Abstract
The targeted depletion of potential gut pathogens is often challenging because of their intrinsic ability to thrive in harsh gut environments. Earlier, we showed that Campylobacter jejuni (C. jejuni) exclusively uses the Type-VI Secretion System (T6SS) to target its prey such as Escherichia coli (E. coli), and phenotypic differences between T6SS-negative and T6SS-positive C. jejuni isolates toward bile salt sensitivity. However, it remains unclear how the target-driven T6SS functionality prevails in a polymicrobial gut environment. Here, we investigated the fate of microbial competition in an altered gut environment via bacterial T6SS using a T6SS-negative and -positive C. jejuni or its isogenic mutant of the hemolysin-coregulated protein (hcp). We showed that in the presence of bile salt and prey bacteria (E. coli), T6SS-positive C. jejuni experiences enhanced intracellular stress leading to cell death. Intracellular tracking of fluorophore-conjugated bile salts confirmed that T6SS-mediated bile salt influx into C. jejuni can enhance intracellular oxidative stress, affecting C. jejuni viability. We further investigated whether the T6SS activity in the presence of prey (E. coli) perturbs the in vivo colonization of C. jejuni. Using chickens as primary hosts of C. jejuni and non-pathogenic E. coli as prey, we showed a marked reduction of C. jejuni load in chickens cecum when bile salt solution was administered orally. Analysis of local antibody responses and pro-inflammatory gene expression showed a reduced risk of tissue damage, indicating that T6SS activity in the complex gut environment can be exploited as a possible measure to clear the persistent colonization of C. jejuni in chickens.
Highlights
1. Differential response of C. jejuniT6SS under bile salt stress.
2. T6SS function was probed by deleting its essential functional subunit, Hcp.
3. The presence of prey bacteria facilitates enhanced bile salt influx and oxidative stress in T6SS-positive C. jejuni.
4. Decreased colonization of C. jejuniin chickens by tuning T6SS activity.
Similar content being viewed by others
Background
Commensal bacteria within the gut engage in a symbiotic interaction with the host, significantly influencing gut health and overall fitness [1, 2]. These effects manifest through the modulation of various host functions, such as development, metabolism, and immunity [3, 4]. Conversely, common gut pathogens possess various inherent mechanisms to circumvent host defenses, enhance host pathogenicity, and withstand exposure to diverse environmental stressors [5]. Pathogenic gut bacteria often use committed secretion systems for their survival, disease progression, and competitive advantages [6, 7]. The bacterial Type VI Secretion System (T6SS) is noted for its functional versatility such as self-survival and secretion of effector molecules towards competitive fitness advantages in a complex gut microenvironment [8, 9]. The bacterial T6SS functionality via dynamic cycles of assembly, contraction, and disassembly targets both prokaryotic, eukaryotic and even ‘sister’ populations within the same ecological niche [10,11,12,13,14,15,16]. However, recent studies suggest that the T6SS functionality of a range of gut pathogens including C. jejuni can be influenced by different stress variables, such as oxidative and chemical stressors, heat-shock factors, osmotic and low-pH stress [17,18,19,20,21,22,23].
As key oxidative stressors naturally present in the gut, bile salts share a complex relationship with resident gut-microflora. In particular, bacterial metabolism and their relative abundance shape the spatial composition of bile salts (or vis-à-vis) in the gastrointestinal tract to maintain gut homeostasis [24]. Specifically, gut microbes utilize several mechanisms to adopt bile salt resistance, which is considered an important selective pressure in modulating the gut microbiome population [25]. The differential ability of bacteria to withstand bile salt resistance is mediated by activating efflux pumps [26, 27], alteration of membrane protein [28, 29], lipid composition [30,31,32,33] and cell wall composition [34]. Previously, we found phenotypic differences between T6SS-negative and T6SS-positive C. jejuni isolates toward bile salt sensitivity. Notably, in the presence of a target (prey), T6SS-positive C. jejuni was less tolerant toward bile salts than in the case without prey [35]. However, it remains unclear whether the T6SS functionality could affect C. jejuni survivability under a complex and polymicrobial gut environment in an in vivo conditions. Another question is whether bile salt tolerance relies on the direct or indirect involvement of target-driven T6SS functionality.
To this end, we used non-pathogenic Escherichia coli (E. coli-DH5α) as prey for the T6SS-positive reference strain of C. jejuni (ATCC 43,431-TGH 9011) and another reference strain of C. jejuni (NCTC 11,168; GenBank ID: AL111168.1), which lacks the complete T6SS sequence as a negative control to assess stress tolerance using chickens as an in vivo model host. Given that the hemolysin-coregulated protein (Hcp) of C. jejuni is the key functional protein of the T6SS system [36, 37], we also confirmed the overall functionality of T6SS in an in vitro setup using an hcp deletion mutant (Δhcp) of the isogenic background of T6SS-positive C. jejuni (ATCC 43431-TGH 9011).
The data generated from in vitro competition of C. jejuni and E. coli, and enhanced intracellular accumulation of iridium-conjugated bile salt with high-level production of reactive oxygen species (ROS) suggest that T6SS functionality could entail “a cost” for T6SS-positive C. jejuni cells when bile salts are present. Finally, in vivo chickens experiment demonstrated that the birds that received T6SS-positive C. jejuni along with E. coli, showed ~ 1.0 log reduction in the T6SS-positive population with a low level of local antibody responses (sIgA) and pro-inflammatory cytokine gene expression. Together with efficient clearance of C. jejuni, low-level sIgA responses and pro-inflammatory gene expression, suggest that T6SS functionality could restore chickens gut health and potentially reduce the risk of food-borne transmission of C. jejuni to humans.
In summary, we propose that utilizing the prey-driven T6SS functionality of C. jejuni can help us develop new dietary formulations that protect birds from a range of other T6SS-harboring enteric pathogens. Although the acquisition of bile tolerance is a natural process by major gut microbiota, bile salt adaptation may affect the probiotic traits of useful gut microbes [38]; therefore, further investigation is needed to determine the appropriate form and optimized dose for the exogenous application of bile salt.
Methods
Bacterial strains and plasmids
Bacteria
C. jejuni reference strain (TGH9011) was obtained from BEI Resources, NIAID, NIH: C. jejuni subsp. jejuni, Strain TGH 9011, NR-4082, and the T6SS-negative C. jejuni strain (NCTC 11168; GenBank ID: AL111168.1) was obtained from the NIH Biodefense and Emerging Infections Research Repository, NIAID, NIH: C. jejuni subsp. jejuni, Strain NCTC 11,168, NR-126. The E. coli (DH5α) cells were procured from BioBharati Life Science, India.
Bacterial vectors
The pTurboGFP-B plasmid was transformed into E. coli (DH5α) cells to generate GFP-expressing E. coli (rE. coli). The pTurboGFP-B was kindly provided by Dr. Partha Pratim Datta, IISER Kolkata. Details of the bacterial transformation procedure can be found in our previous work [35, 39]. The pJMK30 plasmid was used as the source of the kanamycin gene cassette to generate an isogenic mutant of C. jejuni (kindly provided by Prof. Andrey Karlyshev, Kingston University, London, UK).
Cell lines
Chicken Embryonic Intestinal Cells (CEICs) were harvested from 19-day-old chicken embryos. Cells were grown in complete RPMI with 10% FBS (v/v), 100 IU/mL of penicillin, and 100 µg/mL of streptomycin at 37 °C with 5% CO2 supplementation [40].
Generation of hcp mutant C. jejuni(Δhcp)
Targeted gene deletion of hcp was performed by homologus recombination methods using a kanamycin-resistant (KmR) cassette from pJMK30 vector [41]. The KmR cassette was amplified using primers containing the EcoRI restriction sites. The upstream and downstream primers (with RE sites, matched to KmR cassette) were used to amplify the flanking regions of the hcp gene. Further, the pBSK-II(+) vector was used to ligate the PCR-amplified fragments before transformation into E. coli (Top10). Next, a purified plasmid from E. coli was used to transform into C. jejuni (TGH 43431) via electroporation. Briefly, C. jejuni (TGH 43431) culture was resuspended in buffer (mixture of 15% glycerol and 272 mM sucrose), followed by three times washing with the same buffer, and then a 50 µL aliquot was used for each transformation. The mixture was shifted to a chilled electroporation cuvette following the addition of ligated plasmid (0.5 µg in 5 µL). After electroporation, fresh Brucella broth (100 µL) was introduced into the cuvette, followed by plating the bacterial suspension on a blood agar plate, which was further followed by incubation at 37 °C under microaerobic conditions. After visible bacterial growth on the Blood Agar plate, the bacteria were spread onto a kanamycin-supplemented blood agar plate and cultured for 5–6 days under microaerobic conditions at 37 °C. The confirmation of correct gene orientation was done by Sanger sequencing and PCR, followed by transcriptome analysis of the target gene (hcp) and western blot analysis of the whole cell lysate of the mutant strain. Primers are listed in Supplementary table S3.
Chemical and reagents
All analytical grade chemicals and reagents were used in this study, obtained from commercial suppliers, and used without further purification unless otherwise mentioned. The chemicals were procured from Sigma-Aldrich (4′-Chloro-2, 2′:6′2′′-terpyridine), Arora-Matthey Limited (IrCl3.3H2O), and Merck (KOH), and were used without further modification. The solvents were obtained from Sigma-Aldrich (d6-DMSO, CDCl3), SD Fine-Chem Limited (dichloromethane, methanol, and acetone), and used after passing through hot water Na2SO4. Bile salt mixture (1:1 mixture of Sodium Deoxycholate and Sodium Cholate) was procured from Himedia, India.
