire (CRQ)
Three studies used the CRQ to assess the quality of life of COPD patients utilizing different pharmaceutical interventions (paroxetine, budesonide, prednisone). Of the three studies, paroxetine (CRQ emotional function domain) and inhaled corticosteroids (budesonide) were found to produce significant improvements in patients’ quality of life; however, inhaled corticosteroids (even in ‘high’ doses) did not appear to provide significant HRQoL improvement over that achieved with oral prednisone [24, 55, 57].
Miscellanous HRQoL instruments
Several studies utilized additional HRQoL instruments to assess the quality of life of COPD patients. A study by HajGhanbari [25] found that pain severity [measured by the McGill Pain Questionnaire (MPQ) and brief pain inventory scale (BPI)] showed moderate to strong negative correlations to the physical component score of the SF-36 (−0.45, −0.61, −0.70, respectively; p < 0.001). In addition, a cross-sectional survey study using the SIP found significant differences in the mean score between patients’ and healthy spouses’ ratings of the SIP physical score (p = 0.009), but non-significant differences in psychosocial score (p = 0.497) [27]. Finally, a single RCT conducted by Aaron [58] using the chronic respiratory disease index HRQoL instrument (CRD) found that prednisone use did not result in a significant (p = 0.14) overall health benefit (total score) when compared to placebo, although prednisone reduced the incidence of relapse and improved both lung function and dyspnea.
Physical activity
Three studies reported on physical activity related to the burden of COPD. A cross-sectional study using the Canadian national health survey data (1994–2007) found that approximately 68 % of obese and 60 % of non-obese COPD patients were inactive. Additionally, approximately 72 % of obese and 60 % of non-obese COPD patients reported activity restriction [23]. Furthermore, a cross-sectional study by Rocker [26] in patients with severe, stable COPD found that scores on the palliative performance scale from semi-structured interviews ranged from 50 to 70 % and that all patients had a score of 5 on the Medical Research Council dyspnea scale (i.e., they were too short of breath to leave their homes or were breathless when dressing or undressing). The significance of pain in COPD patients was reflected in pain-related interference in activities, which may partly account for the lower SF-36 physical component scores in HRQoL and the lower physical activity scores on the community health activities model program for seniors (CHAMPS) questionnaire [25].
Economic burden evidence
Overview
A total of 5 studies contained outcomes of interest and were included in this review. Of the 5 studies, 4 studies reported the patient level direct costs and 2 studies reported population level direct costs for COPD patients (Tables 5, 6).
Patient level direct costs
Overall, the average total cost per patient was reported from both a patient perspective and a society perspective (accounting for inflation) and ranged between CAN $2444.17–CAN $4391.16 (patient perspective) and CAN $3910.39–CAN $6693.37 (societal perspective) annually. The average cost per acute COPD exacerbation reported by Mittmann [3] and Maleki-Yazdi [59] ranged from $718–$11,156 and the cost was found to increase with the severity of the exacerbation. No studies were found to examine the relationship of cost to overall disease severity.
Two studies examined differences in costs based on patient characteristics. Chapman [1] and Wouters [37] both reported female COPD patients incurred more costs compared to male patients from both a patient and a societal perspective (additional $985/patient from a patient perspective, $1513–2138/patient from a societal perspective). In addition, these studies also found that former smokers incurred more costs than current smokers (additional $1992/patient from a patient perspective, $1698–$1744/patient from a societal perspective) and that COPD patients with less education incurred more costs than those who are more highly educated (additional $901/patient from a patient perspective, $879–902/patient from a societal perspective). Lastly, Chapman [1] reported that patients with comorbidities were more costly than those without comorbidities (additional $136/patient from a patient perspective, $1440/patient from a societal perspective).
