Abstract
Background
Researchers have highlighted the importance of early access to concussion care within one week of injury in reducing recovery times. However, a persisting question for concussion researchers is “just how early is important?” The purpose of this study was to examine differences in recovery time as predicted by the number of days elapsed since injury (DSI) to initial evaluation among patients who had access to a specialty concussion clinic within seven days. We hypothesized that DSI group membership, even within seven days, would significantly predict risk of protracted recovery (i.e., beyond 21 days).
Methods
In this archival study, retrospective data were gathered from electronic medical records between September 2020 to March 2022. Records of participants between ages 12–18, those diagnosed with a sports-related concussion based on initial clinic visit diagnosis by a medical provider and those who established care within seven days of injury at a large pediatric specialty concussion clinic were examined. Participants were divided into three DSI groups (patients seen in < 48 h: “acute”, patients seen between 49 h < and < 96 h: “sub-acute”, and patients seen between 97 < and < 168 h: “post-acute”). A general linear model was constructed to examine relationships between relevant concussion factors (e.g., Post Concussion Scale Score, neurodevelopmental history, psychiatric history, concussion history, migraine history, overall VOMS change score, cognitive testing, sex, age, race, and ethnicity) that were either significant in the preliminary analysis or in clinical judgement and recovery time. Adjusted odds ratios (OR) were derived from a binary logistic regression model, in which recovery time was normal (≤ 21 recovery days) or protracted (> 21 recovery days).
Results
A total of 856 participants were eligible. Adolescents in the acute group (M = 15.12, SD = 8.04) had shorter recovery times in days compared to those in the sub-acute (M = 17.98, SD = 10.18) and post-acute (M = 21.12, SD = 10.12; F = 26.00, p < .001) groups. Further, participants in the acute (OR = 4.16) and sub-acute (OR = 1.37) groups who accessed specialty concussion clinics within 48 h were 4 times more likely to have a normal recovery and recovered approximately 6 days faster than the post-acute care group.
Conclusions
Earlier concussion care access predicted recovery times and was associated with lower risk for protracted recovery.
Similar content being viewed by others
Background
The incidence rates of pediatric concussion is increasing, with current estimates ranging from 1.1 to 1.9 million injuries and costs to the economy exceeding $15 M annually [1]. Management of pediatric sports-related concussion (SRC) presents a clinical challenge as symptoms tend to evolve over the course of the recovery period, with a normal recovery window of two to three weeks [2]. While many variables influence pediatric and adolescent concussion recovery rates including prior psychiatric diagnosis [3,4,5], initial concussion symptom severity [6,7,8], headache/migraine history [9,10,11], concussion history [12,13,14,15], sex [16,17,18,19,20], and neurodevelopmental factors (ADHD, learning disability) [21, 22], only recently have researchers examined time to clinic as a critical factor in reducing the risk of protracted recovery [23, 24].
Recent research has shown that individuals who sought specialty concussion care within seven days improved faster than those who received care after seven days [23, 24]. Patients who delayed clinical care were six times more likely to experience protracted recovery [24], which is associated with continuing cognitive difficulties [25, 26], higher levels of psychological distress [27,28,29,30], sleep disruption [31,32,33], and numerous physiological symptoms [6, 32,33,34,35,36]. Additionally, continuing play, without evaluation and treatment, has been consistently linked to higher risk of longer recovery times in contrast to those who immediately report and seek care by a concussion specialist [37, 38]. Indeed, Charek et al., 2020 compared individuals with concussion who (1) were immediately removed from play, (2) continued to play for 15 min or less, or (3) continued to play for more than 15 min. Those who continued to play were between 5 to 11 times more likely to experience protracted recovery [39]. Additionally, in their study focusing on patients aged 5 to 9 years, Trbovich and colleagues (2024) found that their model accurately predicted 78% of patients with protracted recovery identifying the Vestibular/Ocular Motor Screen-Child (VOMS-C) score and days between concussion to first clinic visit as the most significant predictors of recovery duration [40].