In vitro assessment of C. jejuni response to bile salt stress
Assessing the changes in bacterial morphology
Bile salt-induced changes in C. jejuni morphology were visualized by Field Emission Scanning Electron Microscopy (FESEM). Samples were prepared according to a method published elsewhere [39]. In brief, equal number of C. jejuni (1 × 107 CFU/mL), either T6SS-positive or T6SS-negative or Δhcp mutant of C. jejuni was co-incubated with E. coli (2 × 108 CFU/mL) in Mueller-Hinton (MH) broth (HiMedia, India) supplemented with 0.1% (w/v) of bile salt mixture (Grade III, HiMedia). After 7 h of co-incubation under microaerobic conditions, bile salt-treated C. jejuni cells were fixed using 2.5% (v/v) glutaraldehyde, followed by three times washing using PBS. Next, fixed bacterial cells were incubated serially in 35%, 50%, 70%, and 95% ethanol for 10 min each, followed by 1 h incubation with 100% ethanol for complete dehydration. Eventually, the dehydrated cells were vacuum-dried for 1 h, followed by the fixation on aluminum stubs with silver conductive paint, to perform sputter-coating with platinum. Further, microscopy was performed using a Supra 55 Carl Zeiss scanning electron microscope (Carl Zeiss, Germany).
Visualization and quantification of intracellular reactive oxygen species (ROS) by fluorescence imaging
The samples collected from each group were prepared according to a method published elsewhere [39]. In brief, following the coculture of C. jejuni (1 × 107 CFU/mL) and E. coli (2 × 108 CFU/mL) for 7 h at 37 oC (at 10% CO2, 5% O2, and 85% N2), 100 µL from the mixture was plated onto bile salt added (0.1%; w/v) MH agar and grown for ~ 48 h. Further, ~ 10 individual, C. jejuni colonies were picked up from the same plates based on green-white screening (C. jejuni: white colonies vs. rE.coli: green colonies) and dissolved in 1 mL of MH broth. A total of 6 plates were processed under similar conditions (n = 6) and the absorbance of each colony dissolved in MH broth was normalized to ~ 0.5 OD600. A total of 500 µL from each sample was centrifuged and next the pellet was incubated with 200 µL of 2′, 7′-dichlorodihydrofluorescein diacetate (H2DCFDA; 1 µM) and incubated for 1 h. Following washing, 4% PFA was used to fix the cells. Next, 4 µL of the fixed, stained cells were affixed to a glass slide employing Vecta-shield mounting medium (Vector Laboratories, USA) for fluorescence imaging under Axio observer equipped with ApoTome module (Carl Zeiss, Germany) in FITC filter and 100 µL of cell pellet was subjected to fluorescence spectrophotometry using the Spectramax M2e Multi Detection Microplate Readers (USA) (at λex: 485 nm; λem: 535 nm).
Effect of bile salt-induced stress on C. jejuni growth profile
To examine the effects of bile salt-induced stress, T6SS-positive (TGH 9011), Δhcp (TGH 9011), and T6SS-negative (NCTC 11,168) strains of C. jejuni were used. An equal number of C. jejuni (1 × 107 CFU/mL), either T6SS-positive or T6SS-negative or Δhcp mutant of C. jejuni was co-incubated with E. coli (2 × 108 CFU/mL) were cocultured in MH broth (HiMedia, India) supplemented with different concentrations of bile salt ranging from 0.05% to −0.125% (w/v). After 7 h of co-incubation under microaerobic conditions, followed by 1.0 mL from each tube was diluted serially and 50 µL from the last dilution was plated onto MH agar plate. The plates were incubated for ~ 48 h at microaerobic conditions and the colony was counted based on the green-white screening. Further, the colonies on the plates were enumerated individually and plotted as the average CFU/mL with standard deviation (± SD) .
In vitro tracking and visualization of bile salt transport to C. jejuni
Synthesis of iridium conjugated bile salt (Ir-TBS) complex
To prepare TBS, bile salt solution (500 mg, 1.1620 mmol) was initially introduced into a stirred mixture of powdered KOH (391.1 mg, 6.972 mmol) in DMSO (20 mL) at 60 °C. Following a 20-minute interval, 4’-chloro-2,2’:6’,2″-terpyridine (777.7 mg, 2.9051 mmol) was introduced into the mixture and stirred for 3 days at 60 °C. Subsequently, the reaction mixture was poured into 100 mL of deionized water. Adjustment of the solution’s pH to 7 was achieved using concentrated HCl, followed by filtration to remove the aqueous layer. Further, the compound underwent a washing process with deionized water, followed by vacuum drying and purification through column chromatography, employing a DCM: Hexane mixture (95:5) as the eluting solvent. TBS (50 mg, 0.0444 mmol) was subjected to reflux at 80 °C for 4 h with [Ir(ppy)2]2Cl2 (71.5 mg, 0.1334 mmol) in a solution of dichloromethane and methanol (3:1). After removing the solvent at low pressure the compound underwent purification through preparative thin-layer chromatography, utilizing a dichloromethane mixture containing 7% methanol. The final compound was obtained as the second major fraction [42,43,44,45,46].
Biophysical characterization of Ir-TBS complex
The presence and integrity of the Ir-TBS complex were validated using Electrospray ionization - Mass Spectrometer (ESI-MS) (positive mode electrospray ionization with the Bruker maXis IITM instrument, USA) and Nuclear Magnetic Resonance (NMR) spectroscopy. The NMR spectra (1H and 13C) were acquired on JEOL ECS 400 (Japan) and Bruker-500 spectrometers (Bruker, USA), respectively. The Perkin Elmer (USA) instrument was used to obtain the Fourier Transform Infrared (FT-IR) spectra. The Jasco V 670 spectrophotometer (Japan) was used to measure UV-Vis spectra of both TBS and Ir-TBS (1 × 10− 5 M). The fluorescence spectra of TBS and Ir-TBS (1 × 10− 5 M) in solution were recorded using a Fluoromax spectrofluorometer (Horiba Jobin Yvon, Japan). The stability of Ir-TBS was assessed over four days using time-dependent 1H NMR and UV-vis spectroscopy.
In vitro tracking of bile salt influx into C. jejuni
To track and visualize intracellular transport of bile salts, C. jejuni and E. coli were cocultured in MH broth supplemented with Ir-TBS (0.05%, w/v) and kept at 37 °C. To check the Ir-TBS influx, bacterial cultures were collected and washed, and the fluorescence signal of Ir-TBS complex inside the bacteria was measured by scanning the fluorescence spectra (λex:400 nm; λem:580 nm). Further, to visualize the intracellular accumulation of Ir-TBS, C. jejuni cells were harvested 6 h post-treatment and processed for Confocal Laser Scanning Microscopy (CLSM), as described earlier. After fixation, the bacterial cells were examined under a Leica confocal microscope (Germany) (λex:405 nm; λem:580 nm), and images were captured.
Assessing the effect of T6SS-mediated predation on C. jejuni invasion to primary CEICs
The number of C. jejuni associated with CEICs (adhered and invaded) was determined according to the protocol published elsewhere [40]. In brief, a confluent monolayer of primary CEICs was infected with C. jejuni with or without the presence of E. coli at MOI 300:1 for 7 h at 37 °C and 5% CO2 in RPMI supplemented with 10% FBS and 0.05% bile salt. Next, the medium was discarded, followed by washing with 1X PBS. The cells were lysed using 1% Triton X-100 to release intracellular bacteria, enhancing the release into the pool of adhered bacteria. The mixture was then consecutively diluted (10-fold) and plated on MH agar plate. The C. jejuni colonies on the plate were enumerated and reported as the average CFU/mL ± SD. To determine the number of intracellular bacteria, we also performed gentamicin protection assay [40]. For this, after 7 h of infection, the cells were incubated for 2 h with gentamicin (150 µg/mL) to kill adhered bacteria. Further, cells were washed, followed by lysing using Triton X-100 as previously described.
Imaging method
Monolayers of primary CEIC cells were grown on coverslips at the density of 1.2 × 106 cells/well. Prior to infection, C. jejuni cells were labeled with 4′,6-diamidino-2-phenylindole (DAPI; 5 µg/mL). Further, primary CEICs were treated with various sets of treatment groups: C. jejuni only, C. jejuni with bile salt (0.05%, w/v), C. jejuni with E. coli, C. jejuni with E. coli and bile salt (0.05%, w/v) for 7 h under 5% CO2 pressure. After removing the medium, cells underwent a PBS wash before fixation with 4% PFA for 20 min. Subsequent to fixation, phalloidin 647 (Abcam, UK) staining was conducted for 1 h, followed by another wash, and finally, mounting on slides using a mounting medium (Vectashield). Images were captured using a Leica confocal microscope under a DAPI filter (λex:358 nm and λem:461 nm for C. jejuni) and a phalloidin filter (λex: 647 nm, λem: 668 nm for CEICs) and further processed using Fiji software.
In vivo assessment of T6SS functionality in selective killing of C. jejuni in chickens
Experimental birds and housing conditions
For this present study, we used 9 birds per experimental group (a total of 8 groups). The chickens experiments were conducted separately with both T6SS-positive (4 groups) and T6SS-negative (4 groups) under similar experimental conditions. Thus, the total number of birds used = (9 birds per group × 4 × 2 = 72). Throughout the trial, the birds were housed in a deep litter system and provided with unrestricted access to an antibiotic-free mash diet. The specific feed composition used for this study is detailed in Table S2.
Experimental groups
After seven days of acclimatization, the birds were divided into four different groups (Groups A, B, C, and D). From Day 7 to Day 14, Group C and Group D birds were administered with bile salt dissolved in PBS (0.2%, w/v) daily, while Group A and Group B received only PBS. Furthermore, from day 15 to day 35, birds from different groups received the following treatments consecutively for three days per week. Group A: C. jejuni; Group B: Mixture of C. jejuni and E. coli; Group C: Mixture of C. jejuni and bile salt; Group D: Mixture of C. jejuni, E. coli, and bile salt.
