Abstract
Background
Blood volume (BV) is a critical factor for physical endurance in chronic stroke patients, while hypervolemia can worsen hypertension in these patients. This prospective study assessed whether rehabilitation combined with protein supplementation immediately after each exercise for 3 weeks would improve plasma volume (PV) and BV as well as physical endurance without worsening hypertension.
Methods
Ambulatory patients with chronic cerebrovascular disease who received a 3-week rehabilitation program with high protein jelly (intervention group [PG]; n = 8; 10-g protein) or protein-free jelly (control group [CG]; n = 8) consumed within 30 min after each exercise. PV and BV were assessed while measuring the 6-min walking distance (6MWD), peak oxygen consumption (VO2peak), strength of knee extension, and resting blood pressure before and after the intervention. Two-way ANOVA was used to determine whether there was an interaction of time × group. The difference between before and after intervention or between the groups by post-hoc test (Tukey’s test) at the level of P < 0.05.
Results
The 6MWD increased only in the PG (P = 0.001; an interaction of Group and Time, P = 0.037). PV and BV increased only in the PG (P < 0.05). VO2peak and strength of knee extension in the paralysed limb increased in both groups (P < 0.05). The resting blood pressure did not worsen after the intervention.
Conclusions
In chronic post-stroke patients, 3-week rehabilitation combined with protein intake immediately after exercise increased 6MWD simultaneously with increased PV and BV, but it did not increase resting blood pressure. The present regimen is acceptable and effective for ambulatory patients with chronic cerebrovascular disease.
Name of the registry Examining effects of protein supplementation on functional improvement during rehabilitation intervention in chronic stroke patients
Trial registration number UMIN000028009; date of registration: 30/06/2017. This study was registered prospectively.
Similar content being viewed by others
Introduction
The number of patients with cerebrovascular disease is approximately 1.1 million in Japan [1]. Approximately 850,000 patients have received long-term nursing care through government support [2, 3]. Physical function, especially independent walking, plays a key role in improving the quality of life of patients (e.g. continuing to live at home) and reducing medical costs under the Medicare system [4].
Physical function is generally associated with physical endurance and muscle strength of the extremities. In a clinical rehabilitation setting, the 6-min walking distance (6MWD) has been used to assess physical endurance and muscle strength of the extremities also relates it [5]. Patients are reported to walk at a self-selected speed as fast as possible within 6 min. The main outcome was 6MWD; intra-class correlation coefficients < 10% in patients with low physical endurance, and peak oxygen consumption rate (VO2peak) between 14 and 17 mL/kg/min [6]. The 6MWD was applied to patients with chronic cerebrovascular disease [6]. A 30-min treadmill walking intervention performed 3 days per week for 4 weeks increased 6MWD by 11%, with increased muscle strength on both the non-paretic and paretic sides [7]. High-intensity treadmill training that included 3 sessions per week, with each session between 50 and 60 min, increased 6MWD by 20% after 3 months and increased step length of paretic side by 2 cm [8]. These previous studies suggest that improvement in 6MWD can be achieved through rehabilitation for a duration of at least 4 weeks and is associated with increased muscle strength of the non-paretic and/or paretic lower limb. However, it remains unknown whether other mechanisms, such as physical endurance, can result in improved 6MWD in patients with chronic cerebrovascular disease.
VO2peak is one of the indexes for physical endurance, which must be ≥ 14 mL/kg/min to maintain a walking velocity of ≥ 0.9 m/s [9], and is correlated with 6MWD [10,11,12]. In contrast, VO2peak was positively correlated with plasma volume (PV) and/or blood volume (BV) [13]. Therefore, rehabilitation may improve 6MWD simultaneously with increased PV or BV, even in patients with chronic cerebrovascular disease. Recently, Araki et al. (in submission) showed that VO2peak in patients with chronic cerebrovascular disease was 14.5–30.0 mL/kg/min and tended to correlate with BV. PV and/or BV may be critical factors for physical endurance, even in the population.
However, PV or BV expansion is associated with increased blood pressure in elderly and/or hypertensive subjects [14], which are risk factors for cerebrovascular diseases [15]. As previously shown, in healthy elderly participants, a regimen of combined exercise and ingestion of both carbohydrates and proteins immediately after each exercise improved PV and BV [16]. Volume expansion was observed in hypertensive patients after an 8-week aerobic exercise intervention combined with intake of glucose and protein; however, the regimen did not cause increased blood pressure [14]. The previous results suggest that the regimen may increase PV and/or BV without increasing blood pressure, even in hypertensive subjects.
This study aimed to test the hypothesis that a combination of rehabilitation therapy plus protein intake immediately after each exercise session increases PV, BV, and 6MWD in patients with chronic cerebrovascular disease. Furthermore, we assessed that even if BV was increased in patients after the intervention, blood pressure would not increase, as previously shown in elderly hypertensive individuals [14].