Despite the growing evidence regarding time to clinic and its association with lower incidence of protracted recovery, generally, there is a paucity of research evaluating the effects of delayed care on SRC recovery outcomes within and outside the seven-day mark. In this study, we sought to examine differences in recovery time among adolescents who had access to specialty concussion care within seven days as there is currently no established literature evaluating differences in recovery among adolescents who receive SRC care within seven days. As such, we divided participants into three groups defined by days since injury (DSI; ≤ 48 h: acute, > 48 h and ≤ 96 h: sub-acute, and > 96 and < 168 h: post-acute). Considering our sample and the approximately equal increments of time within the seven-day window, it was hypothesized that adolescents in the acute DSI group would recover faster than those in the sub-acute and post-acute DSI groups from SRC. Further, it was hypothesized that DSI group membership would be most predictive of recovery time compared to other variables associated with concussion recovery (initial symptom severity, age, sex, psychiatric diagnosis, neurodevelopmental disorder, history of concussion, overall VOMS change score, cognitive testing).
Methods
Participants
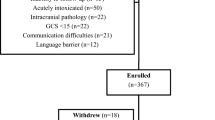
This study involved a retrospective chart review of 1,159 patients evaluated for an SRC September 2020-March 2022 at a large pediatric specialty concussion clinic. Inclusion criteria were as follows: ages 12–18 years, diagnosed with SRC based on CISG 2017 criteria [41], established care within a specialty concussion clinic within seven days, and received medical clearance from the same specialty concussion clinic. In our study, recovery was defined as medical clearance to complete the return to play process. Exclusion criteria included cervicogenic injuries, and congenital or acquired neurological disorders not related to concussion. Patients were referred by emergency departments, athletic trainers, coaches, or self-referral. To increase generalizability of our sample, neurodevelopmental disorders (attention deficit hyperactivity disorder, ADHD, autism spectrum disorder, learning disability), psychiatric diagnoses (depression, anxiety), and individuals with migraines and previous concussion history were all included in our study. This study was approved by the local institutional review board (STU-2021–0334).
Procedure
Of the 1,159 patients, 856 eligible participants were categorized into acute, sub-acute, and post-acute DSI groups. Date of injury was reported directly by the patient based on day of head impact or confirmed by referral source (e.g., athletic trainer, primary care provider, emergency department). All participants were evaluated by clinical staff (sports physician, neuropsychologist, or nurse practitioner) on the date of their initial visit to confirm or establish diagnosis of concussion, and follow-up visits to determine medical clearance. A positive concussion diagnosis was based on clinical history, Post Concussion Symptom Scale score (PCSS) [42], Vestibular Ocular Motor Screening (VOMS) [43], King-Devick test score [44], and brief computerized neuropsychological testing via C3 Logix [45]. Treatment included return-to-learn accommodations, return-to-play protocol implementation, and an individualized rehabilitation program (home exercises and physical therapy) based on PCSS score and clinical evaluation. Data collected from the electronic medical record included concussion diagnosis, age, sex, injury date, date of concussion evaluation, total number of visits, clinical characteristics (e.g., neurodevelopmental disorder, psychiatric diagnosis, history of migraines, history of concussion, PCSS total score), and date of medical clearance.
Data analysis
To establish preliminary group statistical equivalence on potentially confounding risk factors for protracted recovery, we conducted Pearson’s chi-squared tests between categorical variables and one-way ANOVA to test for mean differences between groups, including whether there were significant differences in recovery time due to clinical factors (e.g., gender, concussion history) potentially affecting concussion recovery time. Pearson’s correlational analysis was conducted to evaluate the relationship between recovery time and continuous variables including age and PCSS total. A general linear model was then constructed examining the relationships between recovery time and its prediction by clinically relevant factors including psychiatric diagnosis, history of concussion, migraine diagnosis, neurodevelopmental diagnosis, sex, DSI group membership, race and ethnicity, overall VOMS change score (calculated using Elbin et al., 2018 criteria) [46, 47], average K-D test scores, C3 Logix cognitive testing (i.e., Trails A subtracted from Trails B to better account for cognitive flexibility components), age, and PCSS total. Interaction effects between categorical variables were initially examined but as they were statistically non-significant only main effects are presented for simpler interpretation.