Feeding regimens
Chicks from different groups were orally administered the following treatments. Group A: 1 × 107 CFU C. jejuni in PBS; Group B: 1 × 107 CFU C. jejuni + 2 × 108 CFU E. coli in PBS; Group C: 1 × 107 CFU C. jejuni + 0.2% (w/v) bile salt mixture in PBS; Group D: 1 × 107 CFU C. jejuni + 2 × 108 CFU E. coli + 0.2% (w/v) bile salt mixture in PBS.
Sample collection (cecal content, gastric lavages, and fecal materials)
On day 30 after feeding, fresh fecal samples were obtained from each bird in every group at two distinct time intervals (3 h and 4 h). On day 35, all birds were euthanized, 3 h after the last feeding. After sacrifice, ~ 5 g of cecum tissue sample was obtained from each bird and kept at -20 ºC in RNA-later™ (Qiagen, Germany). The cecal tissue samples for histopathological examination were acquired and 10% neutral formalin buffer (NFB) was used for fixation for 48 h. Approximately 500 mg of cecal content was diluted in MH broth to measure C. jejuni load in the cecum. The intestinal lavages were obtained using a protocol established in our laboratory and were subsequently stored at -20 °C [47].
Assessing local antibody responses (sIgA) against C. jejuni in gastric lavages
To determine anti-C. jejuni sIgA antibody level in the intestine, lavage samples obtained from chickens in various experimental groups to perform an indirect ELISA [47]. In brief, maxisorp ELISA plates were coated with C. jejuni whole-cell lysate overnight at 4 °C prepared in carbonate-bicarbonate coating buffer (pH ~ 9.6). Next day, the plates were washed with PBS-Tween (PBS-T), followed by 1 h blocking at 37 °C with 5% Bovine Serum Albumin (BSA). After thorough washing of the wells, serially diluted intestinal lavage samples were added and allowed for 2 h incubation at room temperature (RT). After washing, the plates were probed with HRP-conjugated goat anti-chicken IgA secondary antibody (dilution: 1:2500; Thermo Fisher Scientific) for 1 h at RT. Subsequently, following three washes, TMB substrate was applied to each well, and the reaction was terminated by the addition of 50 µL of a stop solution comprising 1 M H2SO4. The absorbance was then determined utilizing a microplate reader (at 450 nm) from BioTek.
Determining the C. jejuni load
To enumerate C. jejuni load, fecal and cecal samples of orally administered birds in every group were collected on days 30 and 35, respectively. Approximately 500 mg of feces and cecal content were taken and dissolved in 1 mL of MH broth, followed by serial dilution, and plated onto blood-free Campylobacter selective media. Then, the plates were kept for incubation at 37 °C under microaerobic conditions, and colonies were counted after 48 h of incubation [48].
Determination of cytokine gene expression profile in cecal tissue by RT-qPCR
Total RNA was extracted from the cecal tissues using TRIzol (Invitrogen, USA). Reverse transcription of RNA for each sample was performed using a first-strand cDNA synthesis kit (BioBharati Life Science, India). To evaluate cytokine gene expression, RT-qPCR was performed using a primer set for chicken IL-8, IL-17 A, IL-6, and IL-1β, while the chicken β-actin gene was used as the internal control. The primers details used are listed in Table S3.
Histopathological analysis of cecal tissue
To perform histopathological examination, the cecal tissue was collected from the experimental birds on day 35 and fixed in 10% NFB for 48 h. After fixation, the tissue samples were washed overnight with slow-running tap water and subsequently dehydrated by passing them through a series of acetone grades (70–100%). Thereafter, The dried samples were placed in 100% benzene for 1 h to make them clear and transparent. Next, the samples were impregnated with melted paraffin (temperature 56 °C) and embedded in paraffin using metal molds to make a paraffin block. Paraffin-embedded samples were cut into thin slices (5 µM) using a rotary microtome and further processed for the standard de-paraffinization by immersion in xylene for 2 min. The deparaffinized tissue slices were then hydrated for 2 min with a descending concentration of graded alcohol (100 − 70%), followed by a 2-min immersion in distilled water. The hydrated tissue sections were stained with 1% hematoxylin for 3 min and then rinsed slowly for 5 min in tap water. The stained slides were dipped in HCl: Ethanol (1:1) solution and kept under running tap water for 5 min to remove the additional stain and further counter-stained with 1% eosin for 30 s. The slides were then dehydrated for 2 min with increasing concentrations of graded alcohol (70–100%). The stained slides were then immersed in xylene for 2 min before being mounted using DPX (Di-butylphthalate Polystyrene Xylene) solution. Finally, images of the stained tissue slices were acquired using a (Leica DM 2000, Germany) microscope.
Statistical test
GraphPad Prism statistical software (version 8) was used for graphical presentation and data analysis. The regression (R2) value for the invasion assay was determined using a nonlinear regression curve. To confirm normal distribution, the Shapiro-Wilk test was conducted. Significance among different experimental groups was assessed using Student’s t-test (two-tailed, unpaired) or the non-parametric Mann-Whitney U test. Statistical significance was indicated by *P ≤ 0.05 and **P ≤ 0.01.
Results
Effect of T6SS mediated predation on differential stress tolerance of C. jejuni
We assessed the role of T6SS in bile salt-mediated stress tolerance using two C. jejuni reference strains, one with complete T6SS and the other without T6SS. The whole genome sequence analysis revealed 98.05% similarity between T6SS-positive and -negative strains, while the T6SS-positive strain carried 21 T6SS-related genes, which were absent in the T6SS-negative strain (Supplementary Fig. S1a-b). Given that hemolysin-coregulated protein (hcp) is a major effector among the 13 genes that encode structural and functional units of the hexameric T6SS channel [37, 49], we generated an hcp deletion isogenic mutant of C. jejuni strain (Δhcp) to explore the involvement of the T6SS channel in the intracellular transport of bile salt (Fig. 1a). Deletion of hcp was confirmed by DNA sequencing (Fig. 1b, c), PCR analysis of genomic DNA (Fig. 1d), amplification from mRNA transcript (Fig. 1e), and Western blot analysis (Fig. 1f).
Generation of hcp knockout C. jejuni mutant (Δ hcp C. jejuni). (a) A targeted deletion of hcp (locus tag NMLJCPGM_01019, size: 516 base pairs) was generated in the C. jejuni (ATCC 43431-TGH 9011) by exchanging the gene with a Kanamycin Resistant gene (KmR). Schematic showing homologous recombination between KmR cassette and the flanking regions of hcp in T6SS island of C. jejuni genome to generate Δhcp C. jejuni mutant. The FESEM micrographs showed no morphological changes of C. jejuni after replacing hcp gene with KmR; scale bar: 1 μm. (b, c) Sequencing chromatogram of the hcp knock-out mutant of C. jejuni using upstream and downstream primer set of flanking region of hcp gene showing the insertion of KmR in place of hcp in genomic DNA of T6SS-positive C. jejuni (ATCC 43431-TGH9011). (d) PCR analysis of hcp deletion and replacement of hcp gene with KmR gene showing no amplification of target gene in the genomic DNA extracted from Δhcp C. jejuni (Lane 2, Lane 3) and amplification of KmR gene from genomic DNA of Δhcp C. jejuni (Lane 4). Lane 1 and 5 are kept as positive controls, while Lane 6 is no template control (NTC). Here, Lane 1 shows the amplification of the hcp gene from the genomic DNA of wild-type C. jejuni (TGH 43431), and Lane 5 represents the amplification of the KmR gene from the pBSKII plasmid. (e) Transcriptome analysis from WT-C. jejuni and Δhcp-C. jejuni. Agarose gel image showing the presence of KmR transcript and the absence of the hcp transcript in the hcp knockout mutant while in WT-C. jejuni detects hcp transcript. (f) Western blot analysis of the whole cell lysate of C. jejuni shows the presence of Hcp protein in C. jejuni cell lysate. Detection using an anti-Hcp antibody revealed a protein band at ~ 20 kDa, consistent with Hcp presence. However, the absence of a protein band in the Δhcp C. jejuni strain indicates successful knockout of hcp.
Bile salt has been reported to exhibit antibacterial effects, such as disruption of membrane integrity by altering membrane fluidity and permeability, thus affecting the functions of critical membrane proteins [50]. In particular, the FESEM micrographs suggested bile salt-induced damage in the morphology of T6SS-positive C. jejuni but not in T6SS-negative cells or Δhcp C. jejuni (Fig. 2a-f; Supplementary Fig. S2, S3, S4; Supplementary Table S4). The critical comparison of FESEM images and the percentage of cell damage among the different experimental groups further suggest that the number of damaged cells (sac-like morphology and even complete lysis/disintegration of T6SS-positive C. jejuni cells) was significantly higher (~16.14%) when T6SS-positive C. jejuni grown when E. coli and bile salt were present compared to when C. jejuni grew in bile salt-containing medium but without prey (~0.85%) (Supplementary Fig. S2; Supplementary Table S4).
Such changes in bacterial morphology and physiology are often observed when bacterial cells encounter stress conditions. Since the -OH groups in bile salts are known to cause damage to intercellular organelles and DNA by producing ROS [51], we investigated whether T6SS-positive cells produce a differential amount of intracellular ROS.