Methods
Participants
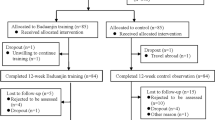
This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the ethics review board of Wakayama Medical University (#2049). All participants signed a consent form voluntarily before participating in the study. The right to withdraw consent at any time without stating the reason was guaranteed without any individual disadvantage for subsequent medical care. The inclusion criteria in the present study were patients who had chronic cerebrovascular disease for > 6 months, walked independently regardless of using foot orthosis or walking aids, and had normal cognitive function (Hasegawa dementia rating scale-revised score > 20 [17]). All patients could communicate effectively and understood the study objectives. Even patients who used β-blockers were included if the resting heart rate was not low and increased over the target heart rate during exercise. We recruited 23 patients who were admitted to the Nachi-Katsuura Balneologic Town Hospital between June 2017 and December 2018. Two patients refused to participate. Six patients withdrew consent after the pre-intervention measurements. Each patient provided signed consent before participating in the study. Exclusion criteria were patients with brainstem stroke, bone and joint problems, heart or respiratory diseases that could worsen with walking, liver, or kidney diseases, poorly controlled diabetes, nephropathy stage 3 or higher, and/or drug allergy. Four patients had mild anaemia and one patient had moderate anaemia [18]; however, all 5 patients were included in the study. None of the patients participated in any rehabilitation therapy in the 2-month period before the study, except for one patient in the intervention group (PG; protein was ingested just after each rehabilitation) who underwent 20-min of rehabilitation therapy at an outpatient clinic at our hospital twice a week for 9 years. Fifteen patients were allocated to the PG (n = 8) and control group (CG; n = 7). One patient in the intervention group discontinued the intervention due to lack of motivation. One patient of each group performed both trials after a wash-out period of 5 or 6 months. Finally, eight patients in each group completed the trial. (Fig. 1).
CONSORT Flow Diagram. We recruited 23 patients, who were admitted to the Nachi-Katsuura Balneologic Town Hospital between June 2017 and December 2018. Two patients refused their participation. Six patients withdrew their consent after the pre-intervention measurements. Fifteen patients were allocated into the intervention (PG; n = 8) and the control groups (CG; n = 7). One patient in the intervention group discontinued intervention due to lost motivation. Each one of both groups performed both trials, after a wash-out period of 5 or 6 months. Finally, 8 patients in each group completed this trial
Assuming that the main effects of d (=|μ1 − μ2|/SD) (d, effect size; μ1 and μ2, two independent means) for PV were 1.75 (44.6 (6.7) and 47.5 (6.3) mL/kg, mean (SD), before and after the intervention in hypertensive elderly participants who ingested protein just after each exercise [14]), the correlation before and after intervention was > 0.96, the statistical power (1 − β) was > 0.95 at an α of 0.05, and the required sample size was 7 for the paired t-test (G*Power 3.1, Kiel, Germany).
Experimental design and intervention
This was a preliminary prospective intervention, and a double-blind study involving one patient from each group who performed both trials, after a wash-out period of 5 or 6 months. Every two patients were admitted to the hospital. Participants were randomly assigned to two groups using the permuted block method (block size 2): protein ingestion immediately after each exercise session (PG; the intervention group; n = 8) or ingestion of protein-free jelly (CG; n = 8) by a study evaluator who worked at another hospital. The doctor who was working in the hospital (C.S.) enrolled participants but was not informed which groups they belonged to. Patients in the PG received a protein-rich jelly (RehaTime Jelly; Clinico Co.; Tokyo; 100 kcal, 10 g protein, 15 g carbohydrate, 0 g fat, and 4 mg sodium in 120 g); meanwhile, those in the CG were protein-free (Minute-Made Qoo; Coca-Cola Japan Co.; Tokyo; 90 kcal, 0 g protein, 18 g carbohydrate, 0 g fat, and 4–16 mg sodium in 125 g). Each participant was handed in a cup with jelly by a nurse who did not participate in the study. Patients ingested one pack of jelly within 30 min of the exercise session. The idea of protein ingestion immediately after each exercise session was based on the results of a previous study [16]. None of the patients, care providers, and investigators were informed about which jelly was administered until the end of the study. The two patients who received both supplements did not notice any difference in flavour between the two supplements.
Rehabilitation program
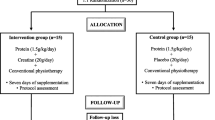
All patients in the study underwent 11 sessions of rehabilitation program per week for 3 weeks. Each rehabilitation session lasted for one hour. The 1st session was conducted in the morning and the other in the afternoon on 5 weekdays per week, and only one session was conducted on Saturday morning per week. The total number of training days was 18 and 33, respectively. The rehabilitation program consisted of standing/sitting exercises, aerobic exercise with a hand cycling ergometer, gait training on a treadmill, and stepping-stairs training (Fig. 2). We attempted to establish an exercise intensity with different parameters, such as the velocity of a treadmill walk, workload of an ergometer, and number of repetitions for standing/sitting; therefore, Borg’s scale was 11–13 (Fig. 3).
Study protocol. Each patient received in-hospital 11-h rehabilitation program per week for 3 weeks. Each rehabilitation session lasted 1 h, the 1st one was conducted in the morning and another in the afternoon on 5 weekdays, and one session was done on Saturday morning. Treadmill; walking on treadmill (WEBG3300, Reha-Tread G, Senoh Corporation, Chiba, Japan) at a speed adjusted according to each patient. Stepping the stairs; climbing up and down the stairs in the hospital. Hand cycling ergometer; cycling upper body ergometer (WBK284740H, Rehab Trainer 881E, Monark Exercise AB, Sverige, Sweden) with their hands at wattage adjusted according to each patient. Standing up repetition; standing up from a chair, sitting down and repeating. The Borg’s scale of each patient’s program was recorded. Patients of the intervention group received protein-rich jelly while those of the control group received protein-free jelly within 30 min after the rehabilitation session. Physical fitness tests were performed in the evening of the day before starting the rehabilitation program and the last day of 3-week program. Blood samples were drawn and plasma volume was measured during 7:30–8:00 am before breakfast on the same days as above. *, blood pressure and pulse rate were checked. CPX, Cardiopulmonary exercise testing.