Adjusted odds ratios (OR) for protracted recovery were derived from a logistic regression model, in which the outcome was normal (≤ 21 days) or protracted (> 21 days) recovery [37,38,39, 48, 49]. Individual predictors were systematically removed from the model if they did not significantly contribute to model performance (p < 0.05). Post hoc model diagnostics were conducted to evaluate the model for any violated assumptions. Statistical significance was set a priori at p < 0.05. Analyses were conducted using SPSS 28 software [50]. Post-hoc (multiple comparison) analyses included Ryan-Einot-Gabriel-Welsch F and Games-Howell tests, both assuming and not assuming equal variance among DSI groups [51, 52].
Results
Demographic and clinical characteristics
Participant demographic information and clinical characteristics can be found in Tables 1 and 2. Results from crosstabulation (chi-square) analyses indicated that all three DSI groups were similar in frequency of group members reporting factors potentially affecting concussion outcomes, including race and ethnicity, sex, and reported history of concussions, neurodevelopmental disorders, psychiatric disorders, and migraines (all χ2s > 0.05; Table 1). Groups were significantly different in terms of protracted recovery such that individuals in the post-acute group had the greatest number of individuals with protracted recovery followed by the sub-acute and acute groups, respectively (χ2s = 18.42; p < 0.001). There were significant differences between groups in terms of age (p = 0.02) and recovery days (p < 0.001), but no significant differences in total visits, PCSS total, overall VOMS change score, average K-D test scores, or cognitive testing (p > 0.05; Table 2). Post-hoc analyses showed that the acute and sub-acute groups differed by age (p = 0.02), while the post-acute group did not significantly differ by either group (p > 0.05). Recovery days were significantly different between all three groups (p < 0.001). Patients who were female, had a history of concussion, had a psychiatric diagnosis, older, and had higher PCSS scores, showed longer mean recovery times (p < 0.05; Tables 3 and 4). There were no differences in recovery time based on race and ethnicity, neurodevelopmental diagnosis, or history of migraines (Table 3).
DSI group membership and recovery days
There were significant differences in recovery times based on DSI group membership (F = 25.99, p < 0.001), with small to nearly medium effect sizes (see Table 5). Participants in the acute group had shorter recovery times in days (15.12 ± 8.04) than those in the sub-acute (17.98 ± 10.18) and post-acute groups (21.12 ± 10.12; Table 3).
Clinical predictors of protracted recovery
The general linear regression model demonstrated that history of concussion, sex, DSI group membership, average K-D test scores, and PCSS total significantly predicted recovery days (see Table 5). When including these significant variables in a model predicting protracted recovery, the logistic regression analysis demonstrated that participants in the acute group, when compared to the post-acute group (OR = 4.16) and those without a concussion history (OR = 2.06), were significantly more likely to have normal recovery (see Table 6). Participants with higher PCSS scores were less likely to experience normal recovery (OR = 0.97). Overall, the model was significant, Nagelkerke Pseudo R2 = 0.18, p < 0.001.
Discussion
The purpose of this study was to compare the clinical recovery of adolescent SRC patients evaluated at a specialty pediatric concussion clinic within seven days since injury. Consistent with our hypotheses, the results showed that adolescents within the acute group were cleared three days faster than those in the sub-acute group, and six days faster than those in the post-acute group. Further, those evaluated within 48 h were approximately 4 times more likely to have normal recovery (recovery less than 21 days). Compared to other relevant variables associated with concussion recovery (initial symptom burden, sex, history of concussion, psychiatric diagnoses, neurodevelopmental diagnosis, Overall VOMS Change score, cognitive testing), early access to specialty concussion clinics, as measured by DSI group membership, was highly predictive of recovery time following PCSS total score.