Population level direct costs
Population level direct costs (in Canadian dollars) were examined in two studies (Table 6). Dormuth [60] found that residents of British Columbia who were dispensed an inhaled anti-cholinergic (IAC) medication (ipratropium or tiotropium) cost $26,298,835 annually over 2.5 years for IACs (Ministry of Health $13,276,279, out of pocket $13,022,556), $310,494,472 for any hospital admission and $59,456,281 for emergency COPD admissions over the 2.5 year period. The second study by Mittmann [3] estimated that moderate COPD exacerbations cost $182.70–$254.44 million annually while severe exacerbations cost $469.64–$642.26 million annually in Canada.
Discussion
COPD is one of the world’s most common health problems [2]. This review found evidence that the clinical, economic and humanistic burden of COPD is substantial in Canada. COPD patients were found to average 0–4 annual emergency department visits, 0.3–1.5 annual hospital visits, and 0.7–5 annual physician visits which are similar to the rates reported worldwide. Variance in these rates across studies may reflect population differences, methodological differences and/or treatment pattern differences between studies. In Canada, the health care services are provided by the private sectors but they are delivered through publicly funded health care systems. For instance, basic services such as physician care are provided by private doctors but the physician fees are paid for by the government. Hospital care is delivered by publicly funded hospitals which are mostly independent institutions incorporated under provincial Corporations Acts. The universal health care system, however, does not include coverage of prescription medication; drug benefit plans for eligible groups are available under provincial and territorial governments.
In terms of ED services, an international survey found that around the world, the percentage of COPD patients using ED services ranges from 1 % (China) to 25 % (Brazil) [61]. The relatively small number of ED visits found for Canadian COPD patients would suggest that the use of ED services for COPD patients may fall on the lower end worldwide. Hospitalization rates, hospital readmission rates, and the number of physician visits for Canadian COPD patients were found to be consistent with rates found in the US [62–64]. Additionally, trends of increasing healthcare resource use as COPD worsens are consistent with worldwide data [61, 65].
Primary care has been reported to have the greatest proportion of worldwide burden in the treatment of COPD. Furthermore, increasing severity of COPD imposes a greater burden on the use of primary care resources [61]. Evidence was found that self-care management programs may help with reducing the number of ED visits, hospitalizations, and physician visits. Additionally, telephone support services were found to reduce the number of physician office visits. Integrated care programs, however, appear to reduce the mean number of hospitalizations but not ED visits.
COPD has a profound impact on patients’ quality of life [66]. Evidence found in this review, while not overwhelming, found that Canadians with COPD have a poorer quality of life. Worldwide data suggests that up to 45 % of COPD patients experience pain and that increases in pain are associated with disease progression [67–72]. The significance of pain in COPD patients was reflected in greater pain-related interference on activities of daily living. In the Canadian Hidden Depths survey, COPD symptoms were found to have a significant effect on a range of daily activities (including climbing stairs, housework, getting dressed and sleeping) for a majority of respondents [73]. Clinicians face challenges in treating COPD related pain in that opioids, common pharmacotherapy, are not recommended for use in COPD patients, presumably due to their effects on the reduction of breathing rates which may further exacerbate COPD [4]. Additionally, this review found evidence that 60–72 % of COPD patients are inactive and/or have activity restrictions with obese patients having the highest percentages.
Obesity is one of the leading causes of overall morbidity and mortality [74, 75]. Thus it is not surprising that health consequences of obesity are seen in the COPD population and coupled with progressively worsening lung function. It is therefore important that more research is performed in order to better understand the impact of interventions on the quality of life and how to maximize patient functioning.
Data from this review found the average total cost per COPD patient ranged between CAN $2444 from a patient perspective to CAN $6693 from a societal perspective. Moreover, data suggests that the costs rise as the disease severity increases. The clinical burden review found evidence which indicates that healthcare resource utilization increases with exacerbation severity [3, 32], increasing age [46, 76], and comorbid cardiovascular disease [53]. Thus, clinicians should focus on ensuring proper diagnosis, optimizing appropriate care, and the importance of personalized medicine.
