Abstract
Background
Metabolic syndrome (MetS) is associated with an increased risk for cardiovascular events and high socioeconomic costs. Despite lifestyle interventions focusing on exercise are effective strategies to improve parameters of the above aspects, many programs fail to show sustained effects in the long-term.
Methods
At visit 2 (V2) 129 company employees with diagnosed MetS, who previously participated in a 6-month telemonitoring-supported exercise intervention, were randomized into three subgroups for a 6-month maintenance treatment phase. A wearable activity device was provided to subgroup A and B to assess and to track physical activity. Further subgroup A attended personal consultations with individual instructions for exercise activities. Subgroup C received neither technical nor personal support. 6 months later at visit (V3), changes in exercise capacity, MetS severity, work ability, health-related quality of life and anxiety and depression were compared between the subgroups with an analysis of variance with repeated measurements.
Results
The total physical activity (in MET*h/week) declined between visit 2 and visit 3 (subgroup A: V2: 48.0 ± 33.6, V3: 37.1 ± 23.0; subgroup B: V2: 52.6 ± 35.7, V3: 43.8 ± 40.7, subgroup C: V2: 51.5 ± 29.7, V3: 36.9 ± 22.8, for all p = 0.00) with no between-subgroup differences over time (p = 0.68). In all three subgroups the initial improvements in relative exercise capacity and MetS severity were maintained. Work ability declined significantly in subgroup C (V2: 40.3 ± 5.0, V3: 39.1 ± 5.7; p < 0.05), but remained stable in the other subgroups with no between-subgroup differences over time (p = 0.38). Health-related quality of life and anxiety and depression severity also showed no significant differences over time.
Conclusions
Despite the maintenance of physical activity could not be achieved, most of the health related outcomes remained stable and above baseline value, with no difference regarding the support strategy during the maintenance treatment phase.
Trial registration The study was completed as a cooperation project between the Volkswagen AG and the Hannover Medical School (ClinicalTrials.gov Identifier: NCT02029131).
Similar content being viewed by others
Background
The metabolic syndrome (MetS) is a cluster of interrelating metabolic risk factors consisting of abdominal obesity with elevated waist circumference, insulin resistance, dyslipidemia and elevated blood pressure [1]. It is estimated that the metabolic syndrome is present in around 20–25% of the world’s adult population and is considered the driving force for a new cardiovascular disease epidemic [2].
People with MetS have a fivefold greater risk of developing type 2 diabetes, which consequently leads to significant higher health care costs and socioeconomic expenses [3]. In 2019, 10% of global health expenditure (760 billion US dollar) is spent upon diabetes. If diabetes prevalence continues to rise as anticipated, 700 million people worldwide will live with type 2 diabetes by 2045 [4]. Further unfavorable outcomes like cardiovascular events, an approximately twice-increased risk of incident cardiovascular mortality and certain cancer types are also linked with the MetS [5, 6].
Reducing risk factors of the MetS on the other hand is associated with reduced health care, pharmacy and short-term disability expenses as well as increased productivity outcomes [7].
The evidence characterizing the benefits of physical activity (PA) in general [8] and in people with MetS specifically is extensive [9]. Yet, in 2016, globally more than 25% of all adults and 36.8% of the population in high-income Western countries show insufficient PA [10]. Lifestyle interventions, including supervised exercise programs, are key components in the treatment of patients with MetS [11, 12], but many exercise programs fail to show sustained gains in the long-term. Previous studies have shown that lifestyle intervention programs based on both nutritional counselling and physical exercise are effective in patients with MetS, but likewise effects diminish over time [12, 13]. In a systematic review by Lynch et al. including twenty-one clinical studies the results imply that wearable devices may enhance PA interventions by using self-monitoring as a meaningful technique for changing PA behavior [14]. Yet, there appears to be no consensus about what contributes to successful physical activity maintenance [15].