The Borg’s scale during intervention in the Black dot, PG; White dot, CG. To determine differences in the Borg’s scale during intervention between groups, unpaired t-test was applied at each time point during day 1 to 18. Because of lacks of data at day 1, 8, 12, 17 and 18, numbers of data at these days are shown in parentheses. Differences were considered statistically significant at P < 0.05. Statistical evaluations were performed using SPSS (version 24.0; IBM, Chicago, IL). Values are represented as mean (SD) unless otherwise stated. All participants performed rehabilitation for 33 sessions
Meals
The patients’ diet was provided during admission with the following composition (values per day): 23.66–35.06 kcal/kg of body mass (BM), carbohydrates 3.23–4.89 g/kg of BM, proteins 1.11–1.38 g/kg of BM, lipids 0.72–1.01 g/kg of BM and salt 0.11–0.14 g/kg of BM.
Protocol for measurements
On the 1st day of admission, each patient reported to the laboratory at 7.30 am normally hydrated but in a fasting state for at least 9 h before the measurement. After emptying their bladder, the patient was weighed and asked to take the supine position throughout the measurement in a temperature-controlled room at 25–28 °C. A 21-gauge butterfly needle was inserted into the antecubital vein for blood sampling and dye injection to assess the PV and baseline values of blood constituents. After a 30-min rest, 14 mL and 12 mL of blood samples were obtained for baseline values before and after the intervention, respectively, followed by injection of sterilised Evans blue dye [19]. Then, 3 mL of blood sample was collected 10 min after the injection. Whole blood samples were used to determine haematocrit (Hct) and haemoglobin concentration ([Hb]). Plasma and serum were separated after centrifugation and were used to determine the PV, plasma hormone concentration, and general biochemistry.
A cardiopulmonary exercise test was performed following PV measurement using a graded exercise method [5, 20]. Before this test, blood pressure was measured once in a seated position after a 60-min rest following PV measurement. Then, the patient sat and rested on an ergometer while all the equipment was attached. After 2-min of rest measurements, the patient started pedalling at 50 revolutions/min without loading. Then, the workload was increased by 5 W every 3 min until it reached 15 W and above this intensity, 5 W every one min for female patients. Meanwhile, for male patients, the workload was increased by 10 W every two min until it reached 30 W, and above this intensity, 15 W every one min. This procedure was performed until male and female patients could not maintain the rhythm due to exhaustion. We measured VO2 every 15 s (MetaMax 3B, Cortex, Leipzig, Germany) and monitored it continuously at rest and during the graded exercise to record heart rate every minute. The same procedure was performed one or two days before discharge at the same time of the day.
Physical fitness tests were performed in the evening of the day before and on the last day of the 3-week program. We measured the 6MWD for each patient and 10-m walking speed at optimum and maximum (10MWS) level to calculate the walking velocity. The patients were allowed to use usual foot orthoses or walking aids if required. The strength of the quadriceps femoris muscle was measured in a seated position. A skilled physiotherapist scored the Fugl-Meyer Assessment (FMA) to estimate the severity of the disability of each patient before the onset of the intervention. The same measurements were performed one day before discharge at the same time of the day.
Measurements
The primary outcome was change in PV before and after the intervention. Also, we assessed changes in BV, plasma concentrations of stress-related hormones or catecholamines, VO2peak and 6MWD at optimal speed, 10MWS at optimal and maximal efforts, and strength of knee extension and resting blood pressure.
PV
Absorbance (620 and 740 nm, SH-1000 Lab; Corona Electric, Hitachinaka, Japan) of plasma at baseline and 10 min after dye injection was measured. PV was calculated as follows [19, 21]:
where EB is the volume (mL) of the dye injected; D is the dilution of the standard dissolved in distilled water (× 1000 in the present study); ODstand is the absorbance of the standard at 620 nm; ODBlank and OD10min are the absorbances of the plasma sampled before the dye injection and at 10 min after injection, respectively; and 1.03 is a factor introduced to correct for slow dye uptake by the tissues [19]. The background absorbance due to turbidity was corrected using a regression equation for the relationship between 620 and 740 nm in the present study [5].
BV
BV was calculated using the following equation: BV = PV/(1 − Hct/ 100) [13]. These values were divided by body weight.
VO2peak and peak HR
VO2peak were determined by averaging the three largest consecutive values at the end of the exercise. Peak HR was applied as the value at the last minute of the VO2peak test.
6MWD
Participants performed a 6-min walking test based on a standardised protocol [22]. The walking course was 30 m in length, and patients were instructed to walk back and forth on the course as fast as possible for 6 min. Each instruction and encouragement was verbalised simultaneously based on the standardised protocol.
10MWS
10-m walking speed at optimum and maximum speed were tested in triplicate. The peak values at each speed were obtained to calculate walking velocity. The course of the 10MWS consisted of a 2 m warm-up, 10 m used for the speed measurement, and 2 m for slowing down to a stop, a total of 14 m. Patients were instructed to walk at a self-selected comfortable pace at the optimum speed and as fast as possible at maximum speed, while measuring 10-m time with a stopwatch [23, 24] to two decimal places.