This review, like all reviews, is limited by publication bias with respect to the articles that are available. In addition, the articles in this review were a priori limited to the English language and restricted to those published since 2000 to examine the most recent data as the practice of medicine and related burden may change over time. Spatial restrictions were also applied, limiting studies to Canadian populations. However, in spite of these limitations, this review was systematic in nature and therefore by reviewing all available and relevant data, it provides a better and comprehensive understanding of the literature with respect to clinical, humanistic and economic burden of COPD in the Canadian population.
Conclusions
COPD is currently the fourth leading cause of death among Canadians. This review found that COPD causes a profound impact on healthcare resources and produces a significant clinical, humanistic and economic burden in Canada. This review found evidence that self-care management programs, telephone support services, and integrated care programs may help limit the overall burden to Canadian patients and society.
Abbreviations
- AECOPD:
-
acute exacerbation of chronic obstructive pulmonary disease
- BMI:
-
body mass index
- BPI:
-
Brief Pain Inventory Scale
- CHAMPS:
-
community health activities model program for seniors
- COPD:
-
chronic obstructive pulmonary disease
- CRD:
-
chronic respiratory disease
- CRQ:
-
Chronic Respiratory Questionnaire
- CV:
-
cardiovascular
- ED:
-
emergency department
- GOLD:
-
global initiative for chronic obstructive lung disease
- HRQoL:
-
health-related quality of life
- IAC:
-
inhaled anti-cholinergic
- NICE:
-
National Institute for Health and Care Excellence
- PRISMA:
-
preferred reporting items for systematic reviews and meta-analyses
- PY:
-
patient years
- RCT:
-
Randomized Controlled Trial
- SF-36:
-
short form 36
- SGRQ:
-
St George’s Respiratory Disease Questionnaire
- SIP:
-
sickness impact profile
- STROBE:
-
strengthening the reporting of observational studies in epidemiology
References
Chapman KR, Bourbeau J, Rance L. The burden of COPD in Canada: results from the Confronting COPD survey. Respir Med. 2003;97(Suppl C):S23–31.
Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412.
Mittmann N, Kuramoto L, Seung SJ, Haddon JM, Bradley-Kennedy C, FitzGerald JM. The cost of moderate and severe COPD exacerbations to the Canadian healthcare system. Respir Med. 2008;102:413–21.
GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2013. http://www.goldcopd.org. Accessed 1 Aug 2011.
Gershon AS, Wang C, Wilton AS, Raut R, To T. Trends in chronic obstructive pulmonary disease prevalence, incidence, and mortality in ontario, Canada, 1996–2007: a population-based study. Arch Intern Med. 2010;170:560–5.
Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD study): a population-based prevalence study. Lancet. 2007;370:741–50.
Celli BR, Halbert RJ, Nordyke RJ, Schau B. Airway obstruction in never smokers: results from the Third National Health and Nutrition Examination Survey. Am J Med. 2005;118:1364–72.
Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest. 2005;128:1239–44.
Bridevaux PO, Probst-Hensch NM, Schindler C, Curjuric I, Felber DD, Braendli O, et al. Prevalence of airflow obstruction in smokers and never-smokers in Switzerland. Eur Respir J. 2010;36:1259–69.
Mannino DM, Watt G, Hole D, Gillis C, Hart C, McConnachie A, et al. The natural history of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:627–43.
Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–9.
McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62:411–5.
National Institute for Health and Clinical Excellence. The guidelines manual (January 2009). 2009. London: National Institute for Health and Clinical Excellence. http://www.nice.org.uk. Accessed 17 March 2011.
National Institute for Health and Clinical Excellence. The guidelines manual (January 2009). 2009. London: National Instutute for Health and Clinical Excellence. http://www.nice.org.uk. Accessed 17 March 2011.
Moher R, Liberati A, Tetzlaff J, Altman D, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e10000097.
Gaebel K, McIvor RA, Xie F, Blackhouse G, Robertson D, Assasi N, et al. Triple therapy for the management of COPD: a review. COPD. 2011;8:206–43.