Our results extend previous research, at a more granular level, and suggest that specialty concussion care, or a provider who is well-equipped to manage concussions, within 48 h, can significantly reduce recovery time compared to those who seek care beyond two days [23, 24, 40]. This is important as the literature has shown that established care with a provider who is familiar with the identification and rehabilitation of concussion is critical to recovery [2]. Historically, active management interventions such as prescribed physical activity or targeted rehabilitations were considered counterproductive or potentially detrimental [53]. For decades, the treatment of choice was complete physical and cognitive rest until the patient was asymptomatic [54]. It is only within the last two decades that research has shown the benefits of sub-symptom threshold physical exertion and vestibular rehabilitation, which too have consequently uncovered the possible deleterious effects of extended cognitive and physical rest [55,56,57,58]. Similarly, the literature has updated return-to-learn protocols showing that early return to school, with accommodations, is associated with lower symptoms at follow-up and faster recovery [58, 59]. This guidance and earlier care are important as optimizing clinical recovery can reduce consequences of post-concussive effects on daily functioning, falling behind in school, and emotional well-being [27,28,29,30, 60]. Indeed, prolonged concussive symptoms can increase psychological distress and compromise cognitive abilities [25, 61]. In addition, increased emotionality due to mechanisms like anxiety sensitivity can amplify physiological symptoms (and vice versa) leading to greater impairment than the initial injury [32, 62]. Protracted recovery has also been shown to increase healthcare costs and financial burden, which compound due to increased number of visits to hospitals and emergency departments [63]. Overall, the benefits of early concussion care include quicker access to education, tailored return-to-play and return-to-learn guidance, and follow-up maintenance appointments, which may reduce risk of protracted recovery.
This study is not without limitations. First, our main outcome variable, recovery time (i.e., time to clearance) is defined as the amount of time from initial appointment to follow-up appointment in which full medical clearance is received. The clinical judgement and expertise of the medical provider based on the patient’s presentation at the initial appointment, was used to determine the scheduled follow-up appointment to increase chances of medical clearance. There are myriad of factors that may affect recovery, which may take more or less time than expected. To increase internal consistency among providers, training regarding rehabilitation guidance, typical windows of recovery based on clinical presentation, and factors influencing recovery was implemented. If ambiguity arose based on a particular patient’s presentation, this was discussed with senior clinical staff to determine best course of action or whether inclusion within the database was appropriate. However, future research may decide to include a standard follow-up timeline to determine windows of recovery more accurately (e.g., check in every 7 days until medical clearance is achieved). Second, differences between clinical and statistical significance should be addressed. As clinical researchers, empirical evidence drives decision making in terms of inclusion or exclusion of predictor variables but may also differ depending on specialty. To address this, we included all factors in the GLM regression analysis that were relevant to the literature, regardless of statistical significance. We then identified the most significant predictors in our final analyses, the logistic regression, to ensure statistical and clinical relevance. Nevertheless, research regarding the ambiguity in clinical and statistical significance should be a point of future research inquiry in SRC. Third, greater inclusion of minoritized ethnic and racial backgrounds in this study are needed to better generalize findings. This underscores issues related to decreased or delayed access to specialty concussion clinics though more work is needed to fully understand these potential health disparities (non-private vs. private insurance) [64, 65]. Fourth, while there were no differences between DSI groups in terms of common variables that influence protracted recovery, we did not differentiate patients based on treatment recommendations (e.g., physical therapy, gradual return to activity guidance), which may have also influenced concussion recovery time. Evaluating recommended rehabilitation guidance in addition to comparing mechanism of injury, motor vehicle accidents, and military populations may be useful in understanding how earlier access to specialty concussion clinics influences recovery beyond SRC. The current study did not include participants who recovered spontaneously without medical guidance. Instead, we focused on individuals who sought specialty care based on concussion symptoms, especially during the acute phase of injury. Future research may include a comparison group of individuals who recover from their concussion naturally, without any specialized medical treatment, from those who seek care from a specialty concussion clinic. The findings may allow clinicians to better understand the factors that contribute to recovery based on medical guidance versus benefits that come from an individual’s daily activities or natural healing process. Additionally, our analysis focused on individuals who underwent their initial evaluation at a concussion clinic within seven days following their injury. Consequently, our dataset lacks information on outcomes for individuals who sought care after this seven-day period. It is important to note that existing studies have demonstrated that individuals receiving specialized concussion care beyond seven days post-injury are at an increased risk for prolonged recovery [23, 24]. Despite this, there is a potential for future research to refine our understanding by further stratifying the timeframe beyond the initial week post-injury (e.g., examining recovery outcomes for intervals such as 6–8 days, 9–12 days, 13–15 days post-injury) to delineate the impact of care timing more precisely on recovery trajectories. Lastly, we compared DSI groups with many similar demographics (sex, race, risks of protracted recovery, comorbidities) and found that early access was impactful in terms of recovery. Future studies should examine whether differences in access to clinic exist based on race and ethnicity, sex, comorbidities, type of insurance, or social determinants.