Knee extension strength
The strength of the quadriceps femoris muscle was measured with a hand-held dynamometer (Power track II MMT commander, MF-104AA, Nihon Medix, Chiba, Japan) in duplicate, and the peak value was obtained. Power track II MMT commander is a battery-operated hand-held device, which measures the peak force in Newtons (N) up to approximately 600 N (125 lb). The patients sat on a chair with their hips and knees flexed at 90° [25,26,27]. An examiner placed the dynamometer 20 cm distal to their knee joint. Patients were instructed to extend the knee joint as much as possible (Fig. 4).
FMA
A skilled physiotherapist scored this test to confirm that the patients’ impairments were comparable between the groups. The Fugl-Meyer Assessment (FMA) is a stroke-specific, performance-based impairment index [28]. This index consists of assessments of motor function of the upper and lower limbs, sensory function, balance, ranges of motion, and joint pain during physical activity of each sub-score was 66, 34, 24, 14, 44, and 44, respectively; the total score was 226.
Cardiovascular responses
Systolic (SBP) and diastolic blood pressures (DBP) were measured using an automatic equipment (UA-787, A&D, Tokyo) once patients were in a seated position after a 60-min rest after completion of PV measurement before and after the intervention period. We reported the blood pressure before the cardiopulmonary exercise test as resting blood pressure (shown in Table 5), while SBP and DBP were also measured during a rehabilitation session before and immediately after each exercise. Blood pressure was remeasured only when the previous measurement was erroneous. The coefficient of variation of the measurement was 4.6–7.1% of SBP, 3.4–6.3% of DBP, and 2.1–3.8% of pulse rate in four healthy or hypertensive adults in our department. The pulse pressure was calculated as SBP–DBP. The mean arterial pressure was calculated as DBP + (SBP − DBP)/3. The rate-pressure product was calculated as the heart rate × SBP.
Blood analyses
A 1-mL aliquot of 14 mL or 12 mL was used to determine Hct (in %, microcentrifuge; the coefficient of variation was 0.69%) and [Hb] (in g/dL, sodium lauryl sulphate haemoglobin method; Sigma Chemical, St. Louis, MO; < 1.5%) in triplicate. Six of 14 mL (before the intervention) or 12 mL (after the intervention), which were obtained at baseline, and 3 mL of blood obtained 10 min after injection were transferred into a heparin-treated tube and centrifuged at 4 °C at 3000 rpm for 30 min. Approximately 1 mL of separated plasmas were used to determine the PV. The remaining plasma at baseline was used to determine cortisol, catecholamine, and aldosterone concentrations. Seven of 14 mL and five of 12 mL of the blood samples at baseline before and after the intervention, respectively, were transferred into a serum separator tube and centrifuged at 24–25 °C for 20 min. The remaining serum obtained before the intervention was used to assess general biochemistry (TBA-120FR, TOSHIBA, Tochigi, Japan) and HbA1c (HLC-723G11, Tosoh, Tokyo). Analysis of cortisol, catecholamine, and aldosterone concentrations in plasma was performed at an external testing laboratory (SRL, Hachioji, Japan). The plasma and serum obtained after PV measurement were stored at − 80 °C until the next assay. Plasma adrenaline and noradrenaline concentrations were measured using high-performance liquid chromatography. The respective intra-assay coefficients of variation (CV) for adrenaline, noradrenaline, and dopamine were 5.04%, 3.59%, and 5.71% at the levels of 255, 248, and 257 pg/mL, respectively. Plasma cortisol concentration was measured using electro chemiluminescence immunoassay, with 4.31% of the intra-assay CV at 11.58 μg/dL. Plasma aldosterone concentration was measured by chemiluminescent enzyme Immunoassay, with the intra-assay CV 3.47% at 66.22 pg/mL.
Statistical analysis
The χ2 test was used to determine the sex and type of disease bias, haemorrhage, or infarction. A normal distribution was assumed. Two-way ANOVA (within factor, time; between factor, group) was used to determine whether there was an interaction of time × group. The difference between before and after intervention or between the groups (PG and CG) was determined by post-hoc test (Tukey). To determine differences in the Borg’s scale during intervention between groups, an unpaired t-test was applied at each time point from day 1 to 18, since several data points were lacking, specifically on days 1, 8, 12, 17, and 18. Differences were considered statistically significant at P < 0.05. All statistical evaluations were performed using SPSS (version 24.0; IBM, Chicago, IL). Values are represented as the mean (SD) unless otherwise stated.
Results
Some examples of potential adverse events in our study include the following: allergy to jelly we provided or to Evans blue dye, injury or onset of joint pain due to rehabilitation, and stroke recurrence; however, no adverse events were observed in the present study. Only one patient with PG dropped out due to a lack of motivation. Table 1 shows the characteristics of patients in the PG and CG. There were no significant differences at baseline in these parameters between the two groups (all P > 0.196). The ratio of female to male patients (PG, 5/3; CG, 3/5; P = 0.317) and the types of disease (haemorrhage/infarction) were not different between the groups: 3/4 in both groups (P = 1.000). The latency between the time of onset and the study was 42 (33) and 66 (38) months in the PG and CG, respectively (P = 0.197). The data of patients at the time of enrollment were not significantly different. The authors failed to obtain FMA data only in one patient in the PG, since the patient refused to provide it, and there was no difference between the groups (P = 0.828).