Recently, we were able to show that a 6-month telemonitoring-guided and wearable activity device-supported exercise intervention with the goal of 150 min of moderate activity per week not only reduced MetS severity, but also has a significant potential to reduce disease risk while also improving mental health, work ability, and productivity-related outcomes in company employees with diagnosed MetS [16].
To assess the long-term effects of this intervention and the potential sustainability of obtained results, participants of the exercise group were assigned to a 6-month maintenance treatment phase in which the originally applied type of intervention was maintained or reduced regarding to personal or technical support.
We hypothesized that the maintenance of personal and technical support, as initially applied is superior for maintaining physical activity-induced benefits compared to lower-intense support strategies.
Material and methods
Study design and participants
The study on which this work is based is divided into two parts, which are presented below as the initial intervention phase and the maintenance treatment phase. Initially, all participants took part in a prospective, randomized, parallel-group and single-blind (assessor blind) controlled trial examining the effects of telemonitoring-supported exercise training on MetS severity and work ability in company employees.
The study was done as a collaborative project between Volkswagen AG and Hannover Medical School and took place at the main Volkswagen factory in Wolfsburg, Germany. After the participants were recruited via a series of information events on the factory premises, the baseline visit started in October 2017, initializing the 6-month exercise intervention. The total of recruited participants (n = 314) consisted of 52% office workers and 36% manual workers (12% unclassified). 21% of the participants worked shifts, 72% were non-shift workers (7% unclassified) [16]. After 6 months at visit 2, participants of the exercise group (19 women, 110 men, age: 48.6 ± 7.6 yrs., BMI: 31.6 ± 4.7 kg/m2) with diagnosed MetS were randomized 1:3 into three subgroups (subgroup A, B and C). Visit 2, starting in April 2018, was the starting point for the maintenance treatment phase which lasted a further 6 months, with each subgroup undergoing a different support strategy during this phase (Fig. 1). The evaluation of the maintenance treatment phase took part at visit 3. The overall course of the study is shown in Fig. 1.
Subgroup A (n = 43, 5 women, 38 men) continuously used wearable devices (Forerunner 35, Garmin, Garching, Germany) with which activities and daily step count were recorded. As during the first intervention, the smartphone application Rebirth Active was used again. Additionally, subgroup A attended personal feedback consultations with individual instructions for exercise activities at month 2 and 4. An exercise scientist performed the individual counseling in order to keep everyday activity on a high level and to adjust the training schedule to participants’ current situation.
Subgroup B (n = 43, 8 women, 35 men) also received technical support from wearable devices, general information via the Rebirth Active application but no further personal consultations.
Subgroup C (n = 43, 6 women, 37 men) received neither technical support, for they had to hand in their wearable device after completing visit 2, nor individualized support by exercise scientists.
The randomization was computer-based generated by an external collaborator. Study nurses and physicians examining participants were blinded for the randomization sequence.
With the start of the initial intervention the exercise group was equipped with a wearable activity device worn on the wrist of the non-dominant hand. After a short introduction, participants were asked to wear the activity device throughout the intervention period. Daily step count was recorded continuously. In addition, sporting activities (eg, cycling, cardio training, walking outdoors, and walking indoors) were asked to be tracked. Activity time, distance, and heart rate ( assessed by an optical heart-rate sensor were recorded. Data was transferred to a server at the Hannover Medical School and was traced and assessed by the exercise scientists.
Right from the start, the aim was to keep everyday activity as high as possible and to meet the WHO target of at least 150 min of moderate activity per week. In order to support the participants in an active lifestyle, but also to individually adapt the training, monthly personal consultations were held on the factory premises. Additionally, general and individual recommendation on health issues, information about the study background and study target dates were communicated via the smartphone application Rebirth Active (d.velop AG, Gescher, Germany).
After the baseline visit as well as after visit 2 and 3, the participants in the exercise group or each subgroup received personal nutritional advice upon a 7-day food diary and general information on healthy nutrition based on the general recommendations of the German Society for Nutrition (https://www.dge.de/index.php?id=52).
