Abstract
Background
Exercise-induced laryngeal obstruction (EILO) is diagnosed by the continuous laryngoscopy during exercise (CLE) test. Whether or how much CLE test scores vary over time is unknown. This study aimed to compare CLE test scores in athletes over time, irrespective of respiratory symptoms and grade of laryngeal obstruction.
Methods
Ninety-eight athletes previously screened for EILO were invited for a follow-up CLE test irrespective of CLE scores and respiratory symptoms. Twenty-nine athletes aged 16–27 did a follow-up CLE test 3–23 months after the baseline test. Laryngeal obstruction at the glottic and supraglottic levels was graded by the observer during exercise, at baseline and follow-up, using a visual grade score (0–3 points).
Results
At baseline, 11 (38%) of the 29 athletes had moderate laryngeal obstruction and received advice on breathing technique; among them, 8 (73%) reported exercise-induced dyspnea during the last 12 months. At follow-up, 8 (73%) of the athletes receiving advice on breathing technique had an unchanged supraglottic score. Three (17%) of the 18 athletes with no or mild laryngeal obstruction at baseline had moderate supraglottic obstruction at follow-up, and none of the 3 reported exercise-induced dyspnea.
Conclusions
In athletes with repeated testing, CLE scores remain mostly stable over 3–24 months even with advice on breathing technique to those with EILO. However, there is some intraindividual variability in CLE scores over time.
Trial registration
ISRCTN, ISRCTN60543467, 2020/08/23, retrospectively registered, ISRCTN – ISRCTN60543467: Investigating conditions causing breathlessness in athletes.
Similar content being viewed by others
Background
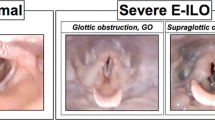
Exercise-induced laryngeal obstruction (EILO) is an important and possibly treatable cause of exertional dyspnea, affecting about 5.7%–7.6% of a general population and 8.1%–27% of athletic populations [1,2,3,4]. EILO causes respiratory symptoms of especially inspiratory character during high-intensity effort [5, 6]. It is important to test for EILO in patients with exercise-induced dyspnea, because EILO is often mistaken for asthma and the two conditions often co-exist [1, 2, 7]. Because symptoms of EILO abate quickly upon exercise cessation, diagnosis requires visualizing the larynx during, or in close association to, a maximal bout of exercise [5, 8, 9]. A common test used in the clinical and research setting is the continuous laryngoscopy during exercise (CLE) test [10]. The test involves the use of a flexible nasolaryngoscope during a maximal effort exercise bout, usually on a treadmill or bicycle [11, 12]. The laryngeal obstruction at the glottic (i.e., vocal cord) and supraglottic levels is then graded, usually by an observer using a 0–3-point visual grade score [12, 13].
Only one study has described the natural course of EILO diagnosed by CLE in teenagers given advice on breathing techniques and having predominantly supraglottic EILO. CLE-scores were normalized in 3 out 14 subjects, respiratory symptom severity decreased, and the teenagers reported a higher ability to perform exercise over a period of 2–5 years [14]. Non-surgical treatment of EILO may be most effective in glottic EILO, as indicated in two recent small studies focusing on inspiratory muscle training and on breathing techniques combined with cognitive behavioral therapy [15, 16]. Nonetheless, advice on breathing techniques during exercise is recommended to patients with mild EILO regardless of the location of obstruction, whereas surgical treatment is an option in selected highly motivated patients with severe supraglottic EILO [9, 14, 17, 18]. Other interventions such as biofeedback, speech therapy, and laryngeal control therapy may also have an effect on EILO [8, 9, 19,20,21]. However, the few longitudinal studies on EILO patients have not included asymptomatic controls. Indeed, there is currently a lack of evidence regarding treatment of EILO, and no randomized controlled trials of treatment have been published.
A recent review on EILO in highlighted our scarce knowledge on the natural history and prognosis of EILO [22].
We need more understanding of the validity of the CLE test, the natural history of EILO and effects of treatment. The aim of this prospective study was to examine the variability of CLE scores in athletes, irrespective of EILO diagnosis, treatment, and respiratory symptoms.