The average Borg’s scale score during intervention was approximately 12 in each group, with no significant differences between the groups (P > 0.783; Fig. 3).
The calorie intake from meals per day, except for the jelly intake just after exercise, were 26.0 (5.9) kcal/kg BM/day and 26.0 (6.5) kcal/kg BM/day of total energy, 1.12 (0.27) g/kg BM and 1.09 (0.23) g/kg BM of proteins, 3.73 (0.84) g/kg BM and 3.69 (0.89) g/kg BM of carbohydrates, 0.70 (0.21) g/kg BM and 0.74 (0.22) g/kg BM of lipids, and 0.11 (0.02) g/kg BM and 0.10 (0.03) g/kg BM of salt in the PG and CG, respectively, with no significant differences between the two groups (all, P > 0.687). (Table 2).
As shown in Table 3, there were no significant medication biases. Table 4 shows the [Hb], Ht, blood sugar, and serum profiles before the intervention; there were no significant differences between the two groups (all P > 0.170).
As shown in Fig. 5, PV and BV increased after the intervention in the PG (P = 0.005 and P = 0.002 of time effect of Two-way ANOVA, respectively), while it remained unchanged in the CG. The 95% confidence intervals for PV were [1.5, 11.6] and [− 1.4, 5.3] and for BV were [2.8, 17.2] and [− 1.3, 7.7] in the PG and CG, respectively.
There were no significant differences in baseline physical fitness variables. Body weight decreased after the intervention in both groups (P = 0.004). Four of the patients in the PG, five in the CG, and one of the patients who performed both trials, took antihypertensive medication. Blood pressure in patients did not increase after the intervention, and no additional antihypertensive medications were used during their admission. Diastolic pressure tended to decrease only in the CG (P = 0.059). The mean arterial pressure and rate-pressure product decreased only in the CG (P = 0.049 and 0.047, respectively). The 6MWD increased only in the PG, with a significant interaction between group and time (P = 0.037). Confidential intervals of 95% were [26, 83] and [− 12.7, 43.2] in the PG and CG, respectively. VO2peak and the strength of knee extension in the paralysed limb increased in both groups (P = 0.035 and P = 0.001, respectively). The 10MWS at optimal speed increased significantly in the PG (P = 0.019); however, there was no significant interaction between group and time (P = 0.597). The 10MWS at maximal speed remained unchanged in both groups (P = 0.116; Table 5).
Table 6 shows that concentrations of cortisol and catecholamines remained unchanged before and after the intervention in both groups (all P > 0.134). However, plasma concentration of aldosterone decreased only in the PG (P = 0.028).
Discussion
This is the first study in patients with chronic cerebrovascular disease that examined whether PV and BV increased with rehabilitation, along with improved physical endurance. The regimen of protein intake immediately after each exercise session in the 3-week rehabilitation program was used in the present study. As a result, PV and BV increased only in the PG, with an interaction of group and time, along with increased 6MWD and decreased plasma aldosterone concentration after the intervention. However, VO2peak and strength of knee extension muscles in the paralysed limb increased regardless of protein intake. Although BV increased in the PG, blood pressure did not increase in patients with no change in plasma concentrations of stress-related hormones or catecholamines.
The 6MWD, as an index of physical endurance, improved only in the PG and was related to increased BV and PV. As previously reported, a 3-week period was sufficient to improve respiratory function and blood flow to the active muscles in the present patients [7, 8, 29,30,31]. Femoral arterial blood flow in the paralysed limb increased after a 2-week training intervention in post-stroke patients [31]. The increase in BV contributes to increased blood flow due to an increase in venous return to the heart [32]. The expansion of BV in the PG was induced by the following regimen: 3-week rehabilitation plus protein ingestion just after each exercise bout, as previously shown in healthy elderly [16] and hypertensive patients [14]. Thus, the present study shows that increased BV and/or PV may result in improved the 6MWD in patients with chronic cerebrovascular disease.
The reduction in plasma aldosterone concentration only in the PG after the intervention may have been due to increased PV and BV. As aldosterone release typically increases after exercise at moderate or high intensities [33, 34], this hormone increased after each rehabilitation session. In the present study, stimulation by aldosterone may have contributed to fluid volume expansion, while a decrease in the baseline level could not be explained. Fluid expansion observed only in the PG seemed to decrease it after the intervention.
Previous studies have described an increase in the rate of liver albumin synthesis following a single bout of intensive exercise [35]. Insulin suppresses proteolysis in the liver and enhances albumin synthesis [36]. Generally, one g of albumin draws 18 [37] or 14–15 mL of water [38]. The increase in PV is associated with an increased gradient of colloid osmotic pressure [38]. Protein synthesis after exercise in groups with a high (1.8 g/kg/day) and low (0.7 g/kg/day) protein intake the day before the exercise session was comparable in young subjects [39]. Protein intake (0.18 g/kg) 2 h after a bout of high-intensity interval exercise did not result in a change in PV 24 h after the exercise; however, ingestion of the same amount of protein just after the exercise resulted in increased PV [40]. Thus, protein intake immediately after exercise would be more beneficial for expanding PV. In the present study, PV increased only in the PG, while [Albumin]s remained unchanged after the intervention, suggesting that albumin content in plasma may increase only in the PG. The reason why PV and BV significantly increased only in the PG is explained by the greater gradient of the colloid osmotic pressure, and the timing of the ingestion worked effectively.