Polisena J, Tran K, Cimon K, Hutton B, McGill S, Palmer K, et al. Home telehealth for chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Telemed Telecare. 2010;16:120–7.
Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756–66.
Keenan SP, Gregor J, Sibbald WJ, Cook D, Gafni A. Noninvasive positive pressure ventilation in the setting of severe, acute exacerbations of chronic obstructive pulmonary disease: more effective and less expensive. Crit Care Med. 2000;28:2094–102.
Chan CK, Maltais F, Sigouin C, Haddon JM, Ford GT. A randomized controlled trial to assess the efficacy of tiotropium in Canadian patients with chronic obstructive pulmonary disease. Can Respir J. 2007;14:465–72.
Sedeno MF, Nault D, Hamd DH, Bourbeau J. A self-management education program including an action plan for acute COPD exacerbations. COPD. 2009;6:352–8.
Moullec G, Favreau H, Lavoie KL, Labrecque M. Does a self-management education program have the same impact on emotional and functional dimensions of HRQoL? COPD. 2012;9:36–45.
Vozoris NT, O’Donnell DE. Prevalence, risk factors, activity limitation and health care utilization of an obese, population-based sample with chronic obstructive pulmonary disease. Can Respir J. 2012;19:e18–24.
Leigh R, Pizzichini MM, Morris MM, Maltais F, Hargreave FE, Pizzichini E. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. 2006;27:964–71.
HajGhanbari B, Holsti L, Road JD, Darlene RW. Pain in people with chronic obstructive pulmonary disease (COPD). Respir Med. 2012;106:998–1005.
Rocker G, Young J, Donahue M, Farquhar M, Simpson C. Perspectives of patients, family caregivers and physicians about the use of opioids for refractory dyspnea in advanced chronic obstructive pulmonary disease. CMAJ. 2012;184:E497–504.
Low G, Gutman G. Couples’ ratings of chronic obstructive pulmonary disease patients’ quality of life. Clin Nurs Res. 2003;12:28–48.
Moullec G, Lavoie KL, Rabhi K, Julien M, Favreau H, Labrecque M. Effect of an integrated care programme on re-hospitalization of patients with chronic obstructive pulmonary disease. Respirology. 2012;17:707–14.
Labrecque M, Rabhi K, Laurin C, Favreau H, Moullec G, Lavoie K, et al. Can a self-management education program for patients with chronic obstructive pulmonary disease improve quality of life? Can Respir J. 2011;18:e77–81.
Rowe BH, Voaklander DC, Marrie TJ, Senthilselvan A, Klassen TP, Rosychuk RJ. Outcomes following chronic obstructive pulmonary disease presentations to emergency departments in Alberta: a population-based study. Can Respir J. 2010;17:295–300.
Rosychuk RJ, Voaklander DC, Senthilselvan A, Klassen TP, Marrie TJ, Rowe BH. Presentations to emergency departments for chronic obstructive pulmonary disease in Alberta: a population-based study. CJEM. 2010;12:500–8.
Johnston NW, McIvor A, Lambert K, Greene JM, Hussack P, erhardsson DV, et al. The Christmas season as a risk factor for chronic obstructive pulmonary disease exacerbations. Can Respir J. 2010;17:275–81.
Tsai CL, Rowe BH, Cydulka RK, Camargo CA Jr. ED visit volume and quality of care in acute exacerbations of chronic obstructive pulmonary disease. Am J Emerg Med. 2009;27:1040–9.
Rowe BH, Cydulka RK, Tsai CL, Clark S, Sinclair D, Camargo CA Jr. Comparison of Canadian versus United States emergency department visits for chronic obstructive pulmonary disease exacerbation. Can Respir J. 2008;15:295–301.
Wang Q, Bourbeau J. Outcomes and health-related quality of life following hospitalization for an acute exacerbation of COPD. Respirology. 2005;10:334–40.