Materials and methods
Study design and study population
This prospective screening study on EILO diagnosed by CLE test with one baseline visit and one follow-up was open to elite cross-country skiers (competing in cross-country skiing, biathlon, or ski-orienteering) aged 15–35 years. Elite skiers were defined as skiers belonging to Swedish national teams, national elite upper secondary sport schools, universities with elite sports agreement, or had competed at national or international level during the last 12 months. Information about the study was distributed to coaches and medical personnel of the Swedish Ski Association, Swedish Biathlon Federation, and Swedish National Elite Sports Schools in the county of Jämtland Härjedalen. Information about the study was also distributed via advertisement at suitable facilities.
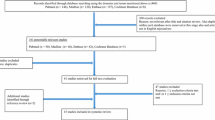
A total of 98 athletes (including 89 cross-country skiers or biathletes) participated in the baseline visit and were screened for EILO, allergy, and asthma [23]. The aim of the follow-up was to re-examine these 98 athletes after 6 months irrespective of airway symptoms and diagnoses. Twenty-nine of the 98 participated in the follow-up. Due to the competition calendar and logistics, the mean duration between first visit and follow-up was 8.4 (min 3, max 23) months.
Setting
The study was carried out at Östersund Hospital, Sweden, in 2015–2017. Both visits included a medical history, clinical examination, dynamic spirometry, eucapnic voluntary hyperventilation (EVH) test, CLE test, and questionnaires.
Study procedures
Spirometry and EVH test
Dynamic spirometry was performed using a Spirare 3 (Diagnostics, Norway) or Jaeger Vyntus IOS (CareFusion, Germany) device [24]. EVH testing was conducted using the AILOS Asthma Test (Ailos Medical AB, Sweden) or Eucapsys (SMTEC, Switzerland). Participants were instructed to hyperventilate dry air with 5% CO2 (Carboair, Air Liquid GAS, Sweden) for 6 min with a target minute ventilation set at 85% of maximum voluntary hyperventilation (30 * FEV1 [Forced Expiratory Volume] in 1 s) [25]. FEV1 was measured before and at 3, 5, 10, 15, and 20 min post EVH, with the highest FEV1 of two approved maneuvers recorded. Exercise-induced bronchoconstriction was defined as a ≥ 10% decrease in FEV1 after EVH [26]. Participants were instructed to avoid vigorous exercise, heavy meals, caffeine, and nicotine for 8 h before the EVH test. They also were instructed to refrain from using asthma medication before the test, within the following time frames: short-acting β2-agonists for 8 h, inhaled corticosteroids for 12 h, long-acting β2-agonists for 24 h, and leukotriene receptor agonists for 3 days. Participants had to be free from respiratory infection for at least 6 weeks before the test.
Questionnaire
The questionnaire was based on the European Community Respiratory Health Survey II (ECHRS II) and covered training background, medications, airway symptoms, allergies, asthma, and family history [27]. Key variables were defined as:
Physician-diagnosed asthma: “Yes” to “Have you ever had asthma?” and “Was it diagnosed by a medical doctor?”.
Asthma medication last 12 months: “Yes” to “Have you used any asthma medication (including inhalers, aerosols, or tablets) in the last 12 months?
Current asthma: Having physician-diagnosed asthma and use of asthma medication in the last 12 months.
Exercise-induced dyspnea (EID): “Yes” to the question “Have you had any shortness of breath following exercise, during the last 12 months?”.
Continuous laryngoscopy during exercise test
The CLE test was performed according to Heimdal et. Al [10]. The larynx was continuously visualized with a nasal fiber-optic nasolaryngoscope (FNL –7RP3, Pentax, Tokyo, Japan) and video-recorded during exercise on a treadmill (Intelligent Suspension 3, Cybex, US). Naphazoline-lidocaine was used for local anesthesia and dilatation of the nasal cavity pre insertion of the nasolaryngoscope. Speed or inclination of the treadmill was increased at every minute after a 3-min warm-up, to reach maximal effort at 6–9 min of incremental exercise. Speed increased by 1 km/h up to a maximum of 15 km/h, and inclination by 2% up to a maximum of 8%. The intention was to exercise the athletes to exhaustion, but we set the lowest acceptable target to > 90% of estimated maximal heart rate. Heart rate was monitored using a chest strap (Polar, Finland).