VO2peak and muscle strength in the paralysed limb improved during the 3-week rehabilitation program in both groups. As patients in the present study were not physically active before the study, they had a high potential for trainability, including one patient who continued rehabilitation therapy twice a week in the PG. The discrepancy between improvements in VO2peak and 6MWD in both groups remains unclear. However, it involves factors other than PV and/or BV.
Since anaemia sometimes disturbs the progress of increasing workload and/or physical activity during rehabilitation [41,42,43], it should be corrected immediately. In this study, two patients in the PG and three patients in the CG were classified as anaemic before the intervention based on the World Health Organization criteria [18]: < 13 and 12 g/dL for men and women, respectively. These patients remained anaemic after the intervention. However, PV also increased in all patients in the PG and some patients in the CG, while [Hb] levels decreased in the patients. [Hb] and haematocrit remained unchanged after the present intervention, and haemoglobin content also increased in the PG. Previous studies showed that a 2-week endurance training in healthy subjects increased plasma concentrations of erythropoietin, a haematopoietic factor secreted by tubular stromal cells in the kidney, which is known to promote erythrocyte production [44]. Protein intake immediately after exercise can also increase haemoglobin content, possibly through enhanced production of haematopoietic hormones.
The patients in the current study performed several resistance exercises, such as squats and calf raise exercises, as well as walking. Improvement in muscle strength in the paralysed limb was achieved after just 3 weeks. Three weeks is generally too short to induce muscle hypertrophy in healthy persons [45, 46]; indeed, at least 6 weeks are needed [47]. Therefore, there was no detectable limb hypertrophy after the 3-week period in the non-paralysed limb. Improvements in neuromuscular recruitment in the paralysed muscles, learning of motor timing and sequencing for locomotion, and coordination of agonist/antagonist muscles are factors that may be responsible for increased muscle strength [48,49,50]. Indeed, approximately 20% of the corticospinal tract fibres do not cross at the medulla and descend on the ipsilateral side as part of the anterior corticospinal tract [51]. Rehabilitation may increase the activity of the tract.
We could not find any reasonable explanation for improvements in 10MWS at the optimal speed only in the PG. Habitual gait speed correlates with VO2peak [9] and maximum gait speed correlates with paretic knee extension muscle strength [52]. In the present study, 6MWD improved only in the PG, suggesting an enhancement of physical endurance. The improvements in 10MWS in the PG could not be explained by muscle strength in the lower limbs; however, improved range of motion of the ankle may partially influence improvement of 6MWD.
An expansion of BV is believed to be a risk factor for increased blood pressure. However, there were no cases in which blood pressure at rest was elevated after the intervention, and no medications were altered during the admission in the present study. Baseline plasma concentrations of catecholamine and stress-related hormones remained unchanged after the present intervention, suggesting that sympathetic nervous activity would not be enhanced, even after BV expansion. This is due to the fact that blood pressure regulation via baroreflexes can be improved by enhancing the reflex responses [14].
Limitations
This was a single-site study, and the sample size was limited. However, the preliminary results of the present study may help support planning of the next multiple-centre study.
Conclusions
Rehabilitation therapy for 3 weeks combined with protein intake immediately after each exercise session increased PV and BV in patients with chronic cerebrovascular disease. Physical endurance, especially 6MWD, improved with protein intake. Furthermore, blood pressure did not deteriorate in the present study, even when BV increased after the intervention.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. The data except for some of indirect identifiers are also available in the Additional file 1: Data.
Abbreviations
- BV:
-
Blood volume
- CG:
-
Control group
- CV:
-
Coefficients of variation
- FMA:
-
The Fugl-Meyer Assessment
- [Hb]:
-
Haemoglobin concentration
- PG:
-
Intervention group (ingested protein immediately after each exercise session)
- PV:
-
Plasma volume
- VO2peak :
-
Peak oxygen consumption rate
- 6MWD:
-
6-min walk distance at optimal speed
- 10MWS:
-
10-M waking speed at optimal and maximal effort
References
Ministry of Health LaW, JAPAN, Health Statistics Office. Number of patients with major diseases, overview of patient survey. 2017. https://www.mhlw.go.jp/toukei/saikin/hw/kanja/14/dl/05.pdf. Accessed 3 Dec 2019.
Ministry of Health LaW, JAPAN, Health and welfare bureau for the elderly. report on the situation of services in long-term care insurance. 2017. https://www.mhlw.go.jp/topics/kaigo/osirase/jigyo/17/dl/h29_gaiyou.pdf. Accessed 3 Dec 2019.
Ministry of Health LaW, JAPAN, Director-general for statistics and information policy, household statistics office. Overview of comprehensive survey of living conditions. 2016. https://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa16/dl/05.pdf. Accessed 3 Dec 2019.
Lonergan ET, Krevans JR. A national agenda for research on aging. N Engl J Med. 1991;324(25):1825–8.
Okazaki K, Kamijo Y, Takeno Y, Okumoto T, Masuki S, Nose H. Effects of exercise training on thermoregulatory responses and blood volume in older men. J Appl Physiol. 2002;93(5):1630–7.
Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, Lee AL, Camillo CA, Troosters T, Spruit MA, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1447–78.