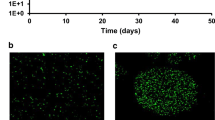
Role of prey (E. coli) in differential bile salt tolerance of T6SS-positive, T6SS-negative and Δhcp C. jejuni. (a-f) FESEM micrographs illustrate clear morphological alterations in C. jejuni when exposed to either the presence or absence of prey (E. coli). Deflated sac-like morphology of C. jejuni was observed, but no such difference was noticed in the case of T6SS-negative C. jejuni and Δhcp C. jejuni (see insets). Moreover, prey cell damages were evident (red box) in the presence of C. jejuni but not in the case of T6SS-negative C. jejuni and Δhcp C. jejuni. Scale bar: 1 μm. (g-o) Epifluorescence images showing intracellular ROS generation in C. jejuni. H2DCFDA-treated C. jejuni cells displaying higher fluorescence signals (green) in the case of T6SS-positive C. jejuni when the prey is present, indicating a high-level intracellular accumulation of ROS. Little or no fluorescence signal was detected in T6SS-negative cells as well as in the hcp-knock out C. jejuni (with or without the presence of prey). The fluorescence signal of T6SS-positive C. jejuni cells incubated with prey and bile salt exhibited a significantly higher mean fluorescence intensity (MFI) compared to conditions without prey (i). Regardless with or without the presence of prey no such elevated MFI was observed in T6SS-negative C. jejuni (l) and in Δhcp C. jejuni (o). Scale bar:10 μm. (p) Quantification of total ROS present in C. jejuni cells indicates enhanced MFI in T6SS-positive C. jejuni when prey bacteria is present. No such difference was noticed for T6SS-negative and Δhcp C. jejuni (n = 6). (q) When prey was present, the count of T6SS-positive C. jejuni colonies (CFU/mL) was notably lower compared to when C. jejuni was grown alone. The C. jejuni cells were cultured in the growth medium containing bile salt solutions (0.05% w/v). However, there was no alteration in CFU counts observed for T6SS-negative and Δhcp C. jejuni regardless with or without the presence of prey. Error bars depict standard deviation (mean ± SD) (n = 6)
The representative apotome images and the mean ROI (region of interest) value for each group indicated that T6SS-positive C. jejuni, when E. coli and bile salt were present, exhibited significantly higher fluorescence signals (Fig. 2g-i; Supplementary Fig. S5 a, b) than using the Δhcp mutant (Fig. 2m-o; Supplementary Fig. S5 e- f) or T6SS-negative C. jejuni (Fig. 2j-l; Supplementary Fig. S5 c, d). Interestingly, we observed higher ROS generation in T6SS-positive cells under bile salt conditions, leading to a loss of bacterial survival when grown together with prey, compared to the case without prey (Fig. 2p). However, when tested across various bile salt concentrations (ranging from 0.05 to 0.125%), the T6SS-negative strain exhibited a higher capacity to withstand stress compared to the T6SS-positive cells (Fig. 2q; Supplementary Fig. S5g). Similar functional attributes in terms of enhanced tolerance to bile salt were detected in the Δhcp mutant, regardless of the presence or absence of prey (Fig. 2q; Supplementary Fig. S5g). Importantly, whether prey was present or absent did not impact the stress tolerance of either the Δhcp mutant or T6SS-negative cells (Fig. 2q). Together, based on our data, it appears that the bile salt susceptibility of C. jejuni is linked to Hcp-driven T6SS functionality, which can modulate the prey population.
As an invasive gut bacterium, C. jejuni can utilize multiple physiological adjustment systems, including activation of CmeABC efflux pumps, to facilitate self-survival by enduring a harsh gut environment [26]. Notably, the T6SS-positive and-negative strains used in this study carried homologous genes encoding CmeABC efflux pumps. Previously, we reported that C. jejuni cells showed less tolerance to predation above the critical bile salt concentration [35]. The present observation further supports that bile salt susceptibility and subsequent ROS generation in C. jejuni are direct outcomes of T6SS-mediated predation, indicating that higher bile salt stress leads to the “depletion” of C. jejuni. However, the question remains whether effects on C. jejuni stress tolerance rely on the intracellular transport of bile salts.
Enhanced intracellular bile salt influx by T6SS-positive C. jejuni in the presence of prey
We postulated that the activation of T6SS by prey could result in the intracellular influx of bile salts through the bidirectional contraction of the T6SS apparatus during the secretion of effectors, as previously documented [35]. We anticipated that visualization of bile salt influx could provide direct evidence that a functional T6SS channel can control the dynamics of intracellular transport of bile salts.
For this, we synthesized iridium conjugated bile salt (Ir-TBS) complex by reacting functionalized bile salt (BS) with 2,2’:6’,2’’-terpyridine (Tpy) molecule (Terpyridine appended bile salt (TBS) followed by conjugation with Ir(ppy)2 [Ir = iridium, ppy = 2-phenyl pyridine) (Fig. 3a). The photophysical property of Ir-TBS was confirmed by UV-Vis spectral analysis, while purity, stability, and higher phosphorescence lifetime (µs) of Ir-TBS complex were confirmed by ESI-MS, and stability was confirmed by time-dependent 1H NMR (Fig. 3b-d; Supplementary Fig. S6 a-m; Supplementary Table S1). Using this Ir-TBS complex when observed for the fluorescence intensity of Ir, we recorded a significantly higher signal for the T6SS-positive C. jejuni compared to the isogenic mutant (Δhcp) and T6SS-negative strain, suggesting that the high-level bile salt influx is mediated by the functional T6SS (Fig. 3e). More interestingly, the Ir signal was enhanced in the case of T6SS-positive C. jejuni when prey was present, compared to without prey (Fig. 3f). This could suggest that the prey population triggers functional enhancement of T6SS, leading to a higher bile salt influx.
T6SS mediates the intracellular influx of fluorescently labeled bile salt. (a) Reaction scheme for synthesizing the iridium (Ir)-conjugated bile salt complex (Ir-TBS). Functionalized bile salt (BS) was reacted with 2,2’:6’,2’’-terpyridine (Tpy), followed by iridium [Ir(ppy)2 [Ir = iridium, ppy = 2-phenyl pyridine)] conjugation to generate Ir-TBS complex. Yield: 31 mg (25.5%). (b) Photophysical profile of Ir-TBS complex at room temperature in acetonitrile. Spectral data showed that Ir-TBS absorbs at 200–520 nm and emits at λmax (λex = 390 nm) = 430 and 600 nm with a shoulder at 436 nm. (c) Observed ESI-MS spectra ([M - Cl− + Na+]/2 = 1359.3811) of Ir-TBS complex (green) with simulated spectra ([M - Cl− + Na+]/2 = 1359.3589) (red) showing the M2+ spectral pattern. (d) Time-dependent 1H NMR showing kinetic stability of Ir-TBS in PBS (pH 7.4) having 0.5% DMSO for 4 days. (e) Comparative analysis of emission fluorescence intensity (EFI) of Ir-TBS complexes present in C. jejuni. Following incubation of C. jejuni cells with Ir-TBS complex for 7 h, the cells were washed and the pellets were processed for spectrophotometry and image analysis. The EFI values indicate higher internalization of Ir-TBS in C. jejuni when prey (E. coli) was present. No differences were recorded for T6SS-negative and Δhcp C. jejuni (n = 6). (f) Representative CLSM images of C. jejuni cells grown in the presence of prey showed a higher influx of Ir-TBS complex in T6SS-positive C. jejuni than in T6SS-negative C. jejuni (Scale bar: 2 μm). Further analysis reveals a significant difference in mean fluorescence intensity (MFI) between T6SS-positive C. jejuni and both Δhcp C. jejuni and T6SS-negative C. jejuni cells under conditions where prey is present. Standard deviations are represented as error bars (mean ± SD) (n = 40)
Since the intracellular accumulation of bile salts is associated with ROS production and subsequent DNA damage [51], the data presented herein suggest the role of T6SS in bile salt sensitivity. Moreover, we showed that the T6SS-mediated predation decreased the self-survival ability of C. jejuni under bile salt stress, supporting the notion that T6SS functionality could entail “a cost” for T6SS-positive C. jejuni cells when bile salts are present. This atypical feature of the T6SS led us to further test the hypothesis in an in vivo set-up. Given that C. jejuni can persistently colonize the chickens gut, leading to a potential source for foodborne transmission to humans, we examined whether these attributes of T6SS could control C. jejuni colonization in poultry. Consequently, we comprehensively explored the effect of T6SS-functionality in reducing C. jejuni in chickens, using an in vitro setup first and then in an in vivo chickens model.
T6SS activity can reduce C. j ejuni load in primary chicken embryonic intestinal cells (CEICs)
To determine T6SS functionality under bile salt stress in vitro, with or without the presence of the prey primary CEICs were infected with C. jejuni (Fig. 4a). Notably, the comparative abundance (adhered and invaded) of T6SS-positive (WT), T6SS-negative, and Δhcp C. jejuni suggested the lower number of bacterial populations when chicken cells were infected with the T6SS-positive strain with E. coli and bile salt (Fig. 4b; Supplementary Fig. S7a). Moreover, irrespective of the prey, C. jejuni load in CEICs remained higher for Δhcp and the T6SS-negative strain (Fig. 4c, d; Supplementary Fig. S7 b, c). This was further confirmed by CLSM imaging, which showed that the cellular integrity of CEICs was not affected when prey (E. coli) and bile salt were present (Fig. 4e; Supplementary Fig. S8a). Alternatively, the cells exhibited a rounded morphology, accompanied by a notable rise in C. jejuni invasion of host cells. Nonetheless, with or without the presence of prey did not markedly influence the interaction of Δhcp C. jejuni or T6SS-negative C. jejuni with host cells (Fig. 4f, g; Supplementary Fig. S88b-c).
Taken together, we can infer that the prey-driven activity of T6SS under stress can significantly reduce C. jejuni association with host cells. Since our in vitro setting did not consider the complexity of the gut environment, we further tested our hypothesis of T6SS-dependent selective killing of C. jejuni in chickens.