Laryngeal obstruction at the glottic and supraglottic levels was assessed at 1 min after warm-up and at maximal effort, using an ordinal visual grade score (0–3 points; none (0), mild (1), moderate (2), severe (3)) according to Maat et. Al [13, 28]. EILO was defined as a CLE score of ≥ 2 points at the glottic or supraglottic level at maximal effort. CLE tests were performed and EILO was diagnosed at the discretion of the study physician (M.R) consultant in otorhinolaryngology, and a physiotherapist (C.B.). M.R. graded the laryngeal obstruction, blinded to results from other parts of the study and to whether the participant had reported any airway symptoms. A second blinded grading of the video laryngoscopies, without access to sound or external video recording, was done by resident physician in pediatrics T.I. A final CLE score was set by M.R. in case of disagreement.
Respiratory distress during the CLE test was graded by the observer ordinally as: “none”, “mild, with audible respiration”, “moderate, with stridor”, and “collapse or panic attack”. Any disagreement between the observers was solved by consensus. Except for the very beginning of the study, participants rated their perceived physical exertion according to the Borg rating of perceived exertion scale (RPE Scale), ranging from 6 (no exertion at all) to 20 (maximal exertion), immediately after cessation of the exercise [29].
Clinical treatment of study participants
All athletes participating in the study were cared for in the same manner as practiced in the clinical setting, and any recommendations were at the discretion of the study physicians. For participants with significant laryngeal obstruction, this included a review of laryngeal findings on video and self-care advice on breathing techniques to use during exercise. For instance, they were advised to focus on diaphragmatic breathing through closed-lip-breathing and to modify the posture e.g. keeping the head high and lifted, and the shoulders low and retracted [30]. There was no follow-up between visits, such as a check on adherence to recommendations. No participant with EILO received speech therapy or surgery.
Statistics
Data analyses were conducted using R Statistical Software (version 3.6.1). The chi-square test was used for categorical variables. The Wilcoxon signed-rank test was used to compare non-parametric data at baseline versus follow-up. Results were considered statistically significant at a P-value of < 0.05.
Results
Population characteristics
Of the 29 athletes (15 females) who completed baseline and follow-up visits, 27 were skiers (cross-country skiers or biathletes), one was an elite orienteer, and one was an elite track-and-field athlete. Prior to the baseline visit, none of the athletes had been diagnosed with EILO, and none had ever smoked regularly. Baseline characteristics are presented in Table 1. The 29 athletes did not differ from the 69 that did not participate in the follow-up regarding age or the proportion of females, physician-diagnosed asthma, current asthma, EID, or EILO (≥ 2 points at the supraglottic or glottic level at maximal effort) (Table 2).
Baseline
Of the 29 athletes, 11 (38%) athletes (ten females) had moderate supraglottic obstruction, visual grade score of 2, at maximal effort during the baseline CLE test (Table 3). These were given self-care advice on breathing techniques. Three (27%) out of the 11 athletes with moderate obstruction did not report any exercise-induced dyspnea in the previous 12 months. The 18 athletes without EILO at baseline are described in Table 4. None of the 29 athletes had glottic EILO, but two had mild glottic obstruction.
Exercise-induced dyspnea was more common in the athletes with moderate obstruction compared to those without (73% vs. 17%, P = 0.003). Only three of 29 athletes had observer-rated respiratory distress during the baseline CLE test. Their respiratory distress was rated as “mild, with audible respiration” and all three had EILO. No athlete had respiratory distress of “moderate, with stridor” or “collapse or panic attack” character.
At the baseline CLE test, median (interquartile range [IQR]) peak heart rate was 96 (94–98) % of estimated maximum heart rate.
Follow-up
Training hours/week at follow-up did not differ from those at baseline (median (quartile 1-quartile 3)), the athletes trained median (quartile 1-quartile 3) 12 (10–14) vs 12 (10–13) hours/week, P = 0.130. Training hours for the athletes with EILO at baseline was also similar between follow-up and baseline, 10 (10–12) vs 10 (10–12), P = 1, In the 11 athletes with moderate obstruction at first visit, 8 (73%) had the same supraglottic score at follow-up, and three athletes were graded with mild obstruction (Table 3). Seven out of 11 athletes with moderate obstruction at follow-up did not report any EID in the previous 12 months. In the 18 athletes with none or mild obstruction at first visit, three athletes had moderate obstruction at follow-up (Table 4).
Two of the three athletes with observer-graded respiratory distress persisted as “mild, with audible respiration” at the second CLE test. No other athlete was observed with respiratory distress at follow-up.