Golmohammadi K, Jacobs P, Sin DD. Economic evaluation of a community-based pulmonary rehabilitation program for chronic obstructive pulmonary disease. Lung. 2004;182:187–96.
Wouters EF. Economic analysis of the Confronting COPD survey: an overview of results. Respir Med. 2003;97(Suppl C):S3–14.
Sin DD, Tu JV. Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:580–4.
Bischoff EW, Hamd DH, Sedeno M, Benedetti A, Schermer TR, Bernard S, et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax. 2011;66:26–31.
Stephenson A, Seitz DP, Fischer HD, Gruneir A, Bell CM, Gershon AS, et al. Cholinesterase inhibitors and adverse pulmonary events in older people with chronic obstructive pulmonary disease and concomitant dementia: a population-based, cohort study. Drugs Aging. 2012;29:213–23.
Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1-year, population-based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): effect on COPD-related exacerbations, emergency department visits and hospitalizations, medication utilization, and treatment adherence. Clin Ther. 2010;32:1320–8.
Gershon A, Croxford R, To T, Stanbrook MB, Upshur R, Sanchez-Romeu P, et al. Comparison of inhaled long-acting beta-agonist and anticholinergic effectiveness in older patients with chronic obstructive pulmonary disease: a cohort study. Ann Intern Med. 2011;154:583–92.
FitzGerald JM, Haddon JM, Bradly-Kennedy C, Kuramoto L, Ford GT. Resource use study in COPD (RUSIC): a prospective study to quantify the effects of COPD exacerbations on health care resource use among COPD patients. Can Respir J. 2007;14:145–52.
Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupré A, Bégin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163:585–91.
Tsai CL, Camargo CA Jr. The role of body mass index in acute exacerbations of chronic obstructive pulmonary disease. Emerg Med J. 2009;26:701–5.
Ohinmaa A, Schopflocher D, Jacobs P, Demeter S, Chuck A, Golmohammadi K, et al. A population-based analysis of health behaviours, chronic diseases and associated costs. Chronic Dis Can. 2006;27:17–24.
Wong AW, Gan WQ, Burns J, Sin DD, van Eeden SF. Acute exacerbation of chronic obstructive pulmonary disease: influence of social factors in determining length of hospital stay and readmission rates. Can Respir J. 2008;15:361–4.
Beaulieu-Genest L, Chretien D, Maltais F, Pelletier K, Parent JG, Lacasse Y. Self-administered prescriptions of oral steroids and antibiotics in chronic obstructive pulmonary disease: are we doing more harm than good? Chron Respir Dis. 2007;4:143–7.
Sin DD, Tu JV. Are elderly patients with obstructive airway disease being prematurely discharged? Am J Respir Crit Care Med. 2000;161:1513–7.
Chen Y, Li Q, Johansen H. Age and sex variations in hospital readmissions for COPD associated with overall and cardiac comorbidity. Int J Tuberc Lung Dis. 2009;13:394–9.
Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128:2640–6.
Goodridge D, Lawson J, Duggleby W, Marciniuk D, Rennie D, Stang M. Health care utilization of patients with chronic obstructive pulmonary disease and lung cancer in the last 12 months of life. Respir Med. 2008;102:885–91.
Mancini GB, Etminan M, Zhang B, Levesque LE, FitzGerald JM, Brophy JM. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006;47:2554–60.
Appleton S, Poole P, Smith B, Veale A, Lasserson TJ, Chan-Matthew MK et al.: Long-acting beta2-agonists for poorly reversible chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;19(3):CD001104
Lacasse Y, Beaudoin L, Rousseau L, Maltais F. Randomized trial of paroxetine in end-stage COPD. Monaldi Arch Chest Dis. 2004;61:140–7.
Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Inter Med. 2007;146:545–55.
Bourbeau J, Rouleau MY, Boucher S. Randomised controlled trial of inhaled corticosteroids in patients with chronic obstructive pulmonary disease. Thorax. 1998;53:477–82.