In vitro T6SS activity on C. jejuni load in primary chickens embryonic intestinal cells (CEICs). (a) Schematic of the in vitro gentamycin protection assay to investigate the effect of T6SS activity on C. jejuni adherence and invasion in CEICs. Confluent monolayers of CEICs were co-incubated for 7 h with T6SS-positive, -negative, or Δhcp C. jejuni in the presence of E. coli and 0.05% bile salt solution. Subsequently, the cells were washed to eliminate extracellular bacteria and any remaining bile salts in the medium, followed by treatment with gentamycin to remove the adhered bacteria. The invading C. jejuni number was calculated (CFU/mL) after lysing the cells using TritonX100. The cells were imaged to visualize cellular changes. (b, c, d) The effect of prey on C. jejuni load on CEICs. Comparative data indicate a significant decrease in the number of T6SS-positive C. jejuni invasions in the presence of prey (E. coli) (b). Irrespective of the prey, no significant difference in C. jejuni population was observed when T6SS-negative (c) and Δhcp C. jejuni (d) strains were used for infection. (e, f, g) Images representing C. jejuni (stained with DAPI, shown in blue) invasion of CEICs (cell membrane stained with phalloidin, shown in red) (scale bar: 50 μm) suggest that when prey and bile salt stress (0.05%, w/v) are present, only a small number of T6SS-positive C. jejuni are detectable (shown in blue), contrasting with cells infected solely with C. jejuni (e). Comparison of the images reveals distinct alterations, notably rounded-off cells when prey and bile salt are absent (e). Regardless of the involvement of prey and bile salt, CEICs exhibited a rounded shape when infected with T6SS-negative C. jejuni (f) and Δhcp C. jejuni (g). All error bars represent standard deviations, denoted as mean ± SD (n = 6)
In vivo perturbation of C. jejuni self-survival utilizing T6SS mediated predation in chickens
To explore how the functionality of T6SS of C. jejuni impacts its ability to prosper within the chicken gut, a total of 72 day-old chicks were maintained on bile salt supplements. From day 14 onwards, birds were orally administered C. jejuni with or without E. coli (as prey), at weekly intervals, as shown in Fig. 5a. To define the effect of different feeding regimens on C. jejuni clearance, we determined the C. jejuni load in freshly collected fecal pellets and cecal content to see the effect of different feeding regimens on C. jejuni clearance. Consistent with in vitro results, a significantly low number of C. jejuni (~ 1 log) was detected in the group of birds that received T6SS-positive C. jejuni along with E. coli and bile salts, compared to the groups that received C. jejuni only. In contrast, oral delivery of T6SS-negative C. jejuni, either alone or with E. coli, or with E. coli and bile salt, failed to show a noticeable reduction in C. jejuni load (Fig. 5b; Supplementary Fig. S9a). The observed differences showed the possibility that if employed under an optimized dose of bile salt, the function of bacterial T6SS can be utilized for purging T6SS-positive C. jejuni from the primary host.
Since the intestinal load of C. jejuni may correlate with C. jejuni-specific antibody response locally, we assessed the secretory IgA (sIgA) antibody titers in the intestinal lavages probed with cell-free lysates of C. jejuni by indirect ELISA. Comparing the mean antibody titer of sIgA among different experimental groups, birds administered C. jejuni along with E. coli and bile salt showed the lowest level of local antibody titer, confirming the previous observation of effective C. jejuni clearance in this group. Notably, no substantial difference in the antibody response against C. jejuni was detected in birds that received T6SS-negative C. jejuni (Fig. 5c; Supplementary Fig. S9b). The persistence of this low-level anti-C. jejuni antibodies in birds administered T6SS-positive C. jejuni along with prey (E. coli), reaffirms the role of functional T6SS in bacterial “depletion.” These outcomes further advocate that the cecal clearance of C. jejuni does not require adaptive immunity if T6SS functionality can be exploited in response to bile salt stress.
Although being a commensal, C. jejuni resides in the chickens gut, recent findings showed that long-term colonization of C. jejuni negatively influences the gut barrier in chickens; particularly, it can lead to a “leaky gut” by an enhanced intestinal permeability [52]. Moreover, persistent colonization of C. jejuni aids the translocation of C. jejuni to the underlying tissues and the spread of other microbiota, including E. coli [53,54,55,56,57,58]. Therefore, we investigated the pro-inflammatory cytokine gene expression profile and histopathological changes in the cecal tissues to further determine whether the T6SS-dependent cecal clearance of C. jejuni positively affects overall gut health.
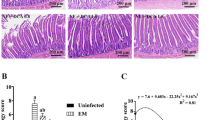
Prey-dependent depletion of C. jejuni in chickens maintained on bile salt supplementation. (a) Schematic of in vivo chickens feeding trial. After 7 days of acclimatization, birds were maintained in a bile salt (0.2%, w/v) containing diet till day 30. Chickens fed with a normal diet without bile salt were kept in control. From day 14 onwards, experimental birds were fed with either C. jejuni or C. jejuni and E. coli at the indicated time points (black circle). On day 35 (open circle), birds were sacrificed, and cecum, its content, and intestinal lavages were collected for the analysis of bacterial load, anti-C. jejuni antibody response, transcriptional profiles of pro-inflammatory cytokines, and cecal tissue histopathological study. The experimental group details were as follows: Group A: C. jejuni only (no bile salt); Group B: C. jejuni + E. coli (no bile salt); Group C: C. jejuni + bile salt and Group D: C. jejuni + E. coli + bile salt. Parallel with this, in vivo feeding with T6SS-negative C. jejuni under similar experimental conditions was performed (n = 9 birds for each group). (b) Cecal load of C. jejuni showing effective clearance of C. jejuni in Group D birds compared to the other experimental groups. No such difference was observed when T6SS-negative C. jejuni was used. (c) Comparative analysis of local antibody (sIgA) responses in the intestinal lavage against C. jejuni indicates a significantly low level of sIgA titer in the birds belonging to Group D compared to the other experimental groups. Reduced sIgA titer in these birds suggests prey-dependent clearance of T6SS-positive C. jejuni in the presence of bile salt. However, no such difference was noticed when the experiment was performed with T6SS-negative C. jejuni. (d) Pro-inflammatory cytokine gene expression profile of caecal tissue collected from different groups of birds indicates low-level expression of IL-1β, IL-8, IL-17A and IL-6 genes in Group D compared to the other experimental groups. (e) Histopathological changes in cecal tissue from different experiment groups. Panels i-iii show higher lesion scores characterized by necrotic lesions (arrow), lymphocytic infiltration (block arrow), disruption in the top layer of epithelium, and unorganized cell boundaries. Panel-iv displays perfectly oriented, continuous, and well-demarked surface epithelium
Given that unregulated inflammation often causes tissue damage, leading to the loss of vital function of intestinal epithelial cells, we assessed the gene expression profiles of IL-6, IL-1β, IL-17A, and IL-8. The birds that received C. jejuni along with E. coli and bile salt showed a significantly lower grade of pro-inflammatory gene expression than the other experimental groups (Fig. 5d). Furthermore, when checking for histopathological changes in these birds, nearly no inflammatory lesions were detected in the cecal tissue. The cecal mucosa of these birds displayed perfectly oriented, continuous, and well-demarked surface epithelium, lamina propria, and muscular mucosae layer, indicating the maintenance of epithelial cell integrity. Furthermore, the epithelial lining consisted of a simple ciliated columnar type, containing an increased population of goblet cells, suggesting an effective barrier function of the mucosal surfaces (Fig. 5e-iv).
In the case of birds that were not maintained in bile salt supplementation, when challenged with either a combination of C. jejuni and E. coli or C. jejuni only, they showed a significantly higher level of cytokine gene expression accompanied by a moderate to high degree of pathological changes in the cecal tissue. A marked discontinuity was observed in the surface epithelia, focal erosion leading to exposure of the lamina propria to the luminal surface, with a high number of monomorphic and polymorphic lymphocyte accumulations in the lamina propria. Together with a decreased height of glandular epithelia, fewer crypts of Lieberkuhn, and more importantly, visible focal necrosis of the inner circular layer of tunica muscularis were indicative of necrotic inflammation in the cecal tissue (Fig. 5e-i and iii).
In contrast, a low to moderate degree of pro-inflammatory signals in terms of cytokine gene expression was detected in birds that received C. jejuni along with bile salt. Distinct inflammatory changes were noted in the lamina propria, with infiltration of monomorphic and polymorphic cells and lymphoid accumulation in the muscularis mucosae layer. Stratification in some sections of the mucosa, with an unusual number of crypts in the lamina propria, was also noticeable. In addition, a bizarre presentation of the muscularis mucosae and degenerative changes in the inner circular layer of the tunica muscularis were visible. Furthermore, the glandular epithelia were found to contain fewer goblet cells, suggesting impaired mucous production and mucosal barrier function in these birds (Fig. 5e-ii).
Discussion
Campylobacter sp. is one of the four major global causes of foodborne diarrheal diseases and is designated a high-priority pathogen on the World Health Organization (WHO) list [59, 60]. Campylobacter infection (campylobacteriosis) is estimated to be 4.4–9.3 per 1000 people annually and is a substantial cause of morbidity among children under five years of age [61]. Multiple case studies have documented a notable association between human infection with C. jejuni and the ingestion of undercooked poultry contaminated with the bacterium [62, 63]. Hence, poultry products contaminated with Campylobacter are regarded as significant contributors to human transmission. Moreover, the prevalence of drug-resistant Campylobacter cases has been increasing in many parts of the world, specifically in Low- and Middle-income countries (LMICs). The increasing resistance of C. jejuni is presumably due to the injudicious use of antimicrobials in poultry, as supported by several studies [64,65,66]. Although C. jejuni remains commensal in chickens, several recent findings showed that persistent colonization of C. jejuni can negatively impact chicken gut health by affecting ion transport, trans-epithelial ion conductance, and increased intestinal permeability [53,54,55,56,57,58]. Furthermore, given that the metabolic processes of the host are intricately connected with resident gut microflora, the use of antibiotics to target gut pathogens, such as C. jejuni can disrupt the gut homeostasis resulting in the overgrowth of potential pathogens [67,68,69].