At follow-up, median (IQR) peak heart rate was 94 (93–97) % of estimated maximum heart rate, which did not significantly differ (P = 0.112) from peak heart rate at baseline.
Asthma
Among the 10 athletes with current asthma at baseline, one had EIB according to the EVH test, and six reported having had an asthma attack during the last 12 months. At follow-up, two out of nine athletes with current asthma had EIB. In total, six (21%) athletes had EIB at baseline or follow-up. Three out of five athletes with both current asthma and EILO at baseline had stopped using asthma medication at follow-up. These five athletes had negative EVH tests at both visits.
Discussion
Main summary
This prospective study assessed the variability in CLE scores over time in athletes irrespective of grade of laryngeal obstruction, respiratory symptoms, and intervention. We found that the CLE scores mainly remained stable over time. However, 27% of those with moderate supraglottic obstruction at baseline and given advice on breathing techniques had mild obstruction at follow-up, and 17% of those with no-to-mild supraglottic obstruction at baseline had moderate obstruction at follow-up. None of the athletes had significant glottic obstruction at baseline or follow-up.
CLE scores remain mostly stable in athletes treated for EILO
In the present study, participants with moderate supraglottic obstruction were given advice on breathing techniques to use during exercise, at the discretion of the study physician. The change in laryngeal obstruction between visits in those with moderate obstruction in the present study is similar to that in 14 teenage patients given advice on breathing techniques for supraglottic EILO, among whom 21% (n = 3) had normalized laryngeal function after 2–5 years [14]. Two recent studies using CLE scores to assess treatment outcome found that inspiratory muscle training and physiotherapy may have an effect, especially on glottic EILO [15, 16]. However, many studies employ methods other than the CLE test to evaluate laryngeal obstruction, often only assessing the glottic level of obstruction post exercise, making comparisons with the present findings difficult [8, 31]. Also, while subjective improvement is important, more objective data related to laryngeal obstruction is needed to give us a better understanding of EILO. To what extent different factors contribute to the remission or incidence of EILO is unknown, and this underlines the need for randomized controlled trials of treatments in these populations.
The breathing technique intervention in the present study appear to have had a limited effect on laryngeal obstruction and respiratory symptoms. The effectiveness of a breathing technique intervention on laryngeal obstruction is largely unknown due to the lack of randomized controlled trials. It is therefore difficult to know whether the apparent lack of effect was a result of poor adherence, an ineffective intervention, or participation bias. Nevertheless, our results indicate that supraglottic EILO in elite skiers does not resolve to any large extent, neither spontaneously nor upon advice on breathing, when reassessed within 2 years.
EILO and sex
Of the 11 athletes with EILO at follow-up, 10 were females. Several studies have shown that EILO is more common in females than men [3, 7, 8, 32]. Other studies do not find sex differences [1, 2]. One explanation for the female dominance may be due to differences in laryngeal size. Prepubertal laryngeal size is similar in boys and girls, but pubertal growth differences result in a larger male larynx [33, 34]. Others have shown that adolescent female athletes with EILO have a reduced respiratory resistance, perhaps due to reduced laryngeal tone [35, 36]. A simpler explanation to the sex difference in the present study is participation bias. We cannot rule out the possibility that female athletes with persistent EILO were more interested, due to unknown reasons, to participate in the follow-up CLE test than the male athletes with EILO.
Screening of EILO may detect asymptomatic supraglottic obstruction
A strength and novelty of the present study is the prospective follow-up of athletes without EID or EILO. In most (83%) of the subjects with no-to-mild laryngeal obstruction, the baseline CLE scores persisted at follow-up. However, three of these athletes had incident moderate supraglottic obstruction at follow-up. Interestingly, all three did not have EID in the previous 12 months and did not have respiratory distress as graded by the observer during the CLE test. Also, three out of 8 athletes with EILO and EID at baseline, reported no EID at follow-up despite persistent laryngeal obstruction fulfilling the study criteria for EILO.
Many epidemiological studies have defined EILO purely based on laryngeal obstruction during a CLE test and many with EILO did not report any self-reported exercise-related respiratory symptoms [2, 3]. Screening has also shown that a substantial proportion of athletes with EILO do not report exercise-induced symptoms [1]. So, asymptomatic subjects with EILO are not uncommon.