According to baseline visit, we distributed questionnaires to collect data on health-related quality of life (short form 36 [SF-36]) [17], on anxiety and depression severity (HADS-D) [18] and on work ability (work ability index [WAI]) [19] at visit 2 and 3. To calculate the total and exercise-related PA metabolic equivalents of task as (MET)-hours per week the Freiburger Physical Activity questionnaire was applied [20]. The HADS-D is composed of fourteen items that cluster into two subscales for anxiety and depression. The score range lies within 0 to 21 points, whereby a higher score indicates more severe symptomatic. The SF-36 consists of a mental- and physical component score. For both sum scores, the score range lies within 0 to 100, with 100 points representing the maximum quality of life (QoL). The WAI questionnaire contains questions concerning work, work ability and health with one total score ranging from 7 (minimum) to 49 (maximum) points.
Further examinations including a general medical examination by a physician (including electrocardiogram, case history, and physical examination) and measurement of bodyweight, waist circumference and height were executed in a standardized way. Body-mass-index (BMI) was calculated with the formula bodyweight (kg) ÷ height (m2).
Fat-free mass and fat mass as markers of body composition were estimated by segmental multi-frequency bioimpedance analysis (InBody720; Biospace, South Korea). Blood pressure was measured after 5 min of rest with a suitable automatic blood pressure cuff (Critikon, Dinamap, USA) as the mean value of two successive recordings.
All examinations were identical on each visit. The description of the measured values from blood samples as well as the calculation of the MetS severity score (MetS-z-score) is described in detail previously [16].
Based on maximum workload and heart rate during the exercise ergometry at visit 2 (Schiller 911 BPplus, Schiller, Feldkirchen, Germany), participants were advised to continue their exercise training established during the initial intervention phase at an individually recommended heart rate. The given task throughout the maintenance treatment phase was to keep PA on a high level, targeting the WHO guidelines of minimum 150 min of moderate activity per week [21]. To evaluate participants’ adherence to these guidelines, the duration of recorded PA with wearable devices were used. The outcomes of the Freiburger Physical Activity questionnaire in terms of everyday, exercise and total PA were used as an aid to evaluate and compare self-reported PA over time and between the subgroups.
Statistical analysis
The analysis for all outcomes was done in the intention-to-treat population, including all patients, who were randomized at visit 2. Missing values were replaced by the Last Observation Carried Forward method. All values are shown as mean ± standard deviation. The normal distribution was tested with a Shapiro–Wilk-test. To test distribution a Chi-squared test was used. Differences between the three groups at visit 2 were calculated with a one-way ANOVA for parametric data or a Kruskal–Wallis-Test for non-parametric data, respectively. Differences within a group over time were calculated with a paired t-Test for parametric data respectively a Wilcoxon test for non-parametric data. The interaction between time and group and differences across three time points within the whole intervention group were tested with an analysis of variance with repeated measurements with η2 as effect size. Post hoc tests were corrected according to Bonferroni. Data were analyzed using SPSS for Windows (SPSS 26, IBM, Armonk, NY, USA). We considered p < 0.05 as statistically significant.
Results
In 113 out of 129 subjects, data for visit 3 was available. Across all three subgroups, there have been 16 recorded dropouts during the maintenance treatment phase. Four subjects were not randomized for they have withdrawn their participation after visit 2 without giving reasons (Fig. 2).
At visit 2, the three subgroups did not differ regarding sex, age, body composition or the five MetS components (Table 1).