Since diverse populations of harmful and beneficial microbes coexist in the chicken gut by sharing resources and space, ideally, alternative antibiotic approaches should be fortified with target-specificity without destabilizing the overall gut homeostasis. Presently, dietary supplementation with probiotics, growth promoters, fecal microbiota transplantation, bacteriophage therapy, and the use of bacteriocins is of note [70,71,72,73]. Among them, probiotics, when given in ample quantities, can confer competitive fitness consequences to the host; however, they cannot selectively kill harmful gut pathogens [74]. Therefore, prioritizing the competitive exclusion of potentially harmful gut pathogens while maintaining the balance of gut microbiota, known as eubiosis, is preferable. In the search for alternative strategies for prophylaxis and control of gut pathogens, in the present study, we relied on the unique and atypical functionality of the bacterial intrinsic secretion system, T6SS of C. jejuni.
As a dynamic secretion system, T6SS functions by cycles of assembly, contraction, and disassembly to deliver various effectors targeting prey cells [10,11,12,13,14, 75,76,77,78,79]. Moreover, in silico analysis further revealed the genetic diversity of C. jejuni T6SS and effector-immunity pair which provide a framework for studying T6SS functionality [80, 81]. In line with these suppositions, using the in vitro “two species competition” model we showed that the T6SS activity of C. jejuni can effectively kill target bacteria (E. coli) and may incur “a cost” during bacterial competition in the presence of environmental stress [35]. However, it remains unclear how the target-driven T6SS functionality prevails in a polymicrobial complex gut environment.
To understand the mechanism of prey-driven T6SS activity under environmental stress, such as bile salt, hcp mutant (Δhcp) with an isogenic background of a T6SS-positive C. jejuni strain was created. Further to confirm the effect of bile salt during T6SS activity, we showed a high intracellular bile salt influx in T6SS-positive C. jejuni compared to the isogenic mutant using fluorophore-tagged bile salt. We propose that higher accumulation of bile salt during predation and subsequent oxidative damage to the DNA caused the morphological changes of C. jejuni and the negative effect of T6SS on its self-survival in the presence of E. coli. Our real-time tracking of the Ir-conjugated bile salt complex also advocates that C. jejuni T6SS can act as a ‘cell-puncturing device’ for effectors on the one hand and a target-driven ‘import system’ on the other hand, possibly by altering the envelope permeability barrier and promoting the intracellular transport of bile salt from the extracellular milieu [14, 35].
While intestinal bacteria utilize bile salts as nutrients, environmental signals, and electron acceptors, C. jejuni can flourish in bile salts due to various mechanisms, including the CmeABC efflux pump [26, 82]. In contrast, prey-induced perturbation of bile salt tolerance of T6SS-positive C. jejuni (but not in Δhcp or T6SS-negative cells) further strengthens our hypothesis that the dynamic and target-driven T6SS activity may exhibit bidirectional effector functions, leading to higher bile salt transport in the presence of prey.
These attributes of T6SS functionality raise the possibility of using stress-induced “depletion” of C. jejuni under in vivo conditions. Since C. jejuni is known to be commensal in chickens, we tested whether T6SS-dependent depletion of C. jejuni during predation can perturb C. jejuni association in chickens. We first confirmed that under in vitro conditions, adhered and invaded C. jejuni populations were substantially reduced when primary chicken embryonic intestinal cells (CEICs) were grown with C. jejuni and its prey (E. coli).
Based on the in vitro results, we next aimed for in vivo dysbiosis of C. jejuni in commercial broiler chickens and showed that the intestinal colonization of C. jejuni can be reduced by oral administration of C. jejuni with its prey and bile salt supplements. Typically, bile acids are produced by the liver and expelled into the digestive tract by the gallbladder when required. Additionally, in their role in digestion, bile salts help in maintaining gut homeostasis by determining the microbial ecology of the intestine [83, 84]. Moreover, bile salts demonstrate antibacterial effects by disrupting bacterial membranes, altering protein structures, binding with iron and calcium ions, inducing oxidative damage to DNA, and regulating the expression of eukaryotic genes associated with host defense and immunity [85]. Previous studies, including ours, have described a generic function of T6SS in competing with neighboring bacteria during interbacterial competition [9, 86]. In contrast, our in vivo trials showed that stress-induced T6SS activities could antagonize C. jejuni colonization in a complex gut environment, supported by positive host responses such as reduced anti-C. jejuni antibodies in the intestine, accompanied by low-grade pro-inflammatory cytokine gene expression. Since intestinal inflammation can perpetuate mild-to-severe pathological changes in the intestinal mucosa, chronic infection with C. jejuni can result in compromised epithelial barrier function in chickens [53, 87, 88]. To this end, histopathological assessment of chicken cecal tissue also suggests that administering C. jejuni along with prey and bile salt can maintain intestinal morphology. Furthermore, we ensured that the chickens were free from C. jejuni colonization from day 1 to day 7 (before the feeding trial started) by molecular and cultural screening of raw fecal samples.
However, as a dynamic and specialized secretion system, despite the cost for “resource sharing”, bacterial T6SS functionality may not incur a cost for intra-species competition, as reported recently [14], possibly due to the intrinsic defense via immunity proteins. In contrast, our data, supported by others, suggest that T6SS activity during intra- or inter-species interaction could be costly under specific conditions and in the presence of certain bacteria [22, 35, 89, 90].
Since infection with T6SS-positive C. jejuni often causes bloody diarrhea more commonly than T6SS-negative C. jejuni [91], our key objective was to selectively target the T6SS-positive C. jejuni in chickens as a measure to restrict their human transmission. Based on our study, ideally, exogenous administration of an optimal amount of bile salt should drive the selective depletion of T6SS-positive C. jejuni in the polymicrobial gut environment, however, studying “two species” competition in an in vivo setup is challenging. Nevertheless, we provide adequate evidence of improved gut health by demonstrating a marked reduction in T6SS-positive C.jejuni in chickens compared to T6SS-negative led us to conclude that careful tuning of T6SS functionality of C. jejuni can result in more favorable outcomes.
In fact, available literature suggests that even a 2–3 log reduction in the cecal load of C. jejuni would reduce approximately 58% of human infections via foodborne transmission, which is considered a therapeutic benchmark from the risk of zoonotic transmission point of view [92]. The influence of gut microbiome on host adaptability primarily hinges on the symbiotic relationship among gut microbes, the nutritional status of the host, and bacterial resilience against natural adversaries [93, 94]. Generally, the evolutionary adaption of the T6SS secretion system provides a unique survival advantage over other bacteria within the same niche [6]. Thus, functional T6SS can have both advantages and disadvantages, depending on the composition of the gut environment.
Conclusion
Our findings suggest that leveraging the functionality of the T6SS in the intricate gut environment could serve as a potential strategy to mitigate the persistent colonization of T6SS-positive C. jejuni in chickens. We suggest that harnessing the prey-driven T6SS of C. jejuni could lead to the development of novel alimentary formulations that protect birds from various enteric pathogens carrying T6SS. While the acquisition of bile tolerance is a natural process for major gut microbiota, adapting to bile salts may impact the probiotic characteristics of beneficial gut microbes. Thus, additional research is necessary to determine the optimal form and dosage for the external application of bile salts.
Data availability
No datasets were generated or analysed during the current study.
References
Belkaid Y, Hand TW. Role of the Microbiota in immunity and inflammation. Cell. 2014;157:121–41.
Wu H-J, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4–14.
Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26. https://doi.org/10.3402/mehd.v26.26191.
Henry LP, Bruijning M, Forsberg SKG, Ayroles JF. The microbiome extends host evolutionary potential. Nat Commun. 2021;12:5141.
Tlaskalová-Hogenová H, Štěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8:110–20.
Chen C, Yang X, Shen X. Confirmed and Potential Roles of Bacterial T6SSs in the Intestinal Ecosystem. Front Microbiol [Internet]. 2019 [cited 2022 Jul 20];10. https://www.frontiersin.org/articles/https://doi.org/10.3389/fmicb.2019.01484.
Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14:685–90.
Gallique M, Bouteiller M, Merieau A, The Type VI. Secretion System: A Dynamic System for Bacterial Communication? Front Microbiol [Internet]. 2017 [cited 2022 Jul 20];8. https://www.frontiersin.org/articles/https://doi.org/10.3389/fmicb.2017.01454.
Sana TG, Lugo KA, Monack DM. T6SS: the bacterial fight club in the host gut. PLoS Pathog. 2017;13:e1006325.
Hernandez RE, Gallegos-Monterrosa R, Coulthurst SJ. Type VI secretion system effector proteins: effective weapons for bacterial competitiveness. Cell Microbiol. 2020;22:e13241.
Ho BT, Dong TG, Mekalanos JJ. A view to a kill: the bacterial type VI secretion system. Cell Host Microbe. 2014;15:9–21.
Song L, Pan J, Yang Y, Zhang Z, Cui R, Jia S, et al. Contact-independent killing mediated by a T6SS effector with intrinsic cell-entry properties. Nat Commun. 2021;12:423.
Kanwal S, Noreen Z, Aalam V, Akhtar J, Masood F, Javed S, et al. Variation in antibiotic susceptibility and presence of type VI secretion system (T6SS) in Campylobacter jejuni isolates from various sources. Comp Immunol Microbiol Infect Dis. 2019;66:101345.
Taillefer B, Giraud JF, Cascales E. No fitness cost entailed by type VI secretion system synthesis, assembly, contraction, or disassembly in enteroaggregative Escherichia coli. J Bacteriol. 2023;0:e00357–23.