Furthermore, we cannot be certain that athletes reporting shortness of breath following exercise during the last 12 months, had these symptoms purely due to EILO. Although breathlessness is a typical symptom of EILO, EILO may also induce other symptoms such as chest tightness and throat discomfort not covered in our questionnaire [3, 5]. Thus, we may have falsely described some athletes with EILO as asymptomatic. Self-reported respiratory symptoms are poor at predicting EILO and also of limited value in discerning EILO from asthma [1,2,3, 7, 37]. However, subjects with EILO have increased work of breathing [38]. Thus, it remains to be established whether mild laryngeal obstruction in an asymptomatic subject constitutes a pathological or normal laryngeal response to high-intensity exercise.
Asthma and EIB
Very few (one out of ten) athletes with current asthma had EIB, indicating a possible overdiagnosis of asthma in the study population [39]. Cross-country skiers are known to have a high prevalence of asthma, probably because of prolonged inhalation of cold dry air leading to airway hyperreactivity [40, 41]. However, the high prevalence of EILO in our study population, indicates that objective testing for both asthma and EILO is important in athletes with exercise-induced symptoms. Also in Norway many athletes diagnosed with EILO use asthma medication and many without objective evidence of variable broncho-obstruction according to retrospective reviews [42].
Limitations
The CLE test protocol used may have led to false low visual-grade scores. Firstly, the target heart rate during the CLE test of 90% of the estimated maximum may simply have been too low to induce EILO in the present study population including athletes competing at the highest national or international level [22]. If the participants had exerted even harder we might have detected more glottic EILO, which tends to develop after supraglottic obstruction [13]. Secondly, the visual grade score might underestimate glottic obstruction [43].
There was a risk of participation bias at both baseline and follow-up. Our study had a high proportion of cross-country skiers with EID at baseline. Recruitment via open invitation possibly attracted athletes with respiratory symptoms, inducing a risk of overestimating the prevalence of EILO and asthma [39]. Furthermore, among the athletes participating in the follow-up, a higher proportion had EID compared with the whole population of cross-country skiers at baseline, indicating that athletes with respiratory symptoms may have been more prone to participate in the follow-up. This could mean that the present results are skewed towards athletes with respiratory symptoms, irrespective of laryngeal obstruction, and underestimate the effects of breathing advice for EILO.
The CLE scoring system has received criticism for not being a robust method of assessing laryngeal obstruction, which if true could have affected our results. Studies assessing its validity and reliability have come to different conclusions [13, 44]. The CLE test score has been found to be comparable to EILOMEA, a more objective method of assessing laryngeal obstruction that uses software to estimate the grade of obstruction from a still frame of the larynx taken during a CLE test [45]. Taken together, there is a need for objective methods with which to assess laryngeal obstruction for use during the CLE test.
One study has shown that athletes in subsequent CLE tests could exercise for a longer time before exhaustion [44]. Our results do not indicate any learning effect. The athletes’ CLE test performance, in terms of self-perceived exertion and peak heart rate percent of estimated maximum heart rate, was similar at baseline and follow-up.
Conclusion
In athletes performing repeated diagnostic tests for EILO, the CLE test score is mostly stable over time, but there is some intra-individual variability in scores. In the present study, the incident cases of moderate supraglottic laryngeal obstruction did not have exercise-related respiratory symptoms, indicating a need to establish what constitutes a normal laryngeal response to exercise in athletes. This study also highlights the need for randomized controlled trials that objectively assess factors associated with treatment effects in patients with EILO.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CLE:
-
Continuous laryngoscopy during exercise
- EID:
-
Exercise-induced dyspnea
- EILO:
-
Exercise-induced laryngeal obstruction
- EVH:
-
Eucapnic voluntary hyperventilation
- FEV1 :
-
Forced expiratory volume first second
- FVC:
-
Forced vital capacity
- IQR:
-
Interquartile range
- RPE:
-
Rating of perceived exertion
References
Ersson K, Mallmin E, Malinovschi A, Norlander K, Johansson H, Nordang L. Prevalence of exercise-induced bronchoconstriction and laryngeal obstruction in adolescent athletes. Pediatr Pulmonol. 2020. https://doi.org/10.1002/ppul.25104.