Conclusions
A concussion may negatively affect quality of life, functionality, and worsening anxiety and depression, especially when time elapsed to care is considered [1, 30, 32, 42, 66]. Our results show that early access to care within a specialty concussion clinic within 48 h reduces recovery time by 3–6 days and increases the likely of normal recovery by 4 times compared to the post-acute group. Overall, receiving care as early as possible, awareness and education regarding concussive symptoms, and tailored rehabilitation protocols are vital in reducing recovery time.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Bryan MA, Rowhani-Rahbar A, Comstock RD, Rivara F. Sports-and recreation-related concussions in US youth. Pediatrics. 2016;138(1):1–8.
Thomas DJ, Coxe K, Li H, Pommering TL, Young JA, Smith GA, Yang J. Length of recovery from sports-related concussions in pediatric patients treated at concussion clinics. Clin J Sport Med. 2018;28(1):56–63.
Legarreta AD, Brett BL, Solomon GS, Zuckerman SL. The role of family and personal psychiatric history in postconcussion syndrome following sport-related concussion: a story of compounding risk. J Neurosurg Pediatr. 2018;22(3):238–43.
Martin AK, Petersen AJ, Sesma HW, Koolmo MB, Ingram KM, Slifko KB, ..., Linabery AM. Concussion symptomology and recovery in children and adolescents with pre-existing anxiety. J Neurol Neurosurg Psychiatry. 2020;91(10):1060–1066.
Master CL, Corwin, DJ, Fedonni D, Ampah SB, Housel KC, McDonald C, ..., Grady MF. Dose-response effect of mental health diagnoses on concussion recovery in children and adolescents. Sports Health. 2024;16:19417381241228870.
Meehan WP III, O’Brien MJ, Geminiani E, Mannix R. Initial symptom burden predicts duration of symptoms after concussion. J Sci Med Sport. 2016;19(9):722–5.
Iverson GL, Gardner AJ, Terry DP, Ponsford JL, Sills AK, Broshek DK, Solomon GS. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med. 2017;51(12):941–8.
Kowalczyk CL, Eagle SR, Holland CL, Collins MW, Kontos AP. Average symptom severity and related predictors of prolonged recovery in pediatric patients with concussion. Appl Neuropsychol Child. 2022;11(2):145–9.
Kontos AP, Elbin RJ, Lau B, Simensky S, Freund B, French J, Collins MW. Posttraumatic migraine as a predictor of recovery and cognitive impairment after sport-related concussion. Am J Sports Med. 2013;41(7):1497–504.
Sufrinko AM, Howie EK, Elbin RJ, Collins MW, Kontos AP. A preliminary investigation of accelerometer-derived sleep and physical activity following sport-related concussion. J Head Trauma Rehabil. 2018;33(5):E64–74.
McEvoy H, Borsook D, Holmes SA. Clinical features and sex differences in pediatric post-traumatic headache: a retrospective chart review at a Boston area concussion clinic. Cephalalgia. 2020;40(7):701–11.
Valovich McLeod TC, Bay RC, Snyder AR. Self-reported history of concussion affects health-related quality of life in adolescent athletes. Athl Train Sports Health Care. 2010;2(5):219–26.
Ellis M, Krisko C, Selci E, Russell K. Effect of concussion history on symptom burden and recovery following pediatric sports-related concussion. J Neurosurg Pediatr. 2018;21(4):401–8.
van Ierssel J, Osmond M, Hamid J, Sampson M, Zemek R. What is the risk of recurrent concussion in children and adolescents aged 5–18 years? A systematic review and meta-analysis. Br J Sports Med. 2021;55(12):663–9.
Cook NE, Gaudet CE, Van Patten R, Kissinger-Knox A, Iverson GL. Clinical outcome following sport-related concussion among children and adolescents with a history of prior concussion: a systematic review. J Neurotrauma. 2022;39(17–18):1146–58.