As one of the major outcomes, relative exercise capacity (W/kgBW) increased significantly for the whole intervention group between baseline and visit 2 and remained above baseline at visit 3 (Fig. 3a). After 6-month maintenance treatment phase, relative exercise capacity remained stable for subgroup A (wearables and personal consultation) and C (no technical or personal support) and increased significantly for subgroup B (Fig. 3a).
a–d Relative exercise capacity (a), total PA (b), WAItotal (c) and MetS-z-score (d) in the whole intervention group across baseline, visit 2 and visit 3, and in the subgroups A, B and C across the maintenance phase from visit 2 to visit 3. Relative exercise capacity (p < 0.001, η2 = 0.46), total PA (p < 0.001, η2 = 0.24) and WAItotal (p < 0.001, η2 = 0.18) increased significantly across the three time points, respectively decreased for MetS-z-score (p < 0.001, η2 = 0.23). WAI work ability index, PA physical activity
Similar findings for the parameters total physical activity (MET*hours/week) (Fig. 3b), work ability (WAI) (Fig. 3c) and MetS-z-score (Fig. 3d) were observed: The results at visit 3 remained above baseline value for the whole intervention group.
Among the MetS components, the systolic blood pressure in subgroup A (wearables and personal consultations) and waist circumference in subgroup C (no technical or personal support) was worsened at visit 3. No significant differences in other MetS components or in the MetS-z-score itself were observed (Table 2).
Though the work ability (WAI) decreased in subgroup C (no technical or personal support) (Table 3) at visit 3, all subgroups showed a good work ability (37–43 points) after maintenance treatment phase.
Everyday and total PA (MET*hours/week) showed a decline in all three subgroups with no differences between subgroups (Table 3).
Mental component sum score of health-related QoL (baseline: 49.2 ± 9.5, visit 2: 53.3 ± 7.3, visit 3: 53.2 ± 7.3; p < 0.001, η2 = 0.19) increased significantly in the whole intervention across all three time points. Mental component sum score (baseline vs visit 2: p < 0.001, baseline vs visit 3: p < 0.001, visit 2 vs visit 3: p = n.s.) increased between baseline and visit 2 and remained above baseline at visit 3. The physical and mental component sum scores of health-related QoL (SF-36) showed no significant changes over time (Table 3).
Anxiety severity (baseline: 5.37 ± 3.22, visit 2: 3.89 ± 2.98, visit 3: 4.13 ± 3.71; p < 0.001, η2 = 0.15) and depression severity (baseline: 4.72 ± 3.32, visit 2: 2.80 ± 2.73, visit 3: 3.09 ± 3.10; p < 0.001, η2 = 0.26) decreased significantly for the whole intervention group across all three time points. Anxiety severity (baseline vs visit 2: p < 0.001, baseline vs visit 3: p < 0.001, visit 2 vs visit 3: p = n.s.) and depression severity (baseline vs visit 2: p < 0.001, baseline vs visit 3: p < 0.001, visit 2 vs visit 3; p = 0.471) decreased between baseline and visit 2 and remained above baseline at visit 3. The mean values of both subscales of the HADS-D questionnaire were within the normal range (0–7 points) for all subgroups (Table 3) with no differences between the subgroups.
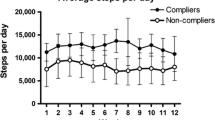
Participants of subgroup A and B recorded their performed exercise unites with wearable devices, the results are displayed as total exercise units and exercise units per week (Table 4). Both data declined significantly for both subgroups, yet with no difference between the subgroups. The same applies for the daily step count, which was consulted to analyze participants’ everyday activity (Table 4). In average, participants in both subgroups failed to reach the given study task of 150 min of PA per week during the initial intervention phase and the following maintenance treatment phase. However, both subgroups were able to maintain over 80% of their previous performance, comparing maintenance treatment phase with the initial intervention phase (A: 83% ± 45%, B: 82% ± 39%, p = 0.964). There was no significant difference between both subgroups.
There is no data available by wearable devices for subgroup C (no technical or personal support) at visit 3, because participants of this subgroup had to give back their wearable device at visit 2. It is to mention though, that 20 participants of subgroup C (57% out of 35 participants completed V3) have bought an own wearable device during the maintenance treatment phase. After excluding these participants from subgroup C in the final evaluation, the interaction between time and group showed no difference in contrast to the entire subgroup C regarding the analysis of variance with repeated measurements in all observed parameters.