Pissaridou P, Allsopp LP, Wettstadt S, Howard SA, Mavridou DAI, Filloux A. The Pseudomonas aeruginosa T6SS-VgrG1b spike is topped by a PAAR protein eliciting DNA damage to bacterial competitors. Proc Natl Acad Sci U S A. 2018;115:12519–24.
Li M, Trong IL, Carl MA, Larson ET, Chou S, Leon JAD, et al. Structural basis for type VI Secretion Effector Recognition by a Cognate immunity protein. PLOS Pathog. 2012;8:e1002613.
Weber B, Hasic M, Chen C, Wai SN, Milton DL. Type VI secretion modulates quorum sensing and stress response in Vibrio anguillarum. Environ Microbiol. 2009;11:3018–28.
Wan B, Zhang Q, Ni J, Li S, Wen D, Li J, et al. Type VI secretion system contributes to Enterohemorrhagic Escherichia coli virulence by secreting catalase against host reactive oxygen species (ROS). PLoS Pathog. 2017;13:e1006246.
Wang T, Si M, Song Y, Zhu W, Gao F, Wang Y, et al. Type VI Secretion System transports Zn2 + to Combat multiple stresses and host immunity. PLoS Pathog. 2015;11:e1005020.
Lertpiriyapong K, Gamazon ER, Feng Y, Park DS, Pang J, Botka G, et al. Campylobacter jejuni Type VI Secretion System: roles in adaptation to deoxycholic acid, host cell adherence, Invasion, and in vivo colonization. PLoS ONE. 2012;7:e42842.
Bachmann V, Kostiuk B, Unterweger D, Diaz-Satizabal L, Ogg S, Pukatzki S. Bile salts modulate the mucin-activated type VI Secretion System of Pandemic Vibrio cholerae. PLoS Negl Trop Dis. 2015;9:e0004031.
Lin L, Ringel PD, Vettiger A, Dürr L, Basler M. DNA uptake upon T6SS-Dependent prey cell lysis induces SOS Response and reduces fitness of Acinetobacter baylyi. Cell Rep. 2019;29:1633–e16444.
Liaw J, Hong G, Davies C, Elmi A, Sima F, Stratakos A et al. The Campylobacter jejuni Type VI Secretion System Enhances the Oxidative Stress Response and Host Colonization. Front Microbiol [Internet]. 2019 [cited 2023 Jul 8];10. https://www.frontiersin.org/articles/https://doi.org/10.3389/fmicb.2019.02864.
Larabi AB, Masson HLP, Bäumler AJ. Bile acids as modulators of gut microbiota composition and function. Gut Microbes 15:2172671.
Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–8.
Lin J, Sahin O, Overbye Michel L, Zhang Q. Critical role of Multidrug Efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect Immun. 2003;71:4250–9.
Yokota A, Veenstra M, Kurdi P, van Veen HW, Konings WN. Cholate resistance in Lactococcus lactis is mediated by an ATP-dependent multispecific organic anion transporter. J Bacteriol. 2000;182:5196–201.
Liu Y, An H, Zhang J, Zhou H, Ren F, Hao Y. Functional role of tlyC1 encoding a hemolysin-like protein from Bifidobacterium longum BBMN68 in bile tolerance. FEMS Microbiol Lett. 2014;360:167–73.
Ruiz L, Couté Y, Sánchez B, de Los Reyes-Gavilán CG, Sanchez J-C, Margolles A. The cell-envelope proteome of Bifidobacterium longum in an in vitro bile environment. Microbiol Read Engl. 2009;155:957–67.
Fernández Murga ML, Bernik D, Font de Valdez G, Disalvo AE. Permeability and stability properties of membranes formed by lipids extracted from Lactobacillus acidophilus grown at different temperatures. Arch Biochem Biophys. 1999;364:115–21.
Kimoto H, Ohmomo S, Okamoto T. Enhancement of bile tolerance in lactococci by tween 80. J Appl Microbiol. 2002;92:41–6.
Gómez Zavaglia A, Kociubinski G, Pérez P, Disalvo E, De Antoni G. Effect of bile on the lipid composition and surface properties of bifidobacteria. J Appl Microbiol. 2002;93:794–9.
Taranto MP, Perez-Martinez G, Font de Valdez G. Effect of bile acid on the cell membrane functionality of lactic acid bacteria for oral administration. Res Microbiol. 2006;157:720–5.
Prouty AM, Schwesinger WH, Gunn JS. Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect Immun. 2002;70:2640–9.
Gupta S, Ray S, Khan A, China A, Das D, Mallick AI. The cost of bacterial predation via type VI secretion system leads to predator extinction under environmental stress. iScience. 2021;24:103507.
Bleumink-Pluym NMC, van Alphen LB, Bouwman LI, Wösten MMSM, van Putten JPM. Identification of a functional type VI Secretion System in Campylobacter jejuni Conferring Capsule Polysaccharide sensitive cytotoxicity. PLOS Pathog. 2013;9:e1003393.
Noreen Z, Jobichen C, Abbasi R, Seetharaman J, Sivaraman J, Bokhari H. Structural basis for the pathogenesis of Campylobacter jejuni Hcp1, a structural and effector protein of the type VI Secretion System. FEBS J. 2018;285:4060–70.
Burns P, Reinheimer J, Vinderola G. Impact of bile salt adaptation of Lactobacillus delbrueckii subsp. lactis 200 on its interaction capacity with the gut. Res Microbiol. 2011;162:782–90.
Gupta S, Khan A, Biswas P, Mondal K, Das D, Sharif S, et al. A combined protocol for isolation of T6SS-positive Campylobacter jejuni and assessment of interspecies interaction. STAR Protoc. 2022;3:101368.
Singh A, Mallick AI. Role of putative virulence traits of Campylobacter jejuni in regulating differential host immune responses. J Microbiol Seoul Korea. 2019;57:298–309.
van Vliet AH, Wooldridge KG, Ketley JM. Iron-responsive gene regulation in a campylobacter jejuni fur mutant. J Bacteriol. 1998;180:5291–8.
Das B, Borah ST, Ganguli S, Gupta P. Phosphorescent trinuclear Pt–Ir–Pt complexes: insights into the Photophysical and Electrochemical Properties and Interaction with Guanine Nucleobase. Chem – Eur J. 2020;26:14987–95.
Das B, Gupta P. Luminescent terpyridine appended geminal bisazide and bistriazoles: multinuclear pt(II) complexes and AIPE-based DNA detection with the naked eye. Dalton Trans. 2021;50:10225–36.
Das B, Gupta P. Trinuclear Organometallic Pt – Ir – Pt complexes: insights into Photophysical properties, amino acid binding and protein sensing. Chem – Asian J. 2021;16:2495–503.
Pritchard VE, Rota Martir D, Zysman-Colman E, Hardie MJ. Multimetallic and mixed environment Iridium(III) complexes: a Modular Approach to Luminescence tuning using a host platform. Chem – Eur J. 2017;23:8839–49.
Sarkar A, Kumar R, Das B, Sarothi Ray P, Gupta P. A cyclometalated trinuclear ir(iii)/Pt(ii) complex as a luminescent probe for histidine-rich proteins. Dalton Trans. 2020;49:1864–72.
Gorain C, Singh A, Bhattacharyya S, Kundu A, Lahiri A, Gupta S, et al. Mucosal delivery of live Lactococcus lactis expressing functionally active JlpA antigen induces potent local immune response and prevent enteric colonization of Campylobacter jejuni in chickens. Vaccine. 2020;38:1630–42.
Singh A, Nisaa K, Bhattacharyya S, Mallick AI. Immunogenicity and protective efficacy of mucosal delivery of recombinant hcp of Campylobacter jejuni Type VI secretion system (T6SS) in chickens. Mol Immunol. 2019;111:182–97.
Douzi B, Spinelli S, Blangy S, Roussel A, Durand E, Brunet YR, et al. Crystal structure and Self-Interaction of the type VI secretion tail-tube protein from Enteroaggregative Escherichia coli. PLoS ONE. 2014;9:e86918.
Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–51.
Negretti NM, Gourley CR, Clair G, Adkins JN, Konkel ME. The food-borne pathogen Campylobacter jejuni responds to the bile salt deoxycholate with countermeasures to reactive oxygen species. Sci Rep. 2017;7:15455.
Awad WA, Ruhnau D, Hess C, Hess M. Campylobacter jejuni increases the paracellular permeability of broiler chickens in a dose-dependent manner. Poult Sci. 2020;99:5407–14.
Awad W, Hess C, Hess M. Enteric pathogens and their Toxin-Induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins. 2017;9:60.
Awad WA, Molnár A, Aschenbach JR, Ghareeb K, Khayal B, Hess C, et al. Campylobacter infection in chickens modulates the intestinal epithelial barrier function. Innate Immun. 2015;21:151–60.
Awad WA, Dublecz F, Hess C, Dublecz K, Khayal B, Aschenbach JR, et al. Campylobacter jejuni colonization promotes the translocation of Escherichia coli to extra-intestinal organs and disturbs the short-chain fatty acids profiles in the chicken gut. Poult Sci. 2016;95:2259–65.
Kalischuk LD, Inglis GD, Buret AG. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog. 2009;1:2.
Kalischuk LD, Leggett F, Inglis GD. Campylobacter jejuni induces transcytosis of commensal bacteria across the intestinal epithelium through M-like cells. Gut Pathog. 2010;2:14.
Lamb-Rosteski JM, Kalischuk LD, Inglis GD, Buret AG. Epidermal growth factor inhibits Campylobacter jejuni-induced claudin-4 disruption, loss of epithelial barrier function, and Escherichia coli translocation. Infect Immun. 2008;76:3390–8.
Igwaran A, Okoh AI. Human campylobacteriosis: a public health concern of global importance. Heliyon. 2019;5:e02814.