Cheng J, Ammerman B, Santiago K, Jivanelli B, Lin E, Casey E, Ling D. Sex-based differences in the incidence of sports-related concussion: systematic review and meta-analysis. Sports Health. 2019;11(6):486–91.
Desai N, Wiebe DJ, Corwin DJ, Lockyer JE, Grady MF, Master CL. Factors affecting recovery trajectories in pediatric female concussion. Clin J Sport Med. 2019;29(5):361–7.
Merritt VC, Padgett CR, Jak AJ. A systematic review of sex differences in concussion outcome: what do we know? Clin Neuropsychol. 2019;33(6):1016–43.
Hannah TC, Li AY, Spiera Z, Kuohn L, Dai J, McAuley F,..., Choudhri T. Sex-related differences in the incidence, severity, and recovery of concussion in adolescent student-athletes between 2009 and 2019. Am J Sports Med. 2021;49(7):1929–1937.
Sharma B, Nowikow C, DeMatteo C, Noseworthy MD, Timmons BW. Sex-specific differences in resting-state functional brain activity in pediatric concussion. Sci Rep. 2023;13(1):3284.
Gunn BS, McAllister TW, McCrea MA, Broglio SP, Moore RD, CARE Consortium Investigators. Neurodevelopmental disorders and risk of concussion: findings from the National collegiate athletic Association Department of defense grand alliance concussion assessment, research, and education (NCAA-DOD CARE) consortium (2014–2017). J Neurotrauma. 2022;39(5–6):379–89.
Martin AK, Petersen AJ, Sesma HW, Koolmo MB, Ingram KM, Slifko KB, ..., Linabery AM. Learning and attention deficit/hyperactivity disorders as risk factors for prolonged concussion recovery in children and adolescents. J Int Neuropsychol Soc. 2022;28(2):109–122.
Eagle SR, Puligilla A, Fazio-Sumrok V, Kegel N, Collins MW, Kontos AP. Association of time to initial clinic visit with prolonged recovery in pediatric patients with concussion. J Neurosurg Pediatr. 2020;26(2):165–70.
Kontos AP, Jorgensen-Wagers K, Trbovich AM, Ernst N, Emami K, Gillie B, ..., Collins MW. Association of time since injury to the first clinic visit with recovery following concussion. JAMA Neurol. 2020;77(4):435–440.
Crowe L, Collie A, Hearps S, Dooley J, Clausen H, Maddocks D, ..., Anderson V. Cognitive and physical symptoms of concussive injury in children: a detailed longitudinal recovery study. Br J Sports Med. 2016;50(5):311–316.
Taylor KM, Kioumourtzoglou MA, Clover J, Coull BA, Dennerlein JT, Bellinger DC, Weisskopf MG. Concussion history and cognitive function in a large cohort of adolescent athletes. Am J Sports Med. 2018;46(13):3262–70.
Stein E, Howard W, Rowhani-Rahbar A, Rivara FP, Zatzick D, McCarty CA. Longitudinal trajectories of post-concussive and depressive symptoms in adolescents with prolonged recovery from concussion. Brain Inj. 2017;31(13–14):1736–44.
Brooks BL, Plourde V, Beauchamp MH, Tang K, Yeates KO, Keightley M, ..., PERC Concussion Team. Predicting psychological distress after pediatric concussion. J Neurotrauma. 2019;36(5):679-685.
Ernst N, Eagle S, Trbovich A, Kissinger-Knox A, Bitzer H, Kontos AP. Lower post-injury psychological resilience is associated with increased recovery time and symptom burden following sport-related concussion. Appl Neuropsychol Child. 2022;11(4):781–8.
Ledoux AA, Webster RJ, Clarke AE, Fell DB, Knight BD, Gardner W, ..., Zemek R. Risk of mental health problems in children and youths following concussion. JAMA Netw Open. 2022;5(3):e221235-e221235.
Bramley H, Henson A, Lewis MM, Kong L, Stetter C, Silvis M. Sleep disturbance following concussion is a risk factor for a prolonged recovery. Clin Pediatr. 2017;56(14):1280–5.
Murdaugh DL, Ono KE, Reisner A, Burns TG. Assessment of sleep quantity and sleep disturbances during recovery from sports-related concussion in youth athletes. Arch Phys Med Rehabil. 2018;99(5):960–6.