Discussion
After an initial 6-month telemonitoring-supported exercise intervention in company employees with diagnosed MetS, we divided the participants 1:3 into three subgroups with different support strategies in the maintenance treatment phase. The leading question was to investigate, whether continuous personal support in combination with wearable devices is the superior strategy to help adults maintain their PA. After a maintenance treatment phase of 6 months, we evaluated participant’s compliance to the given task, the anthropometric and cardio-metabolic outcomes as well as work ability, depression severity and health-related QoL by assessments and questionnaires.
It is known that maintaining PA on a high level is a challenging task. Only few interventions report long-term results (≥ 1 year) by showing a positive effect on PA levels, when considering that participation in PA intervention tend to decrease the longer the intervention lasts [22]. Presumed reasons for decreased participation may be the loss of interest, motivation, enjoyment, time and/or perceived benefit [23]. 12 months after the initial intervention has started, our overall dropout rate was low (Fig. 2). This outcome leads to the conclusion that an intervention phase of 6 months with intense personal and technical support is sustainable for at least half a year to keep participant’s motivation on a high level to participate in a program that promotes a healthy lifestyle.
The given task throughout the maintenance treatment phase was to maintain PA on a high level, targeting the recommendation of WHO of 150 min of moderate activity per week. In our investigation we took this task to assess the compliance to the program by taking the amount of recorded PA per week (in minutes) as well as the total amount of weeks, where this task was successfully fulfilled (Table 4) into account. PA per week (in minutes) was found stable in subgroup A (wearables and personal consultation), yet declined in subgroup B (wearables only). Both subgroups maintained over 80% of the minutes of activity they had previously achieved during the initial intervention phase with intense personal and technical support. This finding emphasizes the effectiveness of our maintenance treatment phase and surpasses the findings presented in a recent review by Madigan et al., where 60% to 80% of PA behavior was maintained [15]. However, we did not find a significant difference between subgroup A (wearables and personal consultation) and B (wearables only).
The initial intervention improved participant’s relative exercise capacity (W/kgBW) significantly, which is an important component to reduce the relative mortality risk irrespectively of a stated disease [24]. This health-promoting outcome was found stable for subgroup A (wearables and personal consultation) and C (no technical or personal support) after maintenance treatment phase, which suggests that PA interventions do have the ability to promote longer-term health effects.
PA and physical exercise on a regular basis have a positive influence on mental health and QoL both in people who are healthy [25] and people with diseases [26], considering that there is a correlation between MetS and depression [27] as well as MetS and anxiety [28]. In 2016, major depression was the fifth leading cause of years lived with disability [29], which clarifies the importance of addressing mental health in health care programs especially in workplace settings. Our findings at visit 3 for the mental score of QoL (SF-36) as well as depression severity and anxiety severity (HADS-D) remained stable throughout maintenance treatment phase and above baseline values, which allows the conclusion that a telemonitoring-based exercise intervention can significantly improve components of mental health in the long-term.
Previously, we showed that regular PA improves not only physiological and mental parameters but also work ability [16]. To maintain work ability in identified risk groups, WAI is an effective instrument for initiating and controlling occupational prevention measures. Study participants of all subgroups showed good mean work ability by score (WAI) 12 months after the initial intervention has started. A cross-sectional study with teachers confirms our findings by indicating a relationship between work ability and PA in which female teachers with excellent or good WAI had significantly higher levels of total weekly PA [30]. The results at visit 3 suggest (Table 3) that maintaining good work ability is detached from a specific support strategy including further personal consultation after a successfully implemented supervision for 6 months [30].
Our study has strengths and limitations. As mentioned before, some participants of subgroup C (no technical or personal support) have used an own wearable device, for they had to hand in their given study device at visit 2.
A further limitation is that we cannot rule out the possibility of non-tracked activities. This could limit the number of activities recorded and thereby underestimate overall activities for certain individuals. Non-tracked activities could not be included in the evaluation.