Yang YR, Wang RY, Lin KH, Chu MY, Chan RC. Task-oriented progressive resistance strength training improves muscle strength and functional performance in individuals with stroke. Clin Rehabil. 2006;20(10):860–70.
Broderick P, Horgan F, Blake C, Ehrensberger M, Simpson D, Monaghan K. Mirror therapy and treadmill training for patients with chronic stroke: a pilot randomized controlled trial. Top Stroke Rehabil. 2019;26(3):163–72.
Fiser WM, Hays NP, Rogers SC, Kajkenova O, Williams AE, Evans CM, Evans WJ. Energetics of walking in elderly people: factors related to gait speed. J Gerontol A Biol Sci Med Sci. 2010;65(12):1332–7.
Araújo CO, Makdisse MR, Peres PA, Tebexreni AS, Ramos LR, Matsushita AM, Carvalho AC. Different patterns for the 6-minute walk test as a test to measure exercise ability in elderly with and without clinically evident cardiopathy. Arq Bras Cardiol. 2006;86(3):198–205.
Tang A, Sibley KM, Bayley MT, McIlroy WE, Brooks D. Do functional walk tests reflect cardiorespiratory fitness in sub-acute stroke? J Neuroeng Rehabil. 2006;3:23.
Patterson SL, Forrester LW, Rodgers MM, Ryan AS, Ivey FM, Sorkin JD, Macko RF. Determinants of walking function after stroke: differences by deficit severity. Arch Phys Med Rehabil. 2007;88(1):115–9.
Hagberg JM, Goldberg AP, Lakatta L, O’Connor FC, Becker LC, Lakatta EG, Fleg JL. Expanded blood volumes contribute to the increased cardiovascular performance of endurance-trained older men. J Appl Physiol. 1998;85(2):484–9.
Kataoka Y, Kamijo YI, Ogawa Y, Sumiyoshi E, Nakae M, Ikegawa S, Manabe K, Morikawa M, Nagata M, Takasugi S, et al. Effects of hypervolemia by protein and glucose supplementation during aerobic training on thermal and arterial pressure regulations in hypertensive older men. J Appl Physiol. 2016;121(4):1021–31.
Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American stroke association. Stroke. 2021;52(7):e364–467.
Okazaki K, Ichinose T, Mitono H, Chen M, Masuki S, Endoh H, Hayase H, Doi T, Nose H. Impact of protein and carbohydrate supplementation on plasma volume expansion and thermoregulatory adaptation by aerobic training in older men. J Appl Physiol. 2009;107(3):725–33.
Kato S. Creation of The Revised Hasegawa’s Dementia Scale (HDS-R). Jpn J Geriatr Psychiatry. 1991;2:1339–47.
WHO: Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. In. Edited by System VaMNI. Geneva, World Health Organization, 2011 (WHO/NMH/NHD/MNM/11.1); 2011. https://apps.who.int/iris/handle/10665/85839. Accessed 2 July 2021.
Greenleaf JE, Hinghofer-Szalkay H: Plasma volume methodology: evans blue, hemoglobin-hematocrit, and mass density transformations. 1985. NASA-TM-86834
Okazaki K, Hayase H, Ichinose T, Mitono H, Doi T, Nose H. Protein and carbohydrate supplementation after exercise increases plasma volume and albumin content in older and young men. J Appl Physiol. 2009;107(3):770–9.
Crooke AC, Morris CJ. The determination of plasma volume by the Evans blue method. J Physiol. 1942;101(2):217–23.
Laboratories ACoPSfCPF: ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002; 166(1):111–7.
Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP, Duncan PW. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. 2010;90(2):196–208.
Flansbjer UB, Holmbäck AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37(2):75–82.
Baschung Pfister P, de Bruin ED, Sterkele I, Maurer B, de Bie RA, Knols RH. Manual muscle testing and hand-held dynamometry in people with inflammatory myopathy: an intra- and interrater reliability and validity study. PLoS ONE. 2018;13(3):e0194531.
Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996;76(3):248–59.
Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. 1997;78(1):26–32.
Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient, 1: a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31.
Byun SD, Jung TD, Kim CH, Lee YS. Effects of the sliding rehabilitation machine on balance and gait in chronic stroke patients—a controlled clinical trial. Clin Rehabil. 2011;25(5):408–15.
Bang DH, Son YL. Effect of intensive aerobic exercise on respiratory capacity and walking ability with chronic stroke patients: a randomized controlled pilot trial. J Phys Ther Sci. 2016;28(8):2381–4.
Billinger SA, Gajewski BJ, Guo LX, Kluding PM. Single limb exercise induces femoral artery remodeling and improves blood flow in the hemiparetic leg poststroke. Stroke. 2009;40(9):3086–90.
González-Alonso J, Calbet JA, Nielsen B. Muscle blood flow is reduced with dehydration during prolonged exercise in humans. J Physiol. 1998;513:895–905.
Luger A, Deuster PA, Debolt JE, Loriaux DL, Chrousos GP. Acute exercise stimulates the renin-angiotensin-aldosterone axis: adaptive changes in runners. Horm Res. 1988;30(1):5–9.
Lieu FK, Lin CY, Wang PS, Jian CY, Yeh YH, Chen YA, Wang KL, Lin YC, Chang LL, Wang GJ, et al. Effect of swimming on the production of aldosterone in rats. PLoS ONE. 2014;9(10):e87080.