Chung JS, Zynda AJ, Didehbani N, Hicks C, Hynan LS, Miller SM, ..., Cullum CM. Association between sleep quality and recovery following sport-related concussion in pediatrics. J Child Neurol. 2019;34(11):639–645.
Howell DR, O’Brien MJ, Beasley MA, Mannix RC, Meehan WP III. Initial somatic symptoms are associated with prolonged symptom duration following concussion in adolescents. Acta Paediatr. 2016;105(9):e426–32.
Root JM, Zuckerbraun NS, Wang L, Winger DG, Brent D, Kontos A, Hickey RW. History of somatization is associated with prolonged recovery from concussion. J Pediatr. 2016;174:39–44.
Green KE, Purtzki J, Chapman A, Oberlander TF, Silverberg ND, Dhariwal AK. Somatization in adolescents with persistent symptoms after concussion: a retrospective chart review. J Neuropsychiatry Clin Neurosci. 2022;34(4):378–85.
Elbin RJ, Sufrinko A, Schatz P, French J,Henry L, Burkhart S, Collins MW, Kontos AP. Removal from play after concussion and recovery time. Pediatrics. 2016;138(3):e20160910.
Barnhart M, Bay RC, Valovich McLeod TC. The influence of timing of reporting and clinic presentation on concussion recovery outcomes: a systematic review and meta-analysis. Sports Med. 2021;51:1491–508.
Charek DB, Elbin RJ, Sufrinko A, Schatz P, D’Amico NR, Collins MW, Kontos AP. Preliminary evidence of a dose-response for continuing to play on recovery time after concussion. J Head Trauma Rehabil. 2020;35(2):85–91.
Trbovich AM, Mucha A, Zynda AJ, Farley T, Kegel N, Fazio V, ..., Kontos AP. Multidomain predictors of protracted recovery following concussion among 5-to 9-year-old patients: a preliminary study. J Pediatr. 2024;268:113927.
McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S, ..., Vos PE. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–847.
Lovell MR, Iverson GL, Collins MW, Podell K, Johnston KM, Pardini D, ..., Maroon JC. Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol. 2006;13(3):166–174.
Mucha A, Collins MW, Elbin RJ, Furman JM, Troutman-Enseki C, DeWolf RM, ..., Kontos AP. A brief vestibular/ocular motor screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42(10):2479–2486.
Oride MK, Marutani JK, Rouse MW, DeLand PN. Reliability study of the Pierce and King-Devick saccade tests. Am J Optom Physiol Optics. 1986;63:419–24.
Neurologix Technology, Inc. C3 logix: A concussion management system. 2015. Retrieved from http://www.neurologixtech.com/. Accessed 10 Aug 2023.
Elbin RJ, Sufrinko A, Anderson MN, Mohler S, Schatz P, Covassin T, ..., Kontos AP. Prospective changes in vestibular and ocular motor impairment after concussion. J Neurol Phys Ther. 2018;42(3):142–148.
Elbin RJ, Eagle SR, Marchetti GF, Anderson M, Schatz P, Womble MN, ..., Kontos AP. Using change scores on the vestibular ocular motor screening (VOMS) tool to identify concussion in adolescents. Appl Neuropsychol Child. 2022;11(4):591–597.
Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311–8.
Phillips MM, Reddy CC. Managing patients with prolonged recovery following concussion. Phys Med Rehabil Clin. 2016;27(2):455–74.
IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk: IBM Corp.
Ruxton GD, Beauchamp G. Time for some a priori thinking about post hoc testing. Behav Ecol. 2008;19(3):690–3.
Sauder DC, DeMars CE. An updated recommendation for multiple comparisons. Adv Methods Pract Psychol Sci. 2019;2(1):26–44.
Leddy JJ, Kozlowski K, Fung M, Pendergast DR, Willer B. Regulatory and autoregulatory physiological dysfunction as a primary characteristic of post concussion syndrome: implications for treatment. NeuroRehabilitation. 2007;22(3):199–205.
McCrory P, Johnston K, Meeuwisse W, Aubry M, Cantu R, Dvorak J, ..., Schamasch P. Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004. Br J Sports Med. 2005;39(4):196–204.