Conclusions
Our data strengthen previous results that a lifestyle intervention supporting PA could be a reasonable resource to reduce MetS related health risk factors, health-care costs, may support productivity related outcomes [7, 31] and help adults to maintain their PA [15]. After analyzing different support strategies in the maintenance treatment phase, our results suggest comparable outcomes for all three subgroups in terms of exercise capacity (W/kgBW), components of MetS, as well as health related QoL, anxiety and depression severity and work ability. Most of the health-associated outcomes could be found stable after 6-month maintenance treatment phase, which shows the effectiveness of our intervention. The combination of both personal and technical support during the initial intervention phase and the initial intervention’s duration seem to be the key to long-term success in employees with MetS that lasts for at least another 6 months.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AG:
-
Working group (German „Arbeitsgemeinschaft“)
- BW:
-
Body weight
- BMI:
-
Body mass index
- fig.:
-
Figure
- HADS-D:
-
Hospital anxiety and depression scale questionnaire
- HDL:
-
High density lipoprotein
- IDF:
-
International diabetes federation
- MET:
-
Metabolic equivalents of task
- MetS:
-
Metabolic syndrome
- MetS-z-score:
-
Metabolic syndrome severity score
- PA:
-
Physical activity
- QoL:
-
Quality of life
- SF-36:
-
Short form 36 questionnaire
- V2:
-
Visit 2
- V3:
-
Visit 3
- WAI:
-
Work ability index
- WHO:
-
World health organization
References
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific statement: executive summary. Circulation. 2005;112(17):e285.
International Diabetes Federation. IDF consensus worldwide definition of the metabolic syndrome. 2020. https://www.idf.org/component/attachments/attachments.html?id=705&task=download.
Schultz AB, Edington DW. Analysis of the association between metabolic syndrome and disease in a workplace population over time. Value Health. 2010;13(2):258–64.
IDF DIABETES ATLAS 9th edn. https://www.diabetesatlas.org/en/. Accessed 15 June 2021.
Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CDA, Bouter LM, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn study. Circulation. 2005;112(5):666–73.
O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1–12.
Schultz AB, Edington DW. The association between changes in metabolic syndrome and changes in cost in a workplace population. J Occup Environ Med. 2009;51(7):771–9.
Warburton DER, Bredin SSD. Health benefits of physical activity. Curr Opin Cardiol. 2017;32(5):541–56. https://doi.org/10.1097/HCO.0000000000000437.
Myers J, Kokkinos P, Nyelin E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients. 2019;11(7):1652. https://doi.org/10.3390/nu11071652.
Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health. 2018;6(10):E1077–86.
Ostman C, Smart NA, Morcos D, Duller A, Ridley W, Jewiss D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:110.
Joseph MS, Konerman MA, Zhang M, Wei B, Brinza E, Walden P, et al. Long-term outcomes following completion of a structured nutrition and exercise lifestyle intervention program for patients with metabolic syndrome. Diabet Metab Syndr Obes. 2018;11:753–9.
Gremeaux V, Drigny J, Nigam A, Juneau M, Guilbeault V, Latour E, et al. Long-term lifestyle intervention with optimized high-intensity interval training improves body composition, cardiometabolic risk, and exercise parameters in patients with abdominal obesity. Am J Phys Med Rehabil. 2012;91(11):941–50.
Lynch C, Bird S, Lythgo N, Selva-Raj I. Changing the physical activity behavior of adults with fitness trackers: a systematic review and meta-analysis. Am J Health Promot. 2020;34(4):418–30.
Madigan CD, Fong M, Howick J, Kettle V, Rouse P, Hamilton L, et al. Effectiveness of interventions to maintain physical activity behavior (device-measured): Systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2021;66:e13304.
Haufe S, Kerling A, Protte G, Bayerle P, Stenner HT, Rolff S, et al. Telemonitoring-supported exercise training, metabolic syndrome severity, and work ability in company employees: a randomised controlled trial. Lancet Public Health. 2019;4(7):E343–52.
Bullinger M. Assessment of health related quality of life with the SF-36 Health Survey. Rehabilitation. 1996;35(XVII):6–XXIX.