Johansson H, Norlander K, Berglund L, Janson C, Malinovschi A, Nordvall L, et al. Prevalence of exercise-induced bronchoconstriction and exercise-induced laryngeal obstruction in a general adolescent population. Thorax. 2015;70(1):57–63. https://doi.org/10.1136/thoraxjnl-2014-205738.
Christensen PM, Thomsen SF, Rasmussen N, Backer V. Exercise-induced laryngeal obstructions: prevalence and symptoms in the general public. Eur Arch Otorhinolaryngol. 2011;268(9):1313–9. https://doi.org/10.1007/s00405-011-1612-0.
Irewall T, Söderström L, Lindberg A, Stenfors N. High incidence rate of asthma among elite endurance athletes: a prospective 4-year survey. J Asthma. 2021;58(6):735–41. https://doi.org/10.1080/02770903.2020.1728769.
Shay EO, Sayad E, Milstein CF. Exercise-induced laryngeal obstruction (EILO) in children and young adults: from referral to diagnosis. Laryngoscope. 2019. https://doi.org/10.1002/lary.28276.
Hall A, Thomas M, Sandhu G, Hull JH. Exercise-induced laryngeal obstruction: a common and overlooked cause of exertional breathlessness. Br J Gen Pract. 2016;66(650):e683–5. https://doi.org/10.3399/bjgp16X687001.
Nielsen EW, Hull JH, Backer V. High prevalence of exercise-induced laryngeal obstruction in athletes. Med Sci Sports Exerc. 2013;45(11):2030–5. https://doi.org/10.1249/MSS.0b013e318298b19a.
Marcinow AM, Thompson J, Chiang T, Forrest LA, Desilva BW. Paradoxical vocal fold motion disorder in the elite athlete: experience at a large division I university. Laryngoscope. 2014;124(6):1425–30. https://doi.org/10.1002/lary.24486.
Roksund OD, Heimdal JH, Clemm H, Vollsaeter M, Halvorsen T. Exercise inducible laryngeal obstruction: diagnostics and management. Paediatr Respir Rev. 2017;21:86–94. https://doi.org/10.1016/j.prrv.2016.07.003.
Heimdal JH, Roksund OD, Halvorsen T, Skadberg BT, Olofsson J. Continuous laryngoscopy exercise test: a method for visualizing laryngeal dysfunction during exercise. Laryngoscope. 2006;116(1):52–7. https://doi.org/10.1097/01.mlg.0000184528.16229.ba.
Tervonen H, Niskanen MM, Sovijärvi AR, Hakulinen AS, Vilkman EA, Aaltonen LM. Fiberoptic videolaryngoscopy during bicycle ergometry: a diagnostic tool for exercise-induced vocal cord dysfunction. Laryngoscope. 2009;119(9):1776–80. https://doi.org/10.1002/lary.20558.
Halvorsen T, Walsted ES, Bucca C, Bush A, Cantarella G, Friedrich G, et al. Inducible laryngeal obstruction: an official joint European Respiratory Society and European Laryngological Society statement. Eur Respir J. 2017;50(3):1602221. https://doi.org/10.1183/13993003.02221-2016.
Maat RC, Roksund OD, Halvorsen T, Skadberg BT, Olofsson J, Ellingsen TA, et al. Audiovisual assessment of exercise-induced laryngeal obstruction: reliability and validity of observations. Eur Arch Otorhinolaryngol. 2009;266(12):1929–36. https://doi.org/10.1007/s00405-009-1030-8.
Maat RC, Hilland M, Røksund OD, Halvorsen T, Olofsson J, Aarstad HJ, et al. Exercise-induced laryngeal obstruction: natural history and effect of surgical treatment. Eur Arch Otorhinolaryngol. 2011;268(10):1485–92. https://doi.org/10.1007/s00405-011-1656-1.
Sandnes A, Andersen T, Clemm HH, Hilland M, Vollsaeter M, Heimdal JH, et al. Exercise-induced laryngeal obstruction in athletes treated with inspiratory muscle training. BMJ Open Sport Exerc Med. 2019;5(1):e000436. https://doi.org/10.1136/bmjsem-2018-000436.
Kolnes LJ, Vollsaeter M, Roksund OD, Stensrud T. Physiotherapy improves symptoms of exercise-induced laryngeal obstruction in young elite athletes: a case series. BMJ Open Sport Exerc Med. 2019;5(1):e000487. https://doi.org/10.1136/bmjsem-2018-000487.