Aaron SD, Vandemheen KL, Hebert P, Dales R, Stiell IG, Ahuja J, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. N Engl J Med. 2003;348:2618–25.
Maleki-Yazdi MR, Kelly SM, Lam SY, Marin M, Barbeau M, Walker V. The burden of illness in patients with moderate to severe chronic obstructive pulmonary disease in Canada. Can Respir J. 2012;19:319–24.
Dormuth CR, Morrow RL, Carney G. Trends in health care utilization in British Columbia following public coverage for tiotropium. Value Health. 2011;14:600–6.
Fletcher MJ, Upton J, Taylor-Fishwick J, Buist SA, Jenkins C, Hutton J, et al. COPD uncovered: an international survey on the impact of chronic obstructive pulmonary disease [COPD] on a working age population. BMC Public Health. 2011;11:612.
Siddique HH, Olson RH, Parenti CM, Rector TS, Caldwell M, Dewan NA, et al. Randomized trial of pragmatic education for low-risk COPD patients: impact on hospitalizations and emergency department visits. Int J Chron Obstruct Pulmon Dis. 2012;7:719–28.
Jackson BE, Suzuki S, Lo K, Su F, Singh KP, Coultas D, et al. Geographic disparity in COPD hospitalization rates among the Texas population. Respir Med. 2011;105:734–9.
Aschan-Leygonie C, Baudet-Michel S, Mathian H, Sanders L. Gaining a better understanding of respiratory health inequalities among cities: an ecological case study on elderly males in the larger French cities. Int J Health Geogr. 2013;12:19.
Brown DW, Croft JB, Greenlund KJ, Giles WH. Trends in hospitalization with chronic obstructive pulmonary disease-United States, 1990–2005. COPD. 2010;7:59–62.
Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, et al. Health-related quality of life in patients by COPD severity within primary care in Europe. Respir Med. 2011;105:57–66.
Blinderman CD, Homel P, Billings JA, Tennstedt S, Portenoy RK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manag. 2009;38:115–23.
Lohne V, Heer HC, Andersen M, Miaskowski C, Kongerud J, Rustoen T. Qualitative study of pain of patients with chronic obstructive pulmonary disease. Heart Lung. 2010;39:226–34.
Bentsen SB, Rustoen T, Miaskowski C. Prevalence and characteristics of pain in patients with chronic obstructive pulmonary disease compared to the Norwegian general population. J Pain. 2011;12:539–45.
Boros PW, Lubinski W. Health state and the quality of life in patients with chronic obstructive pulmonary disease in Poland: a study using the EuroQoL-5D questionnaire. Pol Arch Med Wewn. 2012;122:73–81.
Elkington H, White P, Addington-Hall J, Higgs R, Edmonds P. The healthcare needs of chronic obstructive pulmonary disease patients in the last year of life. Palliat Med. 2005;19:485–91.
Skilbeck J, Mott L, Page H, Smith D, Hjelmeland-Ahmedzai S, Clark D. Palliative care in chronic obstructive airways disease: a needs assessment. Palliat Med. 1998;12:245–54.
Chapman K, Kaplan A. Report: breaking the surface–breaking the silence: how the under-reporting of “Lung Attacks” in Canada impacts patient outcomes in COPD. 2012. http://copdcanada.info/resources/Breaking+the+Surface+-+Breaking+the+Silence+2012.pdf. Accessed 6 June 2013.
Eisenberg MJ, Atallah R, Grandi SM, Windle SB, Berry EM. Legislative approaches to tackling the obesity epidemic. CMAJ. 2011;183:1496–500.
Gotay CC, Katzmarzyk PT, Janssen I, Dawson MY, Aminoltejari K, Bartley NL. Updating the canadian obesity maps: an epidemic in progress. Can J Public Health. 2013;104:e64–8.