World Health Organization. WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007–2015 [Internet]. World Health Organization; 2015 [cited 2022 Aug 21]. https://apps.who.int/iris/handle/10665/199350.
Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global Epidemiology of Campylobacter Infection. Clin Microbiol Rev. 2015;28:687–720.
Skarp CPA, Hänninen M-L, Rautelin HIK. Campylobacteriosis: the role of poultry meat. Clin Microbiol Infect off Publ Eur Soc Clin Microbiol Infect Dis. 2016;22:103–9.
SMITH JL, FRATAMICO PM. Fluoroquinolone Resistance in Campylobacter. J Food Prot. 2010;73:1141–52.
Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM, Zhang Q. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol. 2009;4:189–200.
Rossi DA, Dumont CF, Santos AC, de Vaz S, de Prado ME, Monteiro RR et al. GP,. Antibiotic Resistance in the Alternative Lifestyles of Campylobacter jejuni. Front Cell Infect Microbiol [Internet]. 2021 [cited 2022 Aug 21];11. https://www.frontiersin.org/articles/https://doi.org/10.3389/fcimb.2021.535757.
Siddiqui F, Champion O, Akram M, Studholme D, Eqani S a. m. a. s., Wren B w., et al. Molecular detection identified a type six secretion system in Campylobacter jejuni from various sources but not from human cases. J Appl Microbiol. 2015;118:1191–8.
Francino MP, Antibiotics. and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front Microbiol [Internet]. 2016 [cited 2022 Aug 7];6. https://www.frontiersin.org/articles/https://doi.org/10.3389/fmicb.2015.01543.
Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. 2017;279:90–105.
Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as Major Disruptors of Gut Microbiota. Front Cell Infect Microbiol [Internet]. 2020 [cited 2022 Aug 7];10. https://www.frontiersin.org/articles/https://doi.org/10.3389/fcimb.2020.572912.
Kumar M, Sarma DK, Shubham S, Kumawat M, Verma V, Nina PB et al. Futuristic Non-antibiotic Therapies to Combat Antibiotic Resistance: A Review. Front Microbiol [Internet]. 2021 [cited 2022 Aug 7];12. https://www.frontiersin.org/articles/https://doi.org/10.3389/fmicb.2021.609459.
Lopetuso LR, Giorgio ME, Saviano A, Scaldaferri F, Gasbarrini A, Cammarota G. Bacteriocins and bacteriophages: Therapeutic weapons for gastrointestinal diseases? Int J Mol Sci. 2019;20:183.
Sannathimmappa MB, Nambiar V, Aravindakshan R. Antibiotics at the crossroads – do we have any therapeutic alternatives to control the emergence and spread of antimicrobial resistance? J Educ Health Promot. 2021;10:438.
Todorov SD, Kang H-J, Ivanova IV, Holzapfel WH, Bacteriocins From LAB. and Other Alternative Approaches for the Control of Clostridium and Clostridiodes Related Gastrointestinal Colitis. Front Bioeng Biotechnol [Internet]. 2020 [cited 2022 Aug 7];8. https://www.frontiersin.org/articles/https://doi.org/10.3389/fbioe.2020.581778.
Raheem A, Liang L, Zhang G, Cui S. Modulatory Effects of Probiotics During Pathogenic Infections With Emphasis on Immune Regulation. Front Immunol [Internet]. 2021 [cited 2022 Aug 14];12. https://www.frontiersin.org/articles/https://doi.org/10.3389/fimmu.2021.616713.
Basler M, Pilhofer M, Henderson GP, Jensen GJ, Mekalanos JJ. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature. 2012;483:182–6.
Brunet YR, Zoued A, Boyer F, Douzi B, Cascales E. The type VI Secretion TssEFGK-VgrG Phage-Like Baseplate is recruited to the TssJLM membrane complex via multiple contacts and serves as Assembly platform for tail Tube/Sheath polymerization. PLOS Genet. 2015;11:e1005545.
Cianfanelli FR, Monlezun L, Coulthurst SJ. Aim, load, fire: the type VI Secretion System, a bacterial nanoweapon. Trends Microbiol. 2016;24:51–62.
Coulthurst S. The type VI secretion system: a versatile bacterial weapon. Microbiol Read Engl. 2019;165:503–15.
Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc Natl Acad Sci. 2007;104:15508–13.
Robinson L, Liaw J, Omole Z, Corcionivoschi N, Hachani A, Gundogdu O. In silico investigation of the genus Campylobacter type VI secretion system reveals genetic diversity in organization and putative effectors. Microb Genomics. 2022;8:000898.
Robinson L, Liaw J, Omole Z, Xia D, van Vliet AHM, Corcionivoschi N et al. Bioinformatic Analysis of the Campylobacter jejuni Type VI Secretion System and Effector Prediction. Front Microbiol [Internet]. 2021 [cited 2024 Jun 17];12. https://www.frontiersin.org/journals/microbiology/articles/https://doi.org/10.3389/fmicb.2021.694824/full.
Lin J, Cagliero C, Guo B, Barton Y-W, Maurel M-C, Payot S, et al. Bile salts modulate expression of the CmeABC Multidrug Efflux Pump in Campylobacter jejuni. J Bacteriol. 2005;187:7417–24.
Hellström PM, Nilsson I, Svenberg T. Role of bile in regulation of gut motility. J Intern Med. 1995;237:395–402.
Islam KBMS, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773–81.
Urdaneta V, Casadesús J. Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts. Front Med [Internet]. 2017 [cited 2022 Aug 5];4. https://www.frontiersin.org/articles/https://doi.org/10.3389/fmed.2017.00163.
Verster AJ, Ross BD, Radey MC, Bao Y, Goodman AL, Mougous JD, et al. The Landscape of Type VI Secretion across Human Gut Microbiomes reveals its role in Community Composition. Cell Host Microbe. 2017;22:411–e4194.
Song J, Xiao K, Ke YL, Jiao LF, Hu CH, Diao QY, et al. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult Sci. 2014;93:581–8.
Chen J, Tellez G, Richards JD, Escobar J. Identification of Potential Biomarkers for Gut Barrier Failure in Broiler Chickens. Front Vet Sci [Internet]. 2015 [cited 2022 Aug 7];2. https://www.frontiersin.org/articles/https://doi.org/10.3389/fvets.2015.00014.
Unni R, Pintor KL, Diepold A, Unterweger D. Presence and absence of type VI secretion systems in bacteria. Microbiol Read Engl [Internet]. 2022 [cited 2023 Nov 25];168. https://doi.org/10.1099/mic.0.001151.
Lin YL, Smith SN, Kanso E, Septer AN, Rycroft CH. A subcellular biochemical model for T6SS dynamics reveals winning competitive strategies. PNAS Nexus. 2023;2:pgad195.
Harrison JW, Dung TTN, Siddiqui F, Korbrisate S, Bukhari H, Tra MPV, et al. Identification of possible virulence marker from Campylobacter jejuni isolates. Emerg Infect Dis. 2014;20:1026–9.
Koutsoumanis K, Allende A, Alvarez-Ordóñez A, Bolton D, Bover‐Cid S, Davies R, et al. Update and review of control options for Campylobacter in broilers at primary production. EFSA J. 2020;18:e06090.
Ijaz UZ, Sivaloganathan L, McKenna A, Richmond A, Kelly C, Linton M, et al. Comprehensive Longitudinal Microbiome Analysis of the Chicken Cecum reveals a Shift from competitive to Environmental Drivers and a window of opportunity for Campylobacter. Front Microbiol. 2018;9:2452.
McKenna A, Ijaz UZ, Kelly C, Linton M, Sloan WT, Green BD, et al. Impact of industrial production system parameters on chicken microbiomes: mechanisms to improve performance and reduce Campylobacter. Microbiome. 2020;8:128.
Acknowledgements
SG thanks the UGC, MoE, and Govt. of India. PB thanks CSIR, MoST, and Govt. of India, and BD thanks the IISER-Kolkata for their fellowship support. AIM and DD acknowledge IISER-Kolkata for providing the experimental facilities. AIM thanks the Department of Biotechnology, Government of India for funding support. We thank Dr. P. P. Datta for providing pTurbo-GFP-B plasmid, Prof. A. Karlyshev for providing the pJMK30 plasmid, and Dr. Rahul Das (IISER Kolkata) for helpful discussions. We are also thankful to the FESEM facility and the Central Imaging Facility of the IISER-Kolkata.
Funding
This work was supported by the Department of Biotechnology, Govt, India (grant no. BT/PR40625/AAQ/1/799/2020) to AIM.
Author information
Authors and Affiliations
Contributions
SG performed all the experiments. PB assisted in experiments and BD-synthesized Ir-TBS complex with the help of PG and SM performed histopathological studies. AIM and DD conceived the study and wrote the manuscript with the support of SG, PB, PG, and SM. The chemical synthesis was supervised by PG. The biological experiments were supervised by AIM.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The chicken experimentation protocol was approved by the Institutional Animal Ethics Committee (IAEC) of IISER-Kolkata. All procedures were conducted in accordance with the guidelines of the Committee for Control and Supervision of Experiments on Animals (CCSEA), Ministry of Fisheries, Animal Husbandry, and Dairying, Government of India. The permit number for the experimental chicken protocol is IISERK/IAEC/AP/2022/81. The handling and disposal of the reference strains of Campylobacter jejuni were performed according to the guidelines of the Institutional Bio-Safety Committee (IBSC), IISER-Kolkata (Permit no: IISERK/IBSC/2019/02).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gupta, S., Biswas, P., Das, B. et al. Selective depletion of Campylobacter jejuni via T6SS dependent functionality: an approach for improving chickens gut health. Gut Pathog 16, 38 (2024). https://doi.org/10.1186/s13099-024-00628-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13099-024-00628-6