Nagashima K, Cline GW, Mack GW, Shulman GI, Nadel ER. Intense exercise stimulates albumin synthesis in the upright posture. J Appl Physiol. 2000;88(1):41–6.
Ahlman B, Charlton M, Fu A, Berg C, O’Brien P, Nair KS. Insulin’s effect on synthesis rates of liver proteins: a swine model comparing various precursors of protein synthesis. Diabetes. 2001;50(5):947–54.
Scatchard G, Batchelder AC, Brown A. Chemical, clinical, and immunological studies on the products of human plasma fractionation, VI: the osmotic pressure of plasma and of serum albumin. J Clin Invest. 1944;23(4):458–64.
Convertino VA. Blood volume response to physical activity and inactivity. Am J Med Sci. 2007;334(1):72–9.
Bowtell JL, Leese GP, Smith K, Watt PW, Nevill A, Rooyackers O, Wagenmakers AJ, Rennie MJ. Modulation of whole body protein metabolism, during and after exercise, by variation of dietary protein. J Appl Physiol. 1998;85(5):1744–52.
Okazaki K, Takeda R, Suzuki A, Naghavi N, Imai D, Miyagawa T. The effects of increased plasma volume on autonomic control of the circulation after a bout of intense-intermittent exercise in men. The FASEB J. 2013;27:943.915.
Gledhill N. The influence of altered blood volume and oxygen transport capacity on aerobic performance. Exercise Sport Sci Rev. 1985;13(1):75–94.
Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, Noorani M, Leung JM, Fisher DM, Murray WR, Toy P, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217–21.
Tsai KZ, Lai SW, Hsieh CJ, Lin CS, Lin YP, Tsai SC, Chung PS, Lin YK, Lin TC, Ho CL, et al. Association between mild anemia and physical fitness in a military male cohort: The CHIEF study. Sci Rep. 2019;9(1):11165. https://doi.org/10.1038/s41598-019-47625-3.
Montero D, Breenfeldt-Andersen A, Oberholzer L, Haider T, Goetze JP, Meinild-Lundby AK, Lundby C. Erythropoiesis with endurance training: dynamics and mechanisms. Am J Physiol Regul Integr Comp Physiol. 2017;312(6):R894–902.
Lee J, Lee R, Stone AJ. Combined aerobic and resistance training for peak oxygen uptake, muscle strength, and hypertrophy after coronary artery disease: a systematic review and meta-analysis. J Cardiovasc Transl Res. 2019. https://doi.org/10.1007/s12265-019-09922-0.
Schoenfeld BJ, Ogborn DI, Krieger JW. Effect of repetition duration during resistance training on muscle hypertrophy: a systematic review and meta-analysis. Sports Med. 2015;45(4):577–85.
Schuenke MD, Herman JR, Gliders RM, Hagerman FC, Hikida RS, Rana SR, Ragg KE, Staron RS. Early-phase muscular adaptations in response to slow-speed versus traditional resistance-training regimens. Eur J Appl Physiol. 2012;112(10):3585–95.
Shepherd RB. Exercise and training to optimize functional motor performance in stroke: driving neural reorganization? Neural Plast. 2001;8(1–2):121–9.
Rutherford OM, Jones DA. The role of learning and coordination in strength training. Eur J Appl Physiol Occup Physiol. 1986;55(1):100–5.
Carolan B, Cafarelli E. Adaptations in coactivation after isometric resistance training. J Appl Physiol (1985). 1992;73(3):911–7.
Guyton ACHJ. Textbook of medical physiology. Amsterdam: Elsevier; 1999.
Suzuki K, Nakamura R, Yamada Y, Handa T. Determinants of maximum walking speed in hemiparetic stroke patients. Tohoku J Exp Med. 1990;162(4):337–44.
Duncan PW, Goldstein LB, Horner RD, Landsman PB, Samsa GP, Matchar DB. Similar motor recovery of upper and lower extremities after stroke. Stroke. 1994;25(6):1181–8.
Acknowledgements
We thank Ms. Hiroko Nishi for supplying the jellies to each patient.
Funding
This study was supported by grants from the Nachi-Katsuura Research Foundation (L1221) to Fumihiro Tajima (F.T.) and Grants-in-Aid for Scientific Research (21K11364 by Yoshi-ichiro Kamijo). The funding body did not play any roles in the design of the study and collection, analysis, and interpretation of data and writing this manuscript.
Author information
Authors and Affiliations
Contributions
CS, YK, and FT designed this study; CS and SA collected and analysed the data; Yuta. Sakurai (Y.S.1), Yuki. Sakata (Y.S.2), AI, KM, and IY supported this study. CS and YK drafted the manuscript, and all other authors critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Wakayama Medical University (protocol code #2049; date of approval: 27th June, 2017). All participants signed a consent form voluntarily before participating in the study. The right to withdraw consent at any time without stating the reason was guaranteed without any individual disadvantage for subsequent medical care.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Individual data of Figures 3 and 5 and all Tables are shown in the additional file, while data of "Sex" and "Age" are masked.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sato, C., Kamijo, Yi., Sakurai, Y. et al. Three-week exercise and protein intake immediately after exercise increases the 6-min walking distance with simultaneously improved plasma volume in patients with chronic cerebrovascular disease: a preliminary prospective study. BMC Sports Sci Med Rehabil 14, 38 (2022). https://doi.org/10.1186/s13102-022-00429-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13102-022-00429-x