Chen Y, Stewart P, Dales R, Johansen H, Bryan S, Taylor G. Changing age-pattern of hospitalisation risk of chronic obstructive pulmonary disease in men and women in Canada. Age Ageing. 2005;34:373–7.
Beauchesne MF, Julien M, Julien LA, Piquette D, Forget A, Labrecque M, et al. Antibiotics used in the ambulatory management of acute COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2008;3:319–22.
Tu AW, Buxton JA, Stockwell T. Estimates of smoking-attributable mortality and hospitalization in BC, 2002–2007. Can J Public Health. 2012;103:137–41.
Curkendall SM, DeLuise C, Jones JK, Lanes S, Stang MR, Goehring E Jr, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16:63–70.
Gonzalez AV, Suissa S, Ernst P. Gender differences in survival following hospitalisation for COPD. Thorax. 2011;66:38–42.
Macie C, Wooldrage K, Manfreda J, Anthonisen NR. Introduction of leukotriene receptor antagonists in Manitoba. Can Respir J. 2006;13:94–8.
Ernst P, Suissa S. Pneumonia in elderly patients with chronic obstructive pulmonary disease. Curr Infect Dis Rep. 2008;10:223–8.
Monfared AA, Lelorier J. Accuracy and validity of using medical claims data to identify episodes of hospitalizations in patients with COPD. Pharmacoepidemiol Drug Saf. 2006;15:19–29.
Benayoun S, Ernst P, Suissa S. The impact of combined inhaled bronchodilator therapy in the treatment of COPD. Chest. 2001;119:85–92.
Bourbeau J, Ernst P, Cockcoft D, Suissa S. Inhaled corticosteroids and hospitalisation due to exacerbation of COPD. Eur Respir J. 2003;22:286–9.
Disano J, Goulet J, Muhajarine N, Neudorf C, Harvey J. Social-economic status and rates of hospital admission for chronic disease in urban Canada. Can Nurse. 2010;106:24–9.
Keenan SP, Powers CE, McCormack DG. Noninvasive positive-pressure ventilation in patients with milder chronic obstructive pulmonary disease exacerbations: a randomized controlled trial. Respir Care. 2005;50:610–6.
Dormuth CR, Maclure M, Glynn RJ, Neumann P, Brookhart AM, Schneeweiss S. Emergency hospital admissions after income-based deductibles and prescription copayments in older users of inhaled medications. Clin Ther. 2008;30(Spec No):1038–50.
Sin DD, Wells H, Svenson LW, Man SF. Asthma and COPD among aboriginals in Alberta, Canada. Chest. 2002;121:1841–6.
Moineddin R, Nie JX, Domb G, Leong AM, Upshur RE. Seasonality of primary care utilization for respiratory diseases in Ontario: a time-series analysis. BMC Health Serv Res. 2008;8:160.
Authors’ contributions
All authors contributed to the design and protocol of the study. TD, AI, SZ conceived, funded and participated in the design and coordination of the literature review. MB and VZ coordinated and conducted the literature review, analyzed the results and drafted the manuscript. All authors reviewed the results of the analysis and contributed to the development. All authors read and approved the final manuscript.
Acknowledgements
Funding for this study was provided by GlaxoSmithKline Inc. Canada. All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. The authors wish to acknowledge Emma Goodall for her critical review of the final manuscript.
Compliance with ethical guidelines
Competing interests TD, AI, and SZ are employees of GlaxoSmithKline Inc. Canada. ASI is also an assistant professor (part-time) in the Department of Clinical Epidemiology and Biostatistics at McMaster University, Hamilton, Ontario, Canada. VZ and MB are former employees of Optum.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Dang-Tan, T., Ismaila, A., Zhang, S. et al. Clinical, humanistic, and economic burden of chronic obstructive pulmonary disease (COPD) in Canada: a systematic review. BMC Res Notes 8, 464 (2015). https://doi.org/10.1186/s13104-015-1427-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-015-1427-y