Siewers K, Backer V, Walsted ES. A systematic review of surgical treatment for supraglottic exercise-induced laryngeal obstruction. Laryngoscope Investig Otolaryngol. 2019;4(2):227–33. https://doi.org/10.1002/lio2.257.
Famokunwa B, Sandhu G, Hull JH. Surgical intervention for exercise-induced laryngeal obstruction: A UK perspective. Laryngoscope. 2020;130(11):E667–73. https://doi.org/10.1002/lary.28497.
Olin JT, Deardorff EH, Fan EM, Johnston KL, Keever VL, Moore CM, et al. Therapeutic laryngoscopy during exercise: a novel non-surgical therapy for refractory EILO. Pediatr Pulmonol. 2017;52(6):813–9. https://doi.org/10.1002/ppul.23634.
Shaffer M, Litts JK, Nauman E, Haines J. Speech-language pathology as a primary treatment for exercise-induced laryngeal obstruction. Immunol Allergy Clin North Am. 2018;38(2):293–302. https://doi.org/10.1016/j.iac.2018.01.003.
Olin JT, Westhoff CE. Exercise-induced laryngeal obstruction and performance psychology: using the mind as a diagnostic and therapeutic target. Immunol Allergy Clin North Am. 2018;38(2):303–15. https://doi.org/10.1016/j.iac.2018.01.004.
Clemm HH, Olin JT, McIntosh C, Schwellnus M, Sewry N, Hull JH, et al. Exercise-induced laryngeal obstruction (EILO) in athletes: a narrative review by a subgroup of the IOC Consensus on ‘acute respiratory illness in the athlete’. Br J Sports Med. 2022:bjsports-2021–104704. doi: https://doi.org/10.1136/bjsports-2021-104704.
Irewall T, Bäcklund C, Nordang L, Ryding M, Stenfors N. High prevalence of exercise-induced laryngeal obstruction in a cohort of elite cross-country skiers. Med Sci Sports Exerc. 2021;53(6):1134–41. https://doi.org/10.1249/MSS.0000000000002581.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. https://doi.org/10.1183/09031936.05.00034805.
Argyros GJRJ, Hurwitz KM, Eliasson AH, Phillips YY. Eucapnic voluntary hyperventilation as a bronchoprovocation technique: development of a standarized dosing schedule in asthmatics. Chest. 1996;109(6):1520–4.
Anderson SDAG, Magnussen H, Holzer K. Provocation by eucapnic voluntary hyperpnoea to identify exercise induced bronchoconstriction. Br J Sports Med. 2001;35(5):344–7.
European Community Respiratory Health Survey IISC. The European Community Respiratory Health Survey II. Eur Respir J. 2002;20(5):1071–9. https://doi.org/10.1183/09031936.02.00046802.
Røksund OD, Maat RC, Heimdal JH, Olofsson J, Skadberg BT, Halvorsen T. Exercise induced dyspnea in the young. Larynx as the bottleneck of the airways. Respir Med. 2009;103(12):1911–8.
Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–8.
Kolnes Lj PhDPT, Stensrud TP. Exercise-induced laryngeal obstruction in athletes: Contributory factors and treatment implications. Physiother Theory Pract. 2019;35(12):1170–81. https://doi.org/10.1080/09593985.2018.1474306.
Gallena SK, Johnson AT, Vossoughi J. Short-term intensive therapy and outcomes for athletes with paradoxical vocal fold motion disorder. Am J Speech Lang Pathol. 2019;28(1):83–95. https://doi.org/10.1044/2018_AJSLP-17-0223.
Hanks CD, Parsons J, Benninger C, Kaeding C, Best TM, Phillips G, et al. Etiology of dyspnea in elite and recreational athletes. Phys Sportsmed. 2012;40(2):28–33. https://doi.org/10.3810/psm.2012.05.1962.
Castelli WA, Ramirez PC, Nasjleti CE. Linear growth study of the pharyngeal cavity. J Dent Res. 1973;52(6):1245–8. https://doi.org/10.1177/00220345730520061401.
Wysocki J, Kielska E, Orszulak P, Reymond J. Measurements of pre- and postpubertal human larynx: a cadaver study. Surg Radiol Anat. 2008;30(3):191–9. https://doi.org/10.1007/s00276-008-0307-8.