DiFazio M, Silverberg ND, Kirkwood MW, Bernier R, Iverson GL. Prolonged activity restriction after concussion: are we worsening outcomes? Clin Pediatr. 2016;55(5):443–51.
Grool AM, Aglipay M, Momoli F, Meehan WP, Freedman SB, Yeates K O, ..., Pediatric Emergency Research Canada (PERC) Concussion Team. Association between early participation in physical activity following acute concussion and persistent postconcussive symptoms in children and adolescents. JAMA. 2016;316(23):2504-2514.
Leddy JJ, Haider MN, Hinds AL, Darling S, Willer BS. A preliminary study of the effect of early aerobic exercise treatment for sport-related concussion in males. Clin J Sport Med. 2019;29(5):353.
Vaughan CG, Ledoux AA, Sady MD, Tang K, Yeates KO, Sangha G, ..., Grool A. Association between early return to school following acute concussion and symptom burden at 2 weeks postinjury. JAMA Netw Open. 2023;6(1):e2251839-e2251839.
DeMatteo CA, Randall S, Lin CYA, Claridge EA. What comes first: return to school or return to activity for youth after concussion? Maybe we don’t have to choose. Front Neurol. 2019;10: 466703.
Russell K, Selci E, Chu S, Fineblit S, Ritchie L, Ellis MJ. Longitudinal assessment of health-related quality of life following adolescent sports-related concussion. J Neurotrauma. 2017;34(13):2147–53.
Schilling S, Mansour A, Sullivan L, Ding K, Pommering T, Yang J. Symptom burden and profiles in concussed children with and without prolonged recovery. Int J Environ Res Public Health. 2020;17(1):351.
Caze T, Vásquez D, Moffatt K, Waple K, Hope D. A prospective pilot study of anxiety sensitivity and adolescent sports-related concussion. Arch Clin Neuropsychol. 2021;36(6):930–9.
Yengo-Kahn AM, Wallace J, Jimenez V, Totten DJ, Bonfield CM, Zuckerman SL. Exploring the outcomes and experiences of Black and White athletes following a sport-related concussion: a retrospective cohort study. J Neurosurg Pediatr. 2021;28(5):516–25.
Skinner AC, Mayer ML. Effects of insurance status on children’s access to specialty care: a systematic review of the literature. BMC Health Serv Res. 2007;7(1):1–12.
Copley M, Jimenez N, Kroshus E, Chrisman SPD. Disparities in use of subspecialty concussion care based on ethnicity. J Racial Ethn Health Disparities. 2020;7(3):571–6.
McCrea M, Guskiewicz K, Randolph C, et al. Incidence, clinical course, and predictors of prolonged recovery time following sport-related concussion in high school and college athletes. J Int Neuropsychol Soc. 2019;19(1):22–33.
Acknowledgements
We are grateful to Christine Ellis, APRN, PNP, Troy Smurawa, MD, and our neuropsychology postdoctoral fellows for their assistance with the recruitment phase. We also appreciate Lindsay Hartland, M.S. and Nicole Minor, B.S. for their management of this research study. This study would not have been possible without the support of the Children’s Health Andrews Institute.
Funding
The authors declare that they have no funding disclosures.
Author information
Authors and Affiliations
Contributions
A.M.: conceptualization, methodology, formal analysis, writing-original draft. T.C.II: conceptualization, methodology, data curation. A.P: conceptualization, methodology, writing-original draft. D.V: data curation, formal analysis, writing-review & editing. J.A.t: supervision, writing-review & editing. S.B.: conceptualization, methodology, supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors and institution received ethics committee approval for human investigation by the Children’s Health Institutional Review Board (IRB; STU-2021–0334) in Dallas, Texas. All methods were carried out in accordance with IRB guidelines and regulations. Informed consent was obtained for all subjects and/or their legal guardians at the time of their initial evaluation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mathew, A.S., Caze, T., Price, A.M. et al. Association between days for concussion recovery and initial specialty clinic evaluation within 48 hours. BMC Sports Sci Med Rehabil 16, 75 (2024). https://doi.org/10.1186/s13102-024-00866-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13102-024-00866-w