Snaith RP. The hospital anxiety and depression scale. J Psychosom Res. 2002;52(5):401.
van den Berg TIJ, Elders LAM, de Zwart BCH, Burdorf A. The effects of work-related and individual factors on the Work Ability Index: a systematic review. Occup Environ Med. 2009;66(4):211–20.
Frey I, Berg A, Grathwohl D, Keul J. Freiburger Fragebogen zur körperlichen Aktivität-Entwicklung. Prüfung und Anwendung Soz Präventivmed. 1999;44(2):55–64.
WHO guidelines on physical activity and sedentary behavior. https://www.who.int/publications/i/item/9789240015128. 2021.
van der Bij AK, Laurant MGH, Wensing M. Effectiveness of physical activity interventions for older adults—a review. Am J Prev Med. 2002;22(2):120–33.
Rhodes RE, Martin AD, Taunton JE, Rhodes EC, Donnelly M, Elliot J. Factors associated with exercise adherence among older adults—an individual perspective. Sports Med. 1999;28(6):397–411.
Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. Can Med Assoc J. 2006;174(6):801–9.
Fernandez-Montero A, Moreno-Galarraga L, Sánchez-Villegas A, Lahortiga-Ramos F, Ruiz-Canela M, Martínez-González MA, et al. Dimensions of leisure-time physical activity and risk of depression in the “Seguimiento Universidad de Navarra” (SUN) prospective cohort. BMC Psychiatry. 2020;20(1):66.
Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev. 2015;9(3):366–78.
Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, et al. Bidirectional association between depression and metabolic syndrome A systematic review and meta-analysis of epidemiological studies. Diabet Care. 2012;35(5):1171–80.
Tang F, Wang G, Lian Y. Association between anxiety and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Psychoneuroendocrinology. 2017;77:112–21.
Vos T, Abajobir AA, Abbafati C, Abbas KM, Abate KH, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–59.
Grabara M, Nawrocka A, Powerska-Didkowska A. The relationship between physical activity and work ability—a cross-sectional study of teachers. Int J Occup Med Environ Health. 2018;31(1):1–9.
Burton W, Chen C, Schultz A, Edington D. The prevalence of metabolic syndrome in an employed population and the impact on health and productivity. J Occup Environ Med. 2008;50(10):1139–48.
Acknowledgements
This study was supported and funded by grants from Audi BKK health insurance and the German Research Foundation through the Cluster of Excellence “REBIRTH”. We acknowledge the support of the volunteers. We thank the nurses and technicians at Volkswagen occupational healthcare centers for collecting the anthropometric, body composition, and metabolic data, and collecting and analyzing blood samples.
Funding
Open Access funding enabled and organized by Projekt DEAL. The funder of this study had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit for publication. All coauthors had access to the raw data if needed. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
SH, DB, CT, AH and UT planned and designed the study. RE, LN, and DL recruited participants. AK, PB, HB, SR, TS and AH collected the data. AK, PB, HB, SR, TS, CT, CB, AH and MK processed the exercise test, anthropometric, body composition, and metabolic data. MK and PB were responsible for the statistical analyses. PB and AK wrote the first draft of the manuscript. MdZ and UT contributed to the discussion and reviewed/edited the manuscript. All authors participated in data interpretation, commented on subsequent drafts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of the Hannover Medical School approved the study, and all participants gave written informed consent prior to study entry. The study was performed in accordance with the Declaration of Helsinki and current guidelines of good clinical practice. The study was completed as a cooperation project between the Volkswagen AG and the Hannover Medical School (ClinicalTrials.gov Identifier: NCT02029131).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bayerle, P., Kerling, A., Kück, M. et al. Effectiveness of wearable devices as a support strategy for maintaining physical activity after a structured exercise intervention for employees with metabolic syndrome: a randomized controlled trial. BMC Sports Sci Med Rehabil 14, 24 (2022). https://doi.org/10.1186/s13102-022-00409-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13102-022-00409-1