Gallena SJ, Solomon NP, Johnson AT, Vossoughi J, Tian W. The effect of exercise on respiratory resistance in athletes with and without paradoxical vocal fold motion disorder. Am J Speech Lang Pathol. 2015;24(3):470–9. https://doi.org/10.1044/2015_AJSLP-14-0110.
Solomon NP, Pham A, Gallena S, Johnson AT, Vossoughi J, Faroqi-Shah Y. Resting respiratory resistance in female teenage athletes with and without exercise-induced laryngeal obstruction. J Voice. 2022;36(5):734.e1-.e6.
Walsted ES, Hull JH, Sverrild A, Porsbjerg C, Backer V. Bronchial provocation testing does not detect exercise-induced laryngeal obstruction. J Asthma. 2017;54(1):77–83. https://doi.org/10.1080/02770903.2016.1195843.
Walsted ES, Faisal A, Jolley CJ, Swanton LL, Pavitt MJ, Luo YM, et al. Increased respiratory neural drive and work of breathing in exercise-induced laryngeal obstruction. J Appl Physiol (1985). 2018;124(2):356–63. https://doi.org/10.1152/japplphysiol.00691.2017.
Irewall T. Prevalence and incidence of and risk factors for asthma and exercise-induced laryngeal obstruction in elite endurance athletes. Umeå University; 2021.
Larsson K, Ohlsen P, Larsson L, Malmberg P, Rydstrom PO, Ulriksen H. High prevalence of asthma in cross country skiers. BMJ. 1993;307(6915):1326–9. https://doi.org/10.1136/bmj.307.6915.1326.
Carlsen KH, Anderson SD, Bjermer L, Bonini S, Brusasco V, Canonica W, et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis: part I of the report from the Joint Task Force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy. 2008;63(4):387–403. https://doi.org/10.1111/j.1398-9995.2008.01662.x.
Jansrud Hammer I, Halvorsen T, Vollsæter M, Hilland M, Heimdal JH, Røksund OD, et al. Conundrums in the breathless athlete; exercise-induced laryngeal obstruction or asthma? Scand J Med Sci Sports. 2022;32(6):1041–9. https://doi.org/10.1111/sms.14137.
Norlander K, Johansson H, Emtner M, Janson C, Nordvall L, Nordang L. Differences in laryngeal movements during exercise in healthy and dyspnoeic adolescents. International Journal of Pediatric Otorhinolaryngology. 2020;129:109765. https://doi.org/10.1016/j.ijporl.2019.109765.
Walsted ES, Hull JH, Hvedstrup J, Maat RC, Backer V. Validity and reliability of grade scoring in the diagnosis of exercise-induced laryngeal obstruction. ERJ Open Res. 2017;3(3). https://doi.org/10.1183/23120541.00070-2017.
Norlander K, Christensen PM, Maat RC, Halvorsen T, Heimdal JH, Morén S, et al. Comparison between two assessment methods for exercise-induced laryngeal obstructions. Eur Arch Otorhinolaryngol. 2016;273(2):425–30. https://doi.org/10.1007/s00405-015-3758-7.
Acknowledgements
We thank Kristina Nordebo for data cleaning and Anna Lindam for statistical support.
Funding
Open access funding provided by Umea University. This study was supported by funds from the School of Sport Sciences, Umeå University; the Visare Norr Fund, Northern County Councils’ Federation; and the Research & Development Unit, Region Jämtland Härjedalen. The funding sources have no role in the design, analysis and interpretation of the study.
Author information
Authors and Affiliations
Contributions
Conceptualization: NS, MR, Data collection: TI, CB, MR, NS Data curation and analysis: TI, Writing – original draft: TI, NS. Writing – review and editing: TI, CB, EN, MR, NS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All subjects gave written informed consent. The study was approved by the Regional Ethical Review Board at Umeå University, Umeå (Dnr 2015–43-31 M).
Consent for publication
Participants gave consent for publication as part of the written informed consent.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Irewall, T., Bäcklund, C., Naumburg, E. et al. A longitudinal follow-up of continuous laryngoscopy during exercise test scores in athletes irrespective of laryngeal obstruction, respiratory symptoms, and intervention. BMC Sports Sci Med Rehabil 15, 87 (2023). https://doi.org/10.1186/s13102-023-00681-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13102-023